 |
 |
- Search
| Asian Spine J > Volume 14(5); 2020 > Article |
|
Abstract
Purpose
To analyze the factors influencing early disc height loss following lateral lumbar interbody fusion (LLIF).
Overview of Literature
Postoperative disc height loss can occur naturally as a result of mechanical loading. This phenomenon is enabled by the yielding of the polyaxial screw heads and settling of the cage to the endplates. When coupled with cage subsidence, there can be significant reduction in the foraminal space which ultimately compromises the indirect decompression achieved by LLIF.
Methods
Seventy-two cage levels in 37 patients aged 62┬▒10.2 years who underwent single or multilevel LLIF for degenerative spinal conditions were selected. Their preoperative and postoperative follow-up radiographs were used to measure the anterior disc height (ADH), posterior disc height (PDH), mean disc height (MDH), disc space angle (DSA), and segmental angle. Correlations between the loss of disc height and several factors, including age, construct length, preoperative lordosis, postoperative lordosis, disc height, cage dimensions, and cage position, were analyzed.
Results
We found that the lateral interbody cages significantly increased ADH, PDH, MDH, and DSA after surgery (p <0.0001). However, there was a loss of disc height over time. All postoperative disc height parameters, especially the amount of increase in MDH (r =0.413, p <0.0001) after surgery, showed a significant positive association with early disc height loss. The levels demonstrating a significant (Ōēź25%) height loss were those that exhibited a substantial height increase (128.3%, 4.6┬▒3.0 to 10.5┬▒5.6 mm) postoperatively. However, the levels that showed less than 25% height loss were those that exhibited, on average, only a 57.4% height increase post-operatively.
Lumbar interbody fusion is a long-established procedure that can generally be classified as posterior, lateral, or anterior depending on the approach being used. The most commonly used approach is the posterior one, with which either transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion can be performed [1,2]. In the recent past, lumbar interbody fusion via the lateral retroperitoneal approach has gained popularity and has been widely adopted as an alternative (stand-alone modality) or a supplement to posterior surgery [3-7].
Using a lateral approach, interbody cage placement can be conducted either from the front of the psoas muscle (oblique lumbar interbody fusion, OLIF) or through the psoas muscle (direct lateral interbody fusion, DLIF) [8,9]. Both techniques facilitate the insertion of a wide-bodied interbody cage via a minimally invasive approach. Wide-bodied cages not only rest on the thinner central area of the endplate but also occupy the thicker periphery, thereby creating a stronger boneŌĆōcageŌĆōbone interface [10,11]. These cages have a large area for bone graft incorporation so that there can be effective interbody fusion [3,12]. Additionally, better coronal and sagittal correction can be achieved in patients with degenerative deformities [13-16].
The foremost advantage reported by most authors is the indirect decompression that can be achieved without the risk of injury to posterior neural elements [17]. Therefore, stand-alone lateral interbody fusion is performed, although it must be supplemented with posterior instrumentation and decompression when necessary [18]. We generally perform lateral interbody fusion along with posterior instrumentation. Even though this is associated with the morbidity of a second procedure, additional stability can be achieved by building a circumferentially strong construct [18].
Despite these advantages, disc height loss following lateral lumbar interbody fusion (LLIF) is a potential problem, especially during the early follow-up period when fusion has not yet taken place. We consider this period to be the first 6 months after surgery, during which only the posterior construct and the interbody cage offer actual resistance against mechanical loading. Even though minimal postoperative disc height loss can occur naturally as a result of mechanical loading (settling of the cage or the entire construct facilitated by the polyaxial screw heads), when coupled with cage subsidence, it becomes a worrisome phenomenon. It could be an indicator for the loss of indirect decompression that can subsequently lead to the recurrence of radicular symptoms, especially if posterior decompression was not performed. Additionally, it could also compromise stability and lead to implant loosening. Therefore, to study the factors influencing early disc height loss, we conduct a retrospective radiological analysis.
