Value of Hounsfield units measured by chest computed tomography for assessing bone density in the thoracolumbar segment of the thoracic spine
Article information
Abstract
Study Design
A retrospective study.
Purpose
To investigate the correlation between Hounsfield unit (HU) values measured by chest computed tomography (CT) and dual-energy X-ray absorptiometry (DXA) T-scores. HU-based thoracolumbar (T11 and T12) cutoff thresholds were calculated for a cohort of Chinese patients.
Overview of Literature
For patients with osteoporosis, the incidence of fractures in the thoracolumbar segment is significantly higher than that in other sites. However, most current clinical studies have focused on L1.
Methods
This retrospective study analyzed patients who underwent chest CT and DXA at our hospital between August 2021 and August 2022. Thoracic thoracolumbar segment HU values, lumbar T-scores, and hip T-scores were computed for comparison, and thoracic thoracolumbar segment HU thresholds suggestive of potential bone density abnormalities were established using receiver operating characteristic curves.
Results
In total, 470 patients (72.4% women; mean age, 65.5±12.3 years) were included in this study. DXA revealed that of the 470 patients, 90 (19%) had osteoporosis, 180 (38%) had reduced osteopenia, and 200 (43%) had normal bone mineral density (BMD). To differentiate osteoporosis from osteopenia, the HU threshold was established as 105.1 (sensitivity, 54.4%; specificity, 72.2%) for T11 and 85.7 (sensitivity, 69.4%; specificity, 61.1%) for T12. To differentiate between osteopenia and normal BMD, the HU threshold was 146.7 for T11 (sensitivity, 57.5%; specificity, 84.4%) and 135.7 for T12 (sensitivity, 59.5%; specificity, 80%).
Conclusions
This study supports the significance of HU values from chest CT for BMD assessment. Chest CT provides a new method for clinical opportunistic screening of osteoporosis. When the T11 HU is >146.7 or the T12 HU is >135.7, additional osteoporosis testing is not needed unless a vertebral fracture is detected. If the T11 HU is <105.1 or the T12 HU is <85.7, further DXA testing is strongly advised. In addition, vertebral HU values that fall faster than those of the T11 and L1 vertebrae may explain the high incidence of T12 vertebral fractures.
Introduction
Osteoporosis is a systemic bone disease characterized by the loss of bone mass and destruction of bone microstructures. With economic development and social progress, people’s lifestyles have changed dramatically, including bad habits, such as smoking, drinking, lack of physical activity, and staying up late, which are significant triggers for osteoporosis. Patients with osteoporosis, particularly the older population, have a much higher risk of fractures and other problems compared to the general population [1]. With the aging of the population, osteoporosis has become one of the most significant diseases affecting people’s lives, and its incidence is increasing annually. According to statistics [2], in 2015, the incidence of osteoporosis in people aged >50 years in China reached 27.96%.
At present, dual-energy X-ray absorptiometry (DXA) is considered the gold standard for detecting osteoporosis [3]. However, because of the effects of vessel wall calcification, articular process hyperplasia, and degenerative bone spurs, DXA-measured values are high, resulting in false negatives. Quantitative computed tomography (CT) can accurately distinguish the cancellous bone for the early diagnosis of osteoporosis. However, its application in clinical practice is limited because of the high cost of equipment, complex post-processing analysis, and high radiation exposure [4].
Many studies have shown that the Hounsfield unit (HU) values of the bone trabecula obtained by a routine CT can be used to assess osteoporosis [5], such as in the lumbar spine [6–8], cervical spine [9,10], thoracic spine [11], femur [12], pelvis [13], and ulna [14]. In clinical practice, chest CT [15], a widely used and highly accessible test, can be used for lung cancer screening and chronic obstructive pulmonary disease detection. Osteoporosis can be predicted using the HU value of the spine measured by chest CT without additional cost and radiation exposure.
The thoracolumbar segment is susceptible to fractures because of the loss of rib stabilization, change in the spinal curvature (from posterior convexity of the thoracic spine to anterior convexity of the lumbar spine), which causes stress concentration, and change in the direction of the facet joints (from the coronal plane of the thoracic spine to the sagittal plane of the lumbar spine), which increases rotational load. In patients with osteoporosis, the incidence of fractures in the thoracolumbar segment is significantly higher than that in other sites, with T11, T12, and L1 as the most susceptible vertebrae [16]. However, most current clinical studies have focused on L1, with a few opting for T12 replacement because of L1 fractures, and a few involving T11. Therefore, in the present study, chest CT was selected for opportunistic screening of osteoporosis in a Chinese population to obtain optimal HU values for predicting the thoracolumbar segment (T11 and T12) of the thoracic spine in patients with osteoporosis, osteopenia, and normal bone density.
