 |
 |
- Search
| Asian Spine J > Volume 13(6); 2019 > Article |
|
Abstract
Purpose
Overview of Literature
Methods
Results
Conclusions
Fig. 1.
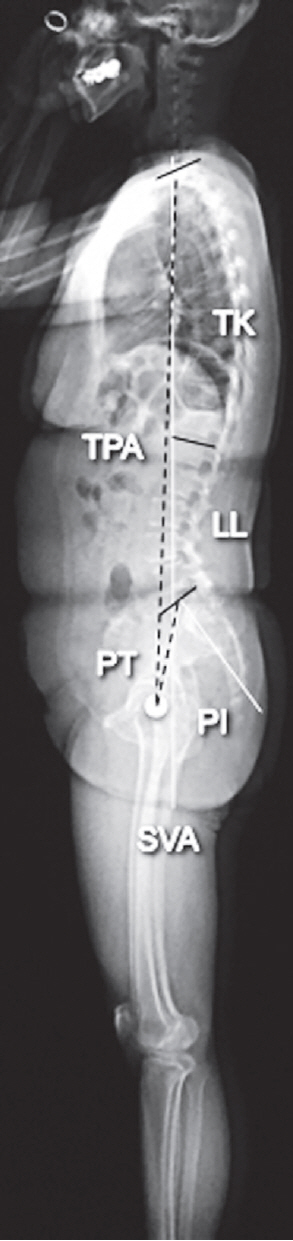
Fig. 2.
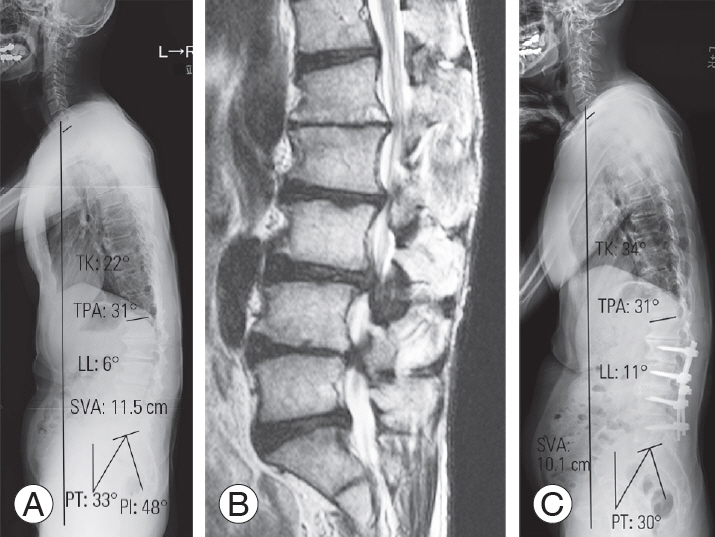
Fig. 3.

Table 1.