After obtaining approval from the institutional review board, the records of patients who underwent single or multilevel DLIF or OLIF along with posterior stabilization for various degenerative lumbar spine conditions between 2012 and 2016 were retrospectively reviewed. This also included some patients in whom TLIF was conducted as part of the multilevel construct due to unfavorable lateral access at a particular level. We shortlisted the patients whose preoperative, immediate postoperative, and follow-up (1 month, 3 months, and 6 months, respectively) true lateral-view standing X-rays were available for analysis. Among these patients, only those cage levels that did not demonstrate any endplate violation or intraoperative subsidence were selected. Cage levels that did not show neutral endplates were excluded when the measurements were conducted. All initial measurements were conducted digitally in the workstation of our picture archiving and communication system by a study team member and were later reviewed by the principal investigator.
The demographic characteristics of the study sample were then tabulated. Preoperative global (L1ŌĆōS1) lordosis was measured from lateral-view X-ray images. Postoperative X-rays were reviewed to note the postoperative global lordosis, posterior construct length, and number of DLIF and OLIF instances in each patient. Then, each DLIF or OLIF cage level was independently analyzed by measuring the preoperative anterior disc height (ADH), posterior disc height (PDH), disc space angle (DSA), and segmental angle (SA) (Fig. 1). The mean disc height (MDH) was derived using ADH and PDH measurements. Cage dimensions, including length, breadth, height, and lordosis, were noted from operative records. Preoperative measurements were compared to corresponding postoperative measurements using t-tests to identify significant changes. Similarly, postoperative measurements were compared with corresponding measurements at the 1-month, 3-month, and 6-month follow-ups to check for variation.
ADH, PDH, and MDH measured using the immediate postoperative standing lateral-view X-rays were considered as baseline disc heights after indirect decompression. Any loss in MDH during early follow-up in comparison with the baseline MDH was measured. Based on this data, disc height loss was divided into four grades: grade 0, 0%ŌĆō24% loss; grade 1, 25%ŌĆō49% loss; grade 2, 50%ŌĆō74% loss; and grade 3, 75%ŌĆō100% loss. The strength of the correlation between disc height loss and all the collected parameters was analyzed by calculating the correlation coefficient (r). In addition, sagittal cage placement was divided into anterior, middle, and posterior according to the visual assessment of the area occupied by the cage. A chisquare test was performed to check whether there was any significant difference in the occurrence of disc height loss with respect to sagittal cage placement.
All statistical analyses were performed using PASW SPSS ver. 18.0 for Windows (SPSS Inc., Chicago, IL, USA). A probability value (p) less than 0.05 was considered statistically significant. A PearsonŌĆÖs correlation coefficient r-value (representing the strength of correlation) greater than zero was considered a positive association, and values closer to +1 were considered a strong positive association. This study was approved by the Domain Specific Review Board, National Healthcare Group, Singapore (reference no., 2018/00714) and a waiver of informed consent was granted. The study was performed in accordance with the ethical standards mentioned in the most recent version of the 1964 Declaration of Helsinki.
A total of 56 patients were shortlisted for this study. Based on our inclusion criteria, 37 patients aged 62┬▒10.2 years (15 males, 22 females) with 72 DLIF or OLIF cages were selected. All patients were operated on for single or multilevel degenerative spondylosis with or without instability. The mean preoperative global lordosis was 35.7┬░┬▒13.2┬░. Of the 72 cages, nine were of a single level, and the rest were multilevel constructs. Regarding the levels, there were five cages in L1ŌĆōL2, 12 cages in L2ŌĆōL3, 30 cages in L3ŌĆōL4, and 25 cages in L4ŌĆōL5. The mean construct length was 4.2┬▒1.5 segments.