Materials and Methods
Patient population
Patients who attended inpatient or outpatient clinics at the Jiangsu Provincial Hospital of Integrative Medicine between August 1, 2021, and August 31, 2022, were retrospectively analyzed. All patients underwent chest CT and DXA. All patient information and image data are visible only to the authors. Moreover, 533 patients underwent chest CT and DXA. The patient screening flow chart is shown in Fig. 1. In addition, 39 patients had thoracic spine fractures and underwent internal fracture fixation. The CT images of 21 patients contained only T11 and above images, and T12 was not scanned. One patient presented with tumor bone metastasis. DXA information was incomplete in two patients. Thus, 63 patients were excluded, and the remaining 470 patients were included in the analysis (Fig. 1).
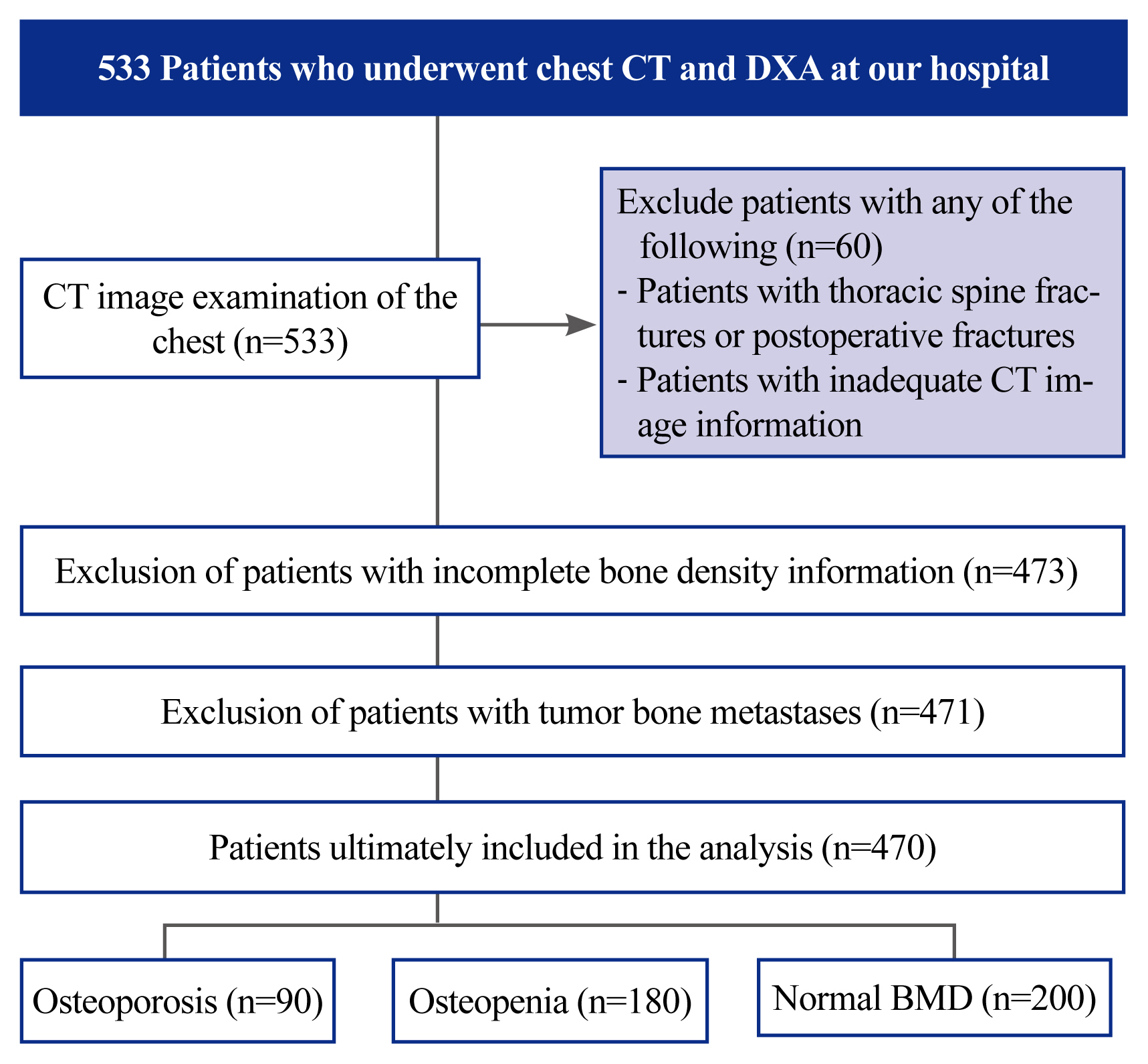
Patient inclusion/exclusion flow chart. A total of 533 patients who underwent chest computed tomography (CT) and bone density examination in our hospital were included. Of these, 470 patients with incomplete imaging, bone density information, and those who had fractures, fractures after internal fixation, or tumor bone metastases were excluded. The patients were divided into three groups according to their T-scores: osteoporosis group, bone loss group, and osteopenia group. DXA, dual-energy X-ray absorptiometry; BMD, bone mineral density.
