 |
 |
- Search
| Asian Spine J > Volume 18(2); 2024 > Article |
|
Abstract
Decompression is a major component of surgical procedures for degenerative lumbar spinal stenosis (LSS). In addition to sufficient decompression to guarantee the relief of neurological pain, compensating surgical instability after wider laminectomy and foraminotomy and instrumentation with caging and fusion with grafting are performed to secure or restore the foraminal dimension and correct coronal/sagittal imbalance for longer survival of the adjacent segment. Endoscopic spinal surgery (ESS) has been developed under the flag of successful decompression while preserving structural integrity as much as possible with the help of magnification and illumination. ESS provides a technical possibility and feasibility for solving LSS by decompression alone. Recently, many endoscopic trials have been conducted to overcome conventional surgical treatment that requires wider dissection, escape inevitable complications from surgical damage, and compensate for the fusion technique. However, biportal ESS has some technical limitations, including clinical difficulties in accessibility for more moderate to severe stenosis and challenges for complicated conditions with segmental ventral slip, isthmic defect, stenosis combined with foraminal stenosis or foraminal disk rupture, or degenerative segmental scoliosis with disk height collapsing and endplate fatigue fracture. Because decompression alone is a skill for eliminating pathologies, there is no function of preserving degenerative structure or stopping the recurrence of disk degeneration or subsidence. This review of clinical reports investigated the possibility of biportal ESS for treating degenerative lumbar disorders by sufficient decompression and adequate elimination of various pathologies and decreasing technical complications. The results of this study may help develop better innovative spinal surgical techniques in the near future.
The best surgical treatment option for lumbar spinal stenosis (LSS) must be decompression [1]. There have been trials, and several techniques have been developed under the name of “sufficient decompression,” showing their own benefits and limitations [2–4]. In early 1990, subtotal laminectomy with wider decompression showed clinical success rates of 78%–88% in the early postoperative period, which decreased to approximately 70% at 1 year and approximately 50% in 5 years [5,6]. On subtotal laminectomy, a larger amount of the surrounding soft and bony tissues should be eliminated to inspect pathologies located deeper in the central canal and foramen under naked-eye identification, causing loss of laminar continuity and facet stability. This is believed to be one of the main reasons for the early deterioration of clinical results and the high incidence of conversion to additional instrumented fusion, despite reports that instrumentation does not affect clinical results [7]. Schaeren et al. [8] studied the concept of an ideal decompression technique that preserves laminar continuity on laminotomy and sufficient foraminal decompression with an intact isthmus. However, ideal decompression has technical limitations in complicated cases using open dissection with the naked eye. Another trial involved wider decompression with subtotal laminectomy and posterolateral fusion (PLF) with instrumentation. Instrumentation could prevent early deterioration of surgical instability. Fusion helps prevent screw loosening and increase the longevity of its structural stability in the mid-to-longer follow-up period. However, the pitfalls of back muscle surgical injury by deeper and wider dissection and loss of normal spinal contouring by instrumentation have not been considered until instrumented PLF became widespread and popular [9]. To overcome laterally wider back muscle dissection for serving bone graft bed on posterolateral side of facets and restoring lower back curvature, posterior interbody fusion (PLIF) was attempted. PLIF in open wider dissection is considered highly invasive with a complication rate of 25%, including 14% of durotomy and 3.5% of infection and is associated with several surgical factors, including more bleeding and longer operating time for intradiscal manipulation, bone harvesting, and grafting [10,11]. As instrumented fusion for degenerative lumbar diseases surged dramatically in early 2000, adjacent segment disorders (ASD) became an unexpected complication with an incidence of >10% 3 years postoperatively, with consequently frequent need of revision extended fusion. Currently, it must be a nonnegligible issue in the era of spinal fusion with popularity [12–15]. Surgical injury of the back muscles with early degeneration or atrophy is an important reason for ASD, which justifies the need for less or minimally invasive surgery [16]. Clinical trials on tubular surgery under microscopic view revealed several techniques, including minimally invasive surgery (MIS)–transforaminal lumbar interbody fusion with established key principles, minimizing soft tissue disruption and destabilization of spinal segments, unilateral laminectomy and bilateral decompression of the central canal (ULBD), and indirect foraminal decompression using the cage technique [17]. Using cage techniques, several MIS trials are dramatically changing and developing for additional benefits, including sagittal balance correction in longer-level instrumented fusion to minimize physical factors on ASD. However, one-sided soft tissue release and elevation of bilateral-sided disk height using a hard cage in osteoporotic vertebrae resulted in a higher rate of cage-related problems, including cage retropulsion, endplate breakage, and foramen collapse, which consequently delayed foraminal stenosis or screw loosening [18–20].