| Variable | PLIF | LLIF | p-value |
|---|---|---|---|
| No. of patients | 109 | 58 | |
| Age (yr) | 66.3±12.8 | 69.1±6.7 | 0.12 |
| Gender (male:female) | 51:58 | 29:29 | 0.75 |
| Body mass index (kg/m2) | 24.5±3.8 | 23.9±3.2 | 0.27 |
| Preop JOA score | 13.0±4.8 | 14.5±4.4 | 0.06 |
| Levels of surgery | 0.46 | ||
| L2/3 | 1 | 2 | |
| L3/4 | 22 | 13 | |
| L4/5 | 86 | 43 | |
| Interbody cage | |||
| Height (mm) | 9.6±1.0 | 9.3±0.9 | 0.60 |
| Angle (°) | 1.3±2.4 | 10.0±0.0 | <0.001a) |
| Preop radiologic measurement | |||
| SLA (°) | 8.0±11.0 | 6.8±8.6 | 0.47 |
| DH (mm) | 6.5±7.9 | 7.5±2.7 | 0.36 |
| PI (°) | 51.9±10.8 | 50.3±10.7 | 0.36 |
| LL (°) | 33.9±15.5 | 36.5±15.3 | 0.30 |
| PI−LL (°) | 18.1±16.3 | 13.8±13.6 | 0.09 |
| PT (°) | 22.1±8.7 | 21.2±9.0 | 0.53 |
| SS (°) | 29.3±8.7 | 29.7±10.0 | 0.79 |
| SVA (mm) | 42.8±34.7 | 52.1±38.9 | 0.12 |
| TK (°) | 32.8±12.6 | 35.4±12.3 | 0.20 |
| TPA (°) | 19.0±9.9 | 22.1±13.8 | 0.10 |
| Changes in radiologic parameters (postop−preop) | |||
| ∆ SLA (°) | 2.1±5.0 | 5.1±5.8 | <0.001a) |
| ∆ DH (mm) | 2.2±2.0 | 4.2±2.3 | <0.001a) |
| ∆ LL (°) | 3.9±8.6 | 7.8±7.6 | 0.004a) |
| ∆ PI−LL (°) | −3.6±10.1 | −6.9±6.8 | 0.03a) |
| ∆ PT (°) | −1.3±6.8 | −2.6±5.6 | 0.22 |
| ∆ SVA (mm) | −11.8±33.5 | −17.5±37.2 | 0.32 |
| ∆ TK (°) | 0.4±10.5 | 1.6±7.2 | 0.44 |
| ∆ TPA (°) | −0.9±12.7 | −3.8±10.5 | 0.14 |
Values are presented as number or mean±standard deviation.
PLIF, posterior lumbar interbody fusion; LLIF, lateral lumbar interbody fusion; preop, preoperative; postop, postoperative; JOA, Japanese Orthopedic Association; SLA, segmental lordotic angle; DH, disc height; LL, lumbar lordosis; PI, pelvic incidence; PT, pelvic tilt; SVA, C7 sagittal vertical axis; TK, thoracic kyphosis; TPA, T1 pelvic angle.
Table 2.
| Variable | PLIF | LLIF | p-value |
|---|---|---|---|
| No. of patients | 21 | 28 | |
| Age (yr) | 69.4±9.3 | 73.1±4.2 | 0.07 |
| Gender (male:female) | 10:21 | 10:18 | 0.79 |
| Body mass index (kg/m2) | 23.4±3.5 | 25.2±2.9 | 0.06 |
| Preop JOA score | 11.3±2.9 | 13.1±4.6 | 0.13 |
| Levels of surgery | 0.77 | ||
| L2/3 | 0 | 1 | |
| L3/4 | 21 | 28 | |
| L4/5 | 21 | 27 | |
| Interbody cage | |||
| Height (mm) | 9.6±1.0 | 9.5±1.0 | 0.73 |
| Angle (°) | 1.3±2.0 | 10.0±0.0 | <0.001a) |
| Preop radiologic measurement | |||
| SLA (°) | 5.2±5.4 | 5.3±5.0 | 0.95 |
| DH (mm) | 6.8±2.1 | 6.1±2.6 | 0.32 |
| PI (°) | 53.0±10.0 | 49.4±6.9 | 0.14 |
| LL (°) | 32.7±11.9 | 31.6±13.4 | 0.77 |
| PI−LL (°) | 15.3±12.4 | 17.8±14.2 | 0.52 |
| PT (°) | 21.4±8.9 | 21.6±6.6 | 0.93 |
| SVA (mm) | 56.3±27.7 | 65.9±45.5 | 0.40 |
| TK (°) | 30.2±14.1 | 32.9±8.6 | 0.41 |
| TPA (°) | 24.1±10.9 | 24.0±8.4 | 0.97 |
| Changes in radiologic parameters (post−preop) | |||
| ∆ SLA (°) | 2.6±3.2 | 4.8±4.0 | 0.04a) |
| ∆ DH (mm) | 2.4±1.9 | 4.0±1.5 | 0.002a) |
| ∆ LL (°) | 2.1±6.7 | 8.4±7.0 | 0.003a) |
| ∆ PI−LL (°) | −3.4±7.4 | −9.0±7.3 | 0.001a) |
| ∆ PT (°) | −2.6±6.1 | −3.0±5.1 | 0.80 |
| ∆ SVA (mm) | −12.1±30.3 | −19.1±31.3 | 0.44 |
| ∆ TK (°) | 3.0±10.1 | 1.8±7.4 | 0.63 |
| ∆ TPA (°) | −0.5±9.7 | −3.8±6.1 | 0.15 |
Values are presented as number or mean±standard deviation.