All data collection and analysis conducted in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. The study protocol was approved by the Institutional Review Board of Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine (2022-LWKY-020). The Institutional Review Board waived the need for written informed consent from the patients because of the retrospective design of this study.
Dual-energy X-ray absorptiometry and computed tomography data acquisition
DXA
All patients underwent DXA of the lumbar spine (L1–L4) and hip. DXA measurements were performed using GE Medical Systems-LUNAR (GE Healthcare, Madison, WI, USA). Osteoporosis was diagnosed when the T-score on either lumbar or hip DXA was low, as lumbar and hip DXA results were considered. According to the criteria of the World Health Organization [17], osteoporosis was defined as a T-score of ≤–2.5, osteopenia as −2.5< T-score ≤–1.0, and normal BMD as a Tscore of >–1.0.
Chest CT
Chest CT examinations were mainly performed on the following two machines: GE 64-row 64-layer (Light-speed VCT; GE Healthcare) CT or Neusoft Medical Systems 64-row 128-layer (NeuViz 128 Precision CT; Neusoft Medical Systems, Lima, Peru) CT. The patient was placed head first, and chest CT was performed. The CT scanning parameters were as follows: tube voltage, 120 kV; automatic milliampere-second technique tube current; collimation, 64×0.625 mm; pitch, 0.9 to 1; rotation time, 0.5 seconds; and field of view, 500 mm. The CT scan data were transferred to the workstation and reconstructed with standard algorithms with reconstruction layer thicknesses of 1.25 (GE VCT) and 1 mm (NeuViz 128 CT), displaying a field of view of 380 mm. All chest CT aminations were performed without a venography. The scanner was calibrated daily to ensure accurate vertebral CT attenuation numbers.
The HU values of the thoracic spine were measured using the authors’ picture archiving and communication system. First, the sagittal plane of the CT image was selected to determine the measurement position. A circular region of interest (ROI) was then drawn on the corresponding vertebral trabecular axial position to make it as large as possible. However, it excluded the cortical bone of the vertebral body, surrounding venous plexus, and trophoblastic foramen. Finally, the HU values of the three parts near the upper, middle, and lower endplates were measured and then averaged (Fig. 2). All measurements were conducted by a physician who was unaware of the DXA results. Another author randomly selected 20 patients for the comparisons of measurements.