Decompression alone was recently reintroduced in the MIS era to perform sufficient decompression for pain relief and decrease instrumented fusion for senile patients under natural spinal balance at their age. Therefore, previous open wider or tubular surgeries used relatively vertical accessibility; therefore, overlying functional bony structures should be widely removed to inspect the central canal and deeper foraminal areas, causing surgical instability that requires instrumented fusion. Biportal ESS can permit accessibility into the sublaminar space, ranging from the central canal to the deeper foramen [21–23]. Even in spondylolisthesis in a relatively well-preserved state of disk integrity with no embrittled endplate, instrumented fusion for observational radiographic instability is no longer the first-line surgical treatment option. Observational radiographic instability, that is, >4° ventral slip or 10° hypermotion in dynamic simple radiographs, is no longer an absolute indication of fusion. Surgeons’ choice of instrumented fusion could mostly depend on their own decompression techniques, that is, wider open, tubular, or endoscopic, rather than considering patients’ anatomically specific configuration [7,24,25].
Biportal ESS uses an arthroscopic system for inspection under higher magnification and brighter illumination, which is different from open wider surgery under naked eyes, requires digging deeper in a dark and narrow path, and faces difficulty in clearly differentiating structural margins as well as securing a working space for instrument handling and careful manipulation of neural structures [4,23,26,27]. An 8-mm sheathed scope guarantees a panoramic view with a wider range of movement under the sublaminar area and foramen and allows for free movements of the instruments, permitting forceful manipulation of hard bone and adhesive tissue to eliminate pathologies. Its accessibility while preserving laminar and isthmic continuity decreases the need for instrumented fusion for LSS in moderate to severe stages. It could also be beneficial to senile patients with higher comorbidities who require multilevel decompression and longer-level instrumented fusion or revision surgery for ASD or delayed foraminal stenosis at index levels due to cage subsidence [28,29]. Another reason for considering decompression alone rather than wider decompression and instrumented fusion is the higher incidence of persistent spinal pain after surgery. Failed back surgery syndrome is defined as all types of back pain, including those of unknown origin. A recent new definition has emerged: persistent spinal pain syndrome (PSPS), which is defined as sustaining back and radicular pain only after a surgical procedure [30,31]. Moreover, reports on this issue have been increasing. The incidence of PSPS has been reported to be >10%, reaching as high as 40% [31]. The surgical outcome of revision surgery is not good, with <50% of patients satisfied [32–34]. The most common reasons for PSPS are inadequate decompression of the spinal canal or foramen (approximately 25%–29%) and judicious decompression with iatrogenic instability (approximately 2.1%–2.9%) [35,36]. Nerve root entrapment in epidural fibrosis or battered root syndrome after careless manipulation with aggressive root traction could be another reason [31]. These are common situations in root and dural protection-and-traction using a root retractor in bone grafting and cage insertion into the interbody space. However, spinal pain is not eliminated by decompression alone, even in cases where laminar continuity is preserved without observational radiographic instability. When the disk endplate is relatively sound with no subtle or fatigue fracture, the segment must be within the normal functional range in daily life. In contrast, a fractured endplate with a fully degenerative disk is observed on one side, particularly the anterior side, with a macerated bony edge of the vertebral body; this phenomenon is called clinical symptomatic instability (Fig. 1). This means that there is still ongoing endplate collapsing, which is not following the stabilizing theory by Kirkaldy-Willis et al. [37] at the final stage. Back and buttock referred pain frequently interferes with daily activities in such conditions. Clinically, the facet function of sustaining against axial or rotational stress is lost, and the disk is fully degenerated and collapsed until the endplate is broken down, bringing about chronic back and buttock pain. Under such conditions, decompression alone cannot prevent axial or rotational stress on the brittle endplate, and the endplate’s subtle fracture progresses. It is the so-called creeping subsidence of endplates, indicating clinical symptomatic instability that requires fusion.