Upon the independent analysis of each cage level, there was no statistically significant change in global L1ŌĆōS1 lordosis (p=0.23) immediately after surgery; however, the ADH, PDH, MDH, and DSA significantly increased (p<0.0001) (Table 1, Fig. 2). This increase was not sustained as we noted a significant decrease in the MDH (p=0.049) and DSA (p=0.03) at 1 month. More pronounced decreases in the ADH, PDH, MDH, and DSA were observed at 3 months and 6 months (Table 2). However, the mean SA remained unchanged throughout the first 6 months.
Based on the above results, it is evident that the lateralaccess cages significantly increased the ADH, PDH, MDH, and DSA after surgery; however, this correction was not sustained as there was a notable decrease in these parameters over time. In total, we had 58 cage levels with grade 0 disc height loss, 13 cage levels with grade 1 disc height loss, and one cage level with grade 2 disc height loss. We did not encounter any grade 3 disc height loss.
There was no correlation between disc height loss and several factors, including age, construct length, preoperative lordosis, postoperative lordosis, cage length, cage breadth, cage height, cage lordosis, DSA, or SA. However, postoperative ADH (r=0.279, p=0.018), PDH (r=0.452, p<0.0001), MDH (r=0.4999, p<0.0001), and the amount of increase in MDH (r=0.413, p<0.0001) after surgery were found to influence the disc height loss by demonstrating an uphill positive correlation (Table 3). No significant difference in the occurrence of disc height loss was noted with respect to sagittal cage placement.
A subgroup analysis was performed, in which our sample of 72 cage levels was divided into two groups according to the grading of disc height loss. Group 1 included those levels that showed grade 1 or above (Ōēź25%) disc height loss. Group 2 included those levels that showed grade 0 (<25%) disc height loss. For interpretation, group 1 will be considered levels with significant disc height loss and group 2 those without significant disc height loss.
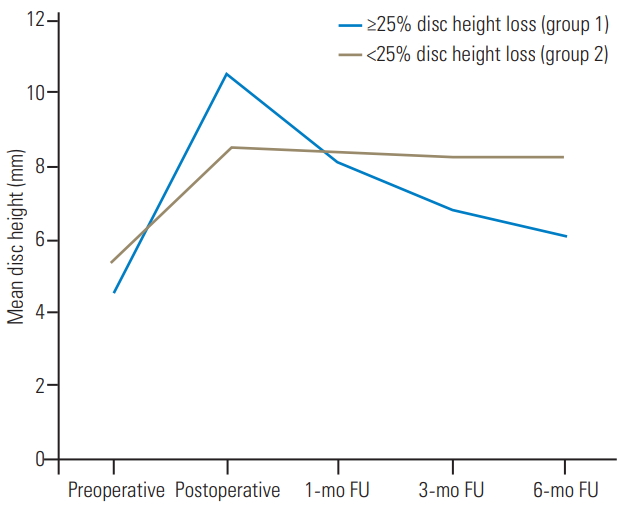
Of those with significant disc height loss (n=14), we noticed that there was a large increase in the ADH (p<0.0001), PDH (p=0.03), and MDH (p=0.0006) after surgery. The MDH increased from 4.6┬▒3.0 mm preoperatively to 10.5┬▒5.6 mm postoperatively (128.3%). However, there was a continual decrease in the MDH throughout early follow-up, and by the end of 6 months, 37.6% of the gained disc height was lost. This was in contrast to that of the other group (n=58, <25% disc height loss), which demonstrated a 57.4% increase in postoperative disc height and only a minimal decrease by the end of early follow-up (Fig. 3).
DLIF and OLIF are lateral-access techniques for lumbar interbody fusion [19]. These fusion techniques can be performed with or without posterior instrumentation [20,21]. However, stand-alone lateral interbody fusion is not advised when there is coexisting facet arthropathy, instability or deformity where high mechanical stress is expected, and when multi-segment fusion is indicated [6,13]. In such cases, when coupled with posterior instrumentation, a circumferentially strong construct that could potentially withstand mechanical forces can be achieved [6,18]. Therefore, considering the available evidence and based on our clinical experience, we always performed lateral interbody fusion along with posterior instrumentation and decompression whenever necessary [22].
In addition to the mechanical stability that lateral-access cages can offer, another significant advantage is the indirect decompression that can be achieved [23]. This is due to the opening up of the foraminal space by the placement of a larger cage. However, there may be a risk of postoperative disc height loss for two main reasons: (1) the settling of the cage construct facilitated by the polyaxial screw heads and (2) cage subsidence [24]. In our study, we noticed that the disc height correction achieved by lateral interbody fusion was not sustained, and there was a significant correction loss during the early follow-up period. Such correction loss may tend to progress, leading to the loss of indirect decompression and subsequent radicular symptoms [24]. Therefore, it is crucial to avoid this complication.