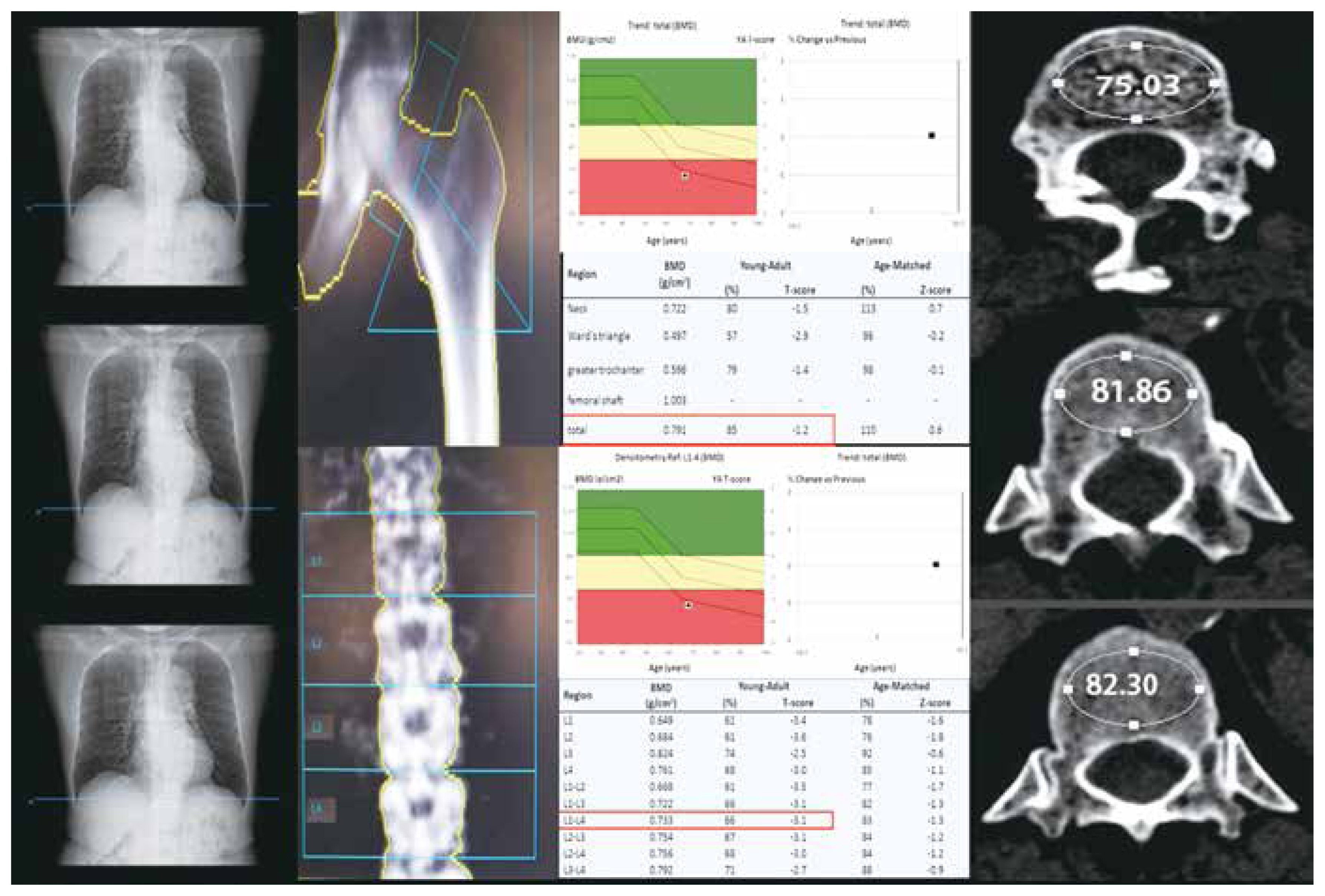
Hounsfield unit (HU) measurements at three different locations in the same vertebral body of the patient; bone mineral density (BMD) and T-scores of the patient’s lumbar spine and hip. Both sides of the Figure show the process of measuring the HU value of the patient’s T12 vertebral body 3 times. Using our own picture archiving and communication system (PACS) system, we first selected the sagittal position of the computed tomography (CT) image to determine the measurement location. Then, we drew a circular area (region of interest) on the corresponding vertebral trabecular axis to make it as large as possible, but excluding the vertebral cortical bone, surrounding venous plexus, and trophoblastic foramen. Finally, the HU values were measured at the proximal superior endplate, middle, and proximal inferior endplate, which were 75.03, 81.86, and 82.30, respectively, with a mean value of 79.73. The middle of the Figure shows the dual-energy X-ray absorptiometry (DXA) test results of the patient’s total hip and lumbar spine. The results were obtained from our bone densitometer. The patient’s hip T-score was −1.2; the lumbar spine T-score was −3.2; −3.2 was used as the patient’s final T-score. The CT images and bone density images were exported from our hospital PACS system and later synthesized by Photoshop.