PLIF, posterior lumbar interbody fusion; LLIF, lateral lumbar interbody fusion; preop, preoperative; postop, postoperative; JOA, Japanese Orthopedic Association; SLA, segmental lordotic angle; DH, disc height; LL, lumbar lordosis; PI, pelvic incidence; PT, pelvic tilt; SVA, C7 sagittal vertical axis; TK, thoracic kyphosis; TPA, T1 pelvic angle.
Table 3.
| Variable | PLIF | LLIF | p-value |
|---|---|---|---|
| No. of patients | 14 | 15 | |
| Age (yr) | 66.4±13.4 | 72.8±5.8 | 0.10 |
| Gender (male:female) | 7:7 | 8:7 | 1.00 |
| Body mass index (kg/m2) | 25.2±5.1 | 25.1±4.2 | 0.93 |
| Preop JOA score | 11.8±5.6 | 13.0±2.5 | 0.48 |
| Levels of surgery | 1.00 | ||
| L2/3 | 14 | 15 | |
| L3/4 | 14 | 15 | |
| L4/5 | 14 | 15 | |
| Interbody cage | |||
| Height (mm) | 9.1±0.9 | 9.1±0.8 | 1.00 |
| Angle (°) | 1.3±2.2 | 10.0±0.0 | <0.001a) |
| Preop radiologic measurement | |||
| SLA (°) | 5.3±7.9 | 4.5±3.6 | 0.72 |
| DH (mm) | 6.5±2.4 | 5.8±2.2 | 0.42 |
| PI (°) | 51.3±11.5 | 47.5±8.5 | 0.32 |
| LL (°) | 34.7±15.1 | 23.9±18.9 | 0.10 |
| PI−LL (°) | 16.9±15.6 | 19.6±17.5 | 0.67 |
| PT (°) | 21.8±8.9 | 26.6±9.0 | 0.16 |
| SVA (mm) | 45.6±35.1 | 66.4±43.9 | 0.17 |
| TK (°) | 31.3±12.9 | 29.2±10.7 | 0.63 |
| TPA (°) | 20.4±10.8 | 27.6±10.6 | 0.08 |
| Changes in radiologic parameters (postop−preop) | |||
| ∆ SLA (°) | 2.6±3.2 | 4.8±3.3 | 0.08 |
| ∆ DH (mm) | 2.9±1.1 | 4.1±2.1 | 0.07 |
| ∆ LL (°) | 4.2±9.1 | 12.1±11.1 | 0.047a) |
| ∆ PI–LL (°) | −3.0±9.3 | −11.2±11.3 | 0.043a) |
| ∆ PT (°) | −2.5±5.3 | −6.4±4.9 | 0.049a) |
| ∆ SVA (mm) | −15.5±45.7 | −18.4±26.8 | 0.84 |
| ∆ TK (°) | −0.3±9.7 | 7.8±11.8 | 0.047a) |
| ∆ TPA (°) | −2.8±9.4 | −5.8±7.6 | 0.35 |
Values are presented as number or mean±standard deviation.
PLIF, posterior lumbar interbody fusion; LLIF, lateral lumbar interbody fusion; preop, preoperative; postop, postoperative; JOA, Japanese Orthopedic Association; SLA, segmental lordotic angle; DH, disc height; PI, pelvic incidence; LL, lumbar lordosis; PT, pelvic tilt; SVA, C7 sagittal vertical axis; TK, thoracic kyphosis; TPA, T1 pelvic angle.