In order to assess the risk factors causing early disc height loss, a correlation analysis was performed. We inferred an uphill positive correlation between disc height loss and the amount of increase in disc height after surgery. Our subgroup analysis showed that the levels with significant disc height loss during early follow-up were those that had exhibited a substantial increase in postoperative disc height. Based on this finding, achieving an optimal disc height rather than overcorrection is an important surgical strategy when placing lateral-access cages. Hence, the selection of an appropriate cage size is crucial.
Therefore, when placing lateral-access cages, surgeons should concentrate on indirect decompression and also be aware of the optimal disc height that needs to be achieved. This way, stability and fusion can be achieved without the complication of early disc height loss. However, we frequently encountered circumstances in which a substantial increase in disc height was absolutely necessary, especially when there was bone on bone (no measurable disc height) preoperatively. In such cases, because disc height loss was anticipated, we always performed primary posterior decompression along with stabilization to avoid the risk of recurrent symptoms. With this strategy, even though disc height loss occurred, we did not encounter recurrent or de novo radicular symptoms during early follow-up among those included in this study.
Overall, we strongly believe that our findings are of clinical importance, especially in circumstances when surgeons opt not to perform posterior decompression and rely entirely on the indirect decompression offered by the cage. In such cases, progressive disc height loss can cause refractory radicular symptoms that may require an additional decompression procedure [22,25]. This study will be the first to consider disc height loss instead of cage subsidence as a potential complication and will be the first to quantify an optimal postoperative increase in disc height that does not lead to subsequent significant disc height loss. However, given that this is a retrospective study, there are a few methodological shortcomings. Because of its limited cohort, potential conclusions should not be drawn from this study. We did not assess the bone mineral density, which may have influenced the occurrence of early disc height loss in the selected elderly population due to osteoporosis. We excluded patients without true lateral-view X-rays from the analysis, which may have led to an underestimation of the rate of disc height loss. In addition, the use of a computed tomography scan to evaluate disc height loss would have been more empirical; however, it was not obtained routinely for all patients.
It is important to recognize the factors influencing early disc height loss following LLIF, as this may affect the outcomes of successful indirect decompression. From this study, we noted that the amount of increase in postoperative disc height was strongly associated with the occurrence of early disc height loss. Therefore, achieving an optimal disc height rather than overcorrection seems to be an important surgical strategy to adopt when placing lateral-access cages.
Fig.┬Ā1.
Measurements of the ADH, PDH, DSA, and SA at a single level. ADH, anterior disc height; PDH, posterior disc height; DSA, disc space angle; SA, segmental angle.
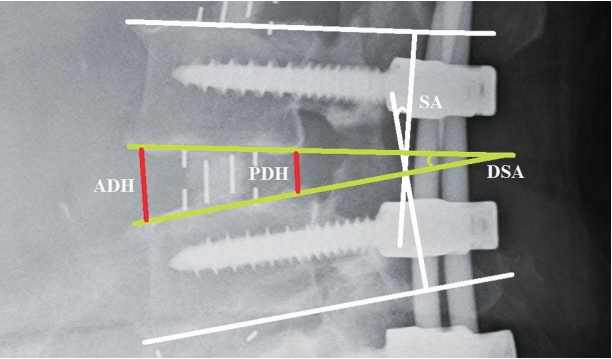
Fig.┬Ā2.
Graph showing the preoperative, postoperative, 1-month, 3-month, and 6-month FU measurements of the ADH, PDH, MDH, and DSA. ADH, anterior disc height; PDH, posterior disc height; MDH, mean disc height; DSA, disc space angle; FU, follow-up.
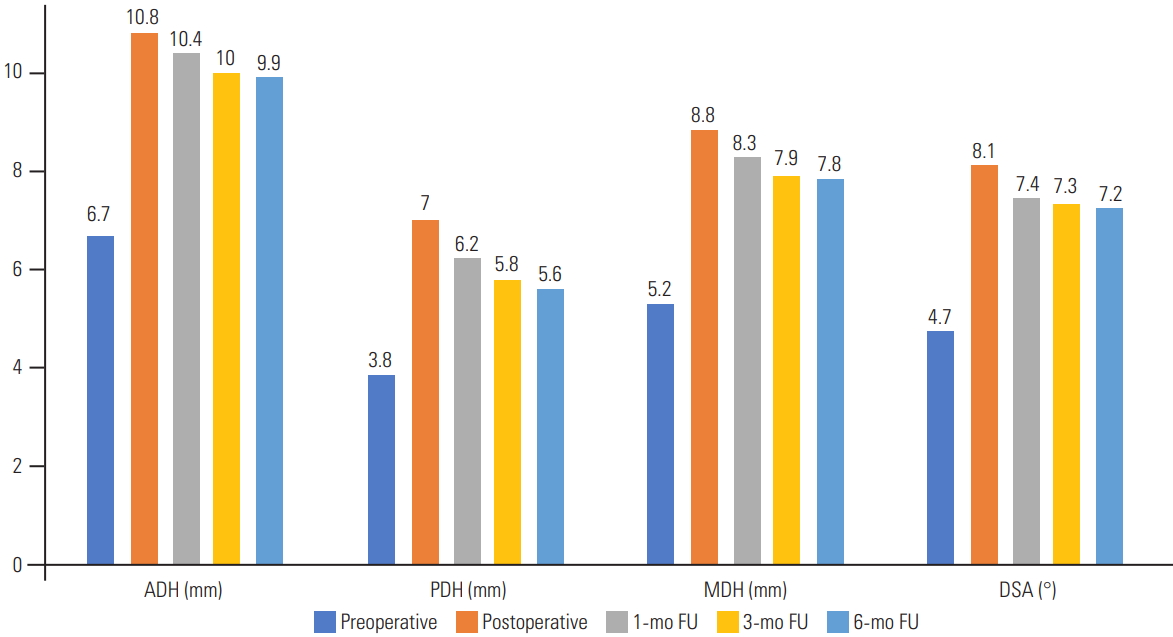
Fig.┬Ā3.
Line chart comparing disc height variations throughout early FUs between levels with and without a significant disc height loss. FU, follow-up.
Table┬Ā1.
Comparison between preoperative and immediate postoperative measurements
Table┬Ā2.
Comparison between immediate postoperative and follow-up measurements
Table┬Ā3.
Influence of various factors over disc height loss during early follow-up
An r-value (correlation coefficient) of greater than 0 was considered as a positive association, and the closer it is to +1 was considered as a strong positive association; a probability value ŌĆ£pŌĆØ of less than 0.05 was considered statistically significant.
Postop, postoperative; MDH, mean disc height.
References
1. Cole CD, McCall TD, Schmidt MH, Dailey AT. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med 2009;2:118ŌĆō26.