Statistical analysis
Descriptive statistics, including means and 95% confidence intervals (CIs), were employed for continuous variables related to patient demographics. The HU values for the three BMD subgroups (normal, osteopenia, and osteoporosis) were expressed as medians. The Spearman correlation coefficient was employed to calculate the correlation between the HU value, BMD, and T-score [18]. To determine statistical differences, a rank-sum test was used to compare the HU values of T11 and T12 with the DXA-measured T-scores. Sensitivity, specificity, and area under the receiver operating characteristic (ROC) curve (AUC) were calculated for the three groups. The maximum value of the Youden index was used as the threshold for selecting the best HU in the ROC curve. All data were collected and stored in standard spreadsheets (Microsoft Excel; Microsoft Corp., Redmond, WA, USA). All statistical results were obtained using IBM SPSS Statistics for Windows ver. 25.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 470 patients were enrolled in the study; 340 were women (72.4%) and 130 were men (27.6%). The average age was 65.5±12.3 years, the average height was 1.62±0.08 m, the average weight was 62.9±11.0 kg, the mean BMD of the lumbar spine was 1.02 g/cm2 (range, 0.56–1.92 g/cm2), the mean T-score was −0.89 (range, −4.6 to 6.2), the mean hip BMD was 0.89 g/cm2 (range, 0.52–1.81 g/cm2), and the mean T-score was −0.38 (range, −3.3 to 4.9). Male patients had significantly higher BMD and T-scores than female patients. The mean HU values of T12 and T11 were 121.8±53.5 and 129.5±57.4, respectively. The mean HU values were significantly higher in male patients than in female patients (Table 1).
Table 2 shows the correlation between the HU values of T11 and T12 and BMD and T-scores in the lumbar spine and hip. A significant positive correlation was observed between HU values and T-scores in the thoracic spine (r=0.576, p<0.01). The correlation between the HU values and total hip was significantly stronger than that of the lumbar spine for both T-scores and BMD.
All patients were classified into the following groups according to the T-scores provided by the DXA test: osteoporosis group (n=90, 19.1%), osteopenia group (n=180, 38.3%), and normal BMD group (n=200, 42.6%). Statistical analysis revealed statistically significant differences in the median T11 and T12 HU values in all three groups (p<0.01).
Fig. 3A shows that the HU values of T11 and T12, which represented the best ratio between sensitivity and specificity for distinguishing osteoporosis from osteopenia, were 105.1 and 85.7, respectively. When the HU value of T11 was ≤105.1, 65 of 169 patients were found to have osteoporosis. The AUC was 0.682 (95% CI, 0.615–0.748), with a sensitivity of 54.4% and specificity of 72.2%. When the HU value of T12 was ≤85.7, osteoporosis was found in 55 of the 120 patients. The AUC at this site was 0.683 (95% CI, 0.616–0.750), with a sensitivity of 69.4% and specificity of 61.1% (Tables 3, 4).
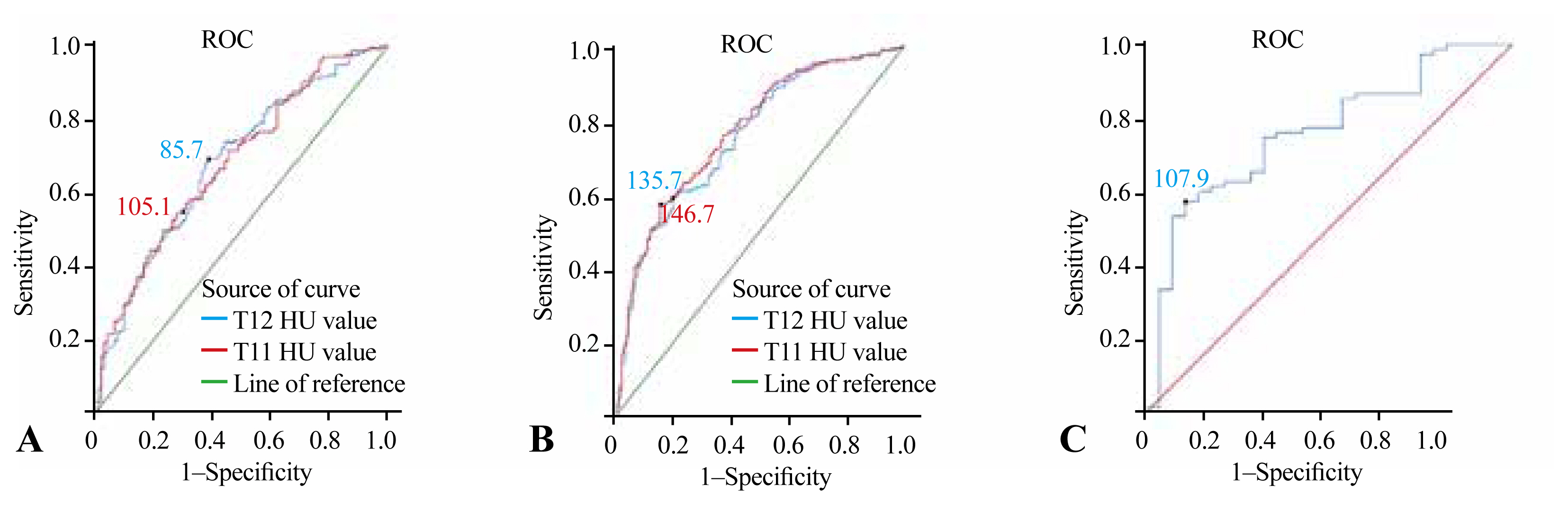
Receiver operating characteristic (ROC) curve of Hounsfield unit (HU) values at T11, T12, and L1. (A) shows the area under ROC curve (AUC) were 0.682 (95% confidence interval [CI], 0.615–0.748) and 0.683 (95% CI, 0.616–0.750), respectively. The HU values for T11 and T12 to distinguish osteoporosis from osteopenia were 105.1 and 85.7, respectively. (B) represents ROC curve of HU values at T11 and T12 showed that the AUC was 0.769 (95% CI, 0.722–0.816) and 0.756 (95% CI, 0.708–0.804), respectively. The HU values of T11 and T12 to distinguish osteopenia from normal bone mineral density (BMD) were 146.7 and 135.7, respectively. (C) shows the AUC of the ROC line was 0.761 (95% CI, 0.660–0.862). Its HU value of L1 to distinguish osteoporosis from non-osteoporosis was 107.9. Using IBM SPSS ver. 25.0 software (IBM Corp., Armonk, NY, USA). (A) represents the HU values of the thoracic lumbar segment of the thoracic spine in the osteoporosis and osteopenia groups were imported, and the ROC curve analysis was run to calculate the HU values corresponding to the specificity and sensitivity in the data of both groups, and to calculate the Yordon index. The HU values corresponding to the maximum Jorden index were taken as the optimal thresholds for differentiating osteoporosis from osteopenia at 105.1 and 85.7, respectively, corresponding to area under the curve of 0.682 and 0.683. Using the IBM SPSS ver. 25.0 software, (B) represents the HU values of the thoracic lumbar segment of the thoracic spine in the bone-reduced and normal BMD groups were imported, and the ROC curves were run to calculate the HU values corresponding to the specificity and sensitivity in the data of the two groups, and to calculate the Yordon index. The HU value corresponding to the maximum Jorden index was taken as the best threshold to distinguish between osteopenia and normal BMD, which was 105.1 and 85.7, respectively, corresponding to area under the curve of 0.682 and 0.683. The L1 HU values of 103 patients were counted. Because of the small number of patients, they were divided into only two groups: osteoporotic and non-osteoporotic groups. Using IBM SPSS ver. 25.0 software, (C) represents that ROC curve analysis was performed to calculate the HU values corresponding to the specificity and sensitivity of the data in the two groups, and to calculate the Youden index. The AUC was 0.761 (95% CI, 0.708–0.804), with a sensitivity of 57.3% and specificity of 89.3%.
Fig. 3B shows that the HU values of T11 and T12, which represented the best ratio between sensitivity and specificity for distinguishing osteopenia from normal BMD, were 146.7 and 135.7, respectively. When the HU value of T11 was ≥146.7, 115 of 146 patients were found to have normal BMD. The AUC was 0.769 (95% CI, 0.722–0.816), with a sensitivity of 57.5% and specificity of 84.4%. When the HU value of T12 was ≥135.7, 119 of 162 patients were found to have normal BMD. The AUC at this site was 0.756 (95% CI, 0.708–0.804), with a sensitivity of 59.5% and specificity of 80% (Tables 3, 4).