2. Sakeb N, Ahsan K. Comparison of the early results of transforaminal lumbar interbody fusion and posterior lumbar interbody fusion in symptomatic lumbar instability. Indian J Orthop 2013;47:255ŌĆō63.



3. Pawar AY, Hughes AP, Sama AA, Girardi FP, Lebl DR, Cammisa FP. A comparative study of lateral lumbar interbody fusion and posterior lumbar interbody fusion in degenerative lumbar spondylolisthesis. Asian Spine J 2015;9:668ŌĆō74.



4. Cheng I, Briseno MR, Arrigo RT, Bains N, Ravi S, Tran A. Outcomes of two different techniques using the lateral approach for lumbar interbody arthrodesis. Global Spine J 2015;5:308ŌĆō14.



5. Cheng JP, Kaliya-Perumal AK, Tandon AA, Oh JY. The oblique corridor at L4-L5: a radiographic-anatomical study into the feasibility for lateral interbody fusion. Spine (Phila Pa 1976) 2019;10.1097/ BRS.0000000000003346.
6. Liu X, Ma J, Park P, Huang X, Xie N, Ye X. Biomechanical comparison of multilevel lateral interbody fusion with and without supplementary instrumentation: a three-dimensional finite element study. BMC Musculoskelet Disord 2017;18:63.




7. Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L. Stand-alone lateral interbody fusion for the treatment of low-grade degenerative spondylolisthesis. ScientificWorldJournal 2012;2012:456346.




8. Gragnaniello C, Seex K. Anterior to psoas (ATP) fusion of the lumbar spine: evolution of a technique facilitated by changes in equipment. J Spine Surg 2016;2:256ŌĆō65.



9. Arnold PM, Anderson KK, McGuire RA Jr. The lateral transpsoas approach to the lumbar and thoracic spine: a review. Surg Neurol Int 2012;3:S198ŌĆō215.



10. Yuan W, Kaliya-Perumal AK, Chou SM, Oh JY. Does lumbar interbody cage size influence subsidence?: a biomechanical study. Spine (Phila Pa 1976) 2020;45:88ŌĆō95.


11. Salzmann SN, Shue J, Hughes AP. Lateral lumbar interbody fusion-outcomes and complications. Curr Rev Musculoskelet Med 2017;10:539ŌĆō46.




12. Berjano P, Langella F, Damilano M, et al. Fusion rate following extreme lateral lumbar interbody fusion. Eur Spine J 2015;24:369ŌĆō71.


13. Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2ŌĆō18.


14. Blizzard DJ, Gallizzi MA, Sheets C, et al. Sagittal balance correction in lateral interbody fusion for degenerative scoliosis. Int J Spine Surg 2016;10:29.



15. Abbasi H, Miller L, Abbasi A, Orandi V, Khaghany K. Minimally invasive scoliosis surgery with oblique lateral lumbar interbody fusion: single surgeon feasibility study. Cureus 2017;9:e1389.



16. Kim JS, Lee HS, Shin DA, Kim KN, Yoon DH. Correction of coronal imbalance in degenerative lumbar spine disease following direct lateral interbody fusion (DLIF). Korean J Spine 2012;9:176ŌĆō80.




17. Malham GM, Parker RM, Goss B, Blecher CM, Ballok ZE. Indirect foraminal decompression is independent of metabolically active facet arthropathy in extreme lateral interbody fusion. Spine (Phila Pa 1976) 2014;39:E1303ŌĆō10.


18. Fukaya K, Hasegawa M. Oblique lumbar interbody fusion combined with minimally invasive percutaneous posterior instrumentation for adult spinal deformity. No Shinkei Geka 2018;46:771ŌĆō81.

19. Jin J, Ryu KS, Hur JW, Seong JH, Kim JS, Cho HJ. comparative study of the difference of perioperative complication and radiologic results: MIS-DLIF (minimally invasive direct lateral lumbar interbody fusion) versus MIS-OLIF (minimally invasive oblique lateral lumbar interbody fusion). Clin Spine Surg 2018;31:31ŌĆō6.


20. Jin C, Jaiswal MS, Jeun SS, Ryu KS, Hur JW, Kim JS. Outcomes of oblique lateral interbody fusion for degenerative lumbar disease in patients under or over 65 years of age. J Orthop Surg Res 2018;13:38.



21. Drazin D, Kim TT, Johnson JP. Simultaneous lateral interbody fusion and posterior percutaneous instrumentation: early experience and technical considerations. Biomed Res Int 2015;2015:458284.




22. Tempel ZJ, McDowell MM, Panczykowski DM, et al. Graft subsidence as a predictor of revision surgery following stand-alone lateral lumbar interbody fusion. J Neurosurg Spine 2018;28:50ŌĆō6.


- TOOLS
-
METRICS

- Related articles in ASJ
-
Bowel Injury and Insidious Pneumoperitoneum after Lateral Lumbar Interbody Fusion2022 August;16(4)