In the calculation of the thoracic spine HU value by chest CT, some chest CT images were found to contain L1. Therefore, the HU values of L1 were also measured. Because there were only 103 patients, they were divided into two groups: osteoporosis and non-osteoporosis groups. Fig. 3C shows the HU value of L1 at 107.9, which represents the best ratio between sensitivity and specificity to distinguish the osteoporosis group from the non-osteoporosis group. The AUC at this site was 0.761 (95% CI, 0.708–0.804), with a sensitivity of 57.3% and specificity of 89.3%.
Seven studies have reported HU thresholds for osteoporosis testing, such as T12 or L1, from different ethnic and geographic populations. The sensitivity and specificity of the study population and the current use of HU thresholds are presented in Table 5 [19–25].
Discussion
This study investigated the opportunistic assessment of the patient’s entire bone mass using vertebral HU values provided by chest CT, calculated the HU value threshold, and determined whether the patient required further examination related to osteoporosis to improve the early detection rate of clinical factors and reduce the risk of fractures.
Chest CT is a commonly used method in daily examinations. Thoracic spine HU values can be measured by chest CT. T11 and T12 are at the turning point of the thoracolumbar spine. These are more similar to the BMD of the lumbar spine. To measure the HU values of L1, previous investigators mostly used abdominal CT [23,26]. In L1 fractures, T12 is used instead of L1. In contrast, most abdominal CT images did not include the level of the thoracic spine. Therefore, the prediction of osteoporosis by thoracic spine HU values is significant. Few studies have evaluated T12, and no study has yet proposed an optimal HU threshold of T11 to predict osteoporosis. Therefore, 470 patients who underwent chest CT were included in this study, and the HU values of T11 and T12 were measured separately for the study.
The researchers represented the entire vertebral body by measuring the HU values of a single oval ROI vertebra. The vertebral body has a three-dimensional structure; therefore, a single ROI may lead to questionable accuracy and reproducibility of measurements [20]. Therefore, in this study, three locations of the same vertebral body were measured, i.e., near the upper middle plate, middle plate, and lower endplates, and the HU value of the whole vertebral body was represented by the mean of three measurements. Cohen et al. [21] found no statistically significant difference in HU values measured in axial and sagittal vertebral bodies. Therefore, in the present study, the axial position closer to the ellipse was chosen for the HU value measurement.
The DXA T-score is now a common way to diagnose osteoporosis. Compared with BMD, the T-score is more accurate for the diagnosis of osteoporosis [25]. Other indicators have been developed to assess osteoporosis, such as the trabecular bone score (TBS). The TBS, which is primarily applied to the lumbar spine, is a texture index used to assess pixel-by-pixel grayscale changes in DXA images of the lumbar spine. Compared with DXA, the TBS reflects the three-dimensional characteristics of the bone to a certain extent and can provide information on bone microstructure and strength and additional information on fractures [27,28]. Using multivariate regression techniques, some researchers have revealed that the TBS is less affected by degenerative changes in the spine than the BMD. The comparison of the TBS with vertebral HU values revealed that both performed similarly in assessing patients’ osteoporosis risk, suggesting that the TBS was able to ignore the effect of spinal degenerative changes on the vertebral BMD [29]. However, differences in the DXA scan acquisition modes, differences between bone densitometer builders, and scanner resolution influence the TBS [30]. Previous studies have shown a correlation between lumbar HU values and DXA T-scores [8]. The present study confirmed that thoracic spine HU values also positively correlated with the T-score. Therefore, the criterion for evaluating the osteoporosis status of patients in this study was based on the minimum T-score at the lumbar spine or hip.
Using ROC curves, the optimal HU thresholds of the T11 and T12 to distinguish osteoporosis from osteopenia were 105.1 and 85.7, respectively. The sensitivity of the HU threshold of T12 was higher than that of T11 in detecting osteoporosis (69.4% versus 54.4%). The correlation between T12 and DXA findings was higher than that of T11. Therefore, the diagnostic value of T12 is higher than that of T11. Other study reported that the HU threshold of T12 to distinguish osteoporosis from non-osteoporosis was 104, which was quite different from the HU value of T12 in this study [19]. This may be due to the specific distinction between osteoporosis and osteopenia in this study and the reduction of the range of cases, resulting in smaller HU values. In addition, BMD values vary among ethnic groups. A study showed the highest mean lumbar spine BMD among African–Caribbeans and African–Americans and the lowest among Hong Kong Chinese [31].
The optimal HU values of the T11 and T12 to distinguish osteopenia from normal BMD were 146.7 and 135.7, respectively. This is similar to the findings of Kim et al. [18] and Kim et al. [24]. Osteoporosis was found in only three of 146 patients (2%) if the HU value was ≥146.7. When using an HU value of ≥135.7, osteoporosis was found in only seven of 162 patients (4%). Therefore, when the vertebral body is greater than these two thresholds, the patient does not need to undergo additional DXA unless the patient has a fracture. In contrast, only nine of 119 patients (8%) with an HU value of <85.7 had normal BMD. Consequently, such patients are highly suspected of having abnormal bone density, and DXA is recommended. A comparison with other populations revealed that the thresholds determined to distinguish osteoporosis from osteopenia were lower than those in other regions. The HU values of the thoracolumbar spine in other areas were between 99 and 146. The HU threshold of T12 reported by Buckens et al. [19] is similar to the HU threshold of T11 in the present study. When the thresholds for distinguishing osteopenia from normal BMD were compared with those of other researchers, they were similar in Chinese, Korean, and American studies but higher than those reported by Israeli and Dutch researchers. Therefore, the threshold values obtained in the present study are consistent with those of the Asian population.
Osteoporotic vertebral compression fractures are common and account for approximately 40% of all osteoporotic fractures [32]. The risk of fracture of the thoracolumbar junctional vertebrae is significantly higher than that of the other vertebrae. The most susceptible vertebrae are T11, T12, and L1, with T12 having the highest incidence of vertebral fractures [16,33,34]. In this study, the HU values of L1 were assessed by chest CT in 103 patients, and the optimal HU value for tabulating the distinction between osteoporosis and non-osteoporosis was 107.9, which was similar to the results of a previous study [6]. Comparing the HU thresholds of the three vertebrae, the HU thresholds of T11 and L1 to distinguish osteoporosis were similar, and the differences when compared with the threshold of T12 were 18.4 and 22.2, respectively. Therefore, a faster decline in the HU value of T12 among the three vertebral bodies may explain the high incidence of T12 fractures.
This study has some limitations. Significantly more women than men were analyzed. This can be attributed to the significantly higher average life expectancy of women than of men and the significantly higher prevalence of osteoporosis in postmenopausal women than in men. We attempted to mitigate the effects of sex imbalance through random grouping. In the future, sex control can be strengthened to explore the differences in HU values between sexes. All clinical factors (smoking, diabetes, race, and anti-osteoporosis treatment) were not considered in this study; thus, further research is required. This study may have been subjected to selection bias because medical conditions were not considered. Therefore, a relatively large number of consecutive patients (n=470) was included. All HU values are subject to measurement errors because of factors such as differences in CT equipment and semiautomatic measurements. To address equipment differences, screening is conducted only after validation of the machine’s effect. The TBS was not included in the indicators of patient records because it is mainly used in the lumbar spine. The correlation between the TBS and thoracic spine HU values can be further explored in the future to expand the use of the TBS.
Conclusions
The HU value obtained from chest CT showed a correlation with the relevant evaluation value obtained by DXA. This indicates that the HU values measured by chest CT have potential for use in BMD assessment. When the HU value of T11 is >146.7 or that of T12 is >135.7, additional osteoporosis testing is not required unless a vertebral fracture is detected. If the HU value T11 is <105.1 or that of T12 <85.7, further DXA testing is necessary. We recommend the inclusion of the thoracic spine HU values in the report at the time of chest CT examination. It helps increase the detection rate of osteoporosis and helps patients who may benefit from further DXA testing. Moreover, the higher incidence of T12 fractures may be explained by the more rapid decline in vertebral HU values compared to the upper and lower vertebral bodies.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was supported by Project of National Clinical Research Base of Traditional Chinese Medicine in Jiangsu Province, China (JD2022SZXMS07), Scientific Research Project of Jiangsu Provincial Health Commission (M2022095), and the Seventh Batch of National Chinese Medicine Experts’ Academic Experience Inheritance Work Project (22QGSC6).
Author Contributions
Conception and study design: CX, ZX. Data acquisition and analysis of data: GS, NW, XL, GH. Drafting of the manuscript: CX. Obtaining funding: YW, ZX. Critical revision: YW, ZX. Final approval of the manuscript: all authors.