For successful decompression of traversing and exiting roots in a segment and selection of the optimal endoscopic approach among ipsilateral posterior, contralateral, and transforaminal ones, the “four-level stenosis protocol” is used to evaluate the foraminal and lateral recess zones. Four magnetic resonance (MR) axial cuts in a segment were used at the levels of four guidelines drawn at the lower endplate level of the upper vertebral body, mid-disk space level, upper endplate level of the lower vertebral body, and 3 mm farther distal (Fig. 2). The upper two levels show the foramen and extraforaminal area where the exiting root can be irritated at the foraminal area (level 1) and the central canal under the sublaminar area where dura compression by the upper lamina occurs in degenerative spondylolisthesis (level 2). The lower two levels reveal the lateral recess area where the traversing root can be compressed at the lateral recess (level 3) and under the distal laminar border (level 4) by the lower lamina. In the higher lumbar spine (L1–4), the traversing roots are not yet divided at level 3, and most roots and the axillar portion can be inspected after distal laminectomy at level 4. The lower lumbar spine (L4–S1) is divided from the dura at level 3, and its axillar portion is exposed proximal to level 4. Accessibility of the ipsilateral posterior approach ranges from the ipsilateral level 1 ventral area (pathology on disk) at the foramen to the contralateral level 1 ventral and dorsal areas (superior articular process (SAP) tip and subpedicular spur) of the foramen and from the central to lateral recess area of levels 2, 3, and 4. Therefore, in cases of extraforaminal disk or foraminal stenosis, a broad endplate spur overlying the foramen to the extraforaminal area without lateral recess stenosis at level 3 or 4 requires a transforaminal approach (TFA). In case of dominant foramen-to-extraforminal stenotic pathology or disk extrusion at level 1 or 2 combined with lateral recess stenosis at level 3 or 4, the surgeon intentionally stands on the side opposite to the dominant foraminal pathology and attempts to decompress the central stenosis at level 3 or 4 bilaterally, the sublaminar area at level 2, and the foramen-to-extraforamen at level 1 contralaterally.
The best points permitting access to targets with no hazard to structural durability are portal positions. Choosing the portal position must not be fixed by our preconception on two-dimensional sagittal or axial radiographic images but should be designed under a three-dimensional surgical field considering anatomical barriers. It could be slightly different depending on the target’s location, depth, surrounding anatomic structures, and the surgeon’s peculiar experiences and skills. Good portal positioning in biportal ESS means that first, normal functional integrity should be preserved as much as possible; second, smooth access to pathology located deeper inside should be achieved; and third, it helps provide an optimal view for making surgical procedures safer and easier. Many studies have shown various portal positions in biportal ESS. All things happening under water on performance are mostly decided from the first step of addressing portal position, resulting in different accessing angles and views and laminectomy size to access the targets. Various portal positions in biportal ESS can be simply differentiated as higher and lower portal positions [38,39]. A higher portal position is derived from the concept of providing a freer working space for instrument handling. It is marked on the back skin depending on the disk space level, for example, 1 cm above the upper margin of the disk space and 1 cm below the distal margin after making the endplate margins parallel on a tilted view of the C-arm (Fig. 3). Two portals are located at the midlevel of the pedicles along the medial margins. During the placement of the scope, the scope faces the dorsal surface of the upper lamina. Wider, rectangular upper laminectomy is mandatory for a scope to be placed and to inspect the deeper spinal canal. The concept of laminectomy comes from tubular surgery performed under a microscope. In contrast, scoping from proximally higher instrument handling is somewhat free for a much wider space, which is performed not to fight with the tip of the scope. The ligamentum flavum (LF) is removed in the en-bloc style after burring off the surrounding upper and lower lamina. If central stenosis is combined with foraminal stenosis, endoscopy-assisted fusion using a cage on the ULBD technique is preferred for decompressing the foramen in a higher portal position. Some difficulties in a higher portal position are that the scoping view is caudally oblique, the laminar borderline changes even in a little rotation of scoping, and surgeons in the learning curve lose orientation and proceed laminectomy not into the laminar medial surface but into its distal surface with facet destruction. Then, the majority of the upper lamina would be resected until the SAP tip is exposed, and consequently, unilateral subtotal laminectomy, the so-called “vacant facet,” is performed on postoperative MR axial view, indicating iatrogenic instability, which consequently requires instrumented fusion (Fig. 4).
In lower portal positions, a proximal portal is located at the lower border of the upper lamina, very close to the lateral margin of the proximal SP base, under a vertical C-arm view. A scope should land onto the distal edge of the upper lamina, ready to slide down the sublaminar space in a cranially oblique direction with less laminar resection, which is a similar concept to the handling of a scope in full-endoscopic spine surgery. Posing a proximal viewing portal at the best point for sublaminar access is more important than creating a distal working portal. A working portal should be a two-finger breath distally from the proximal one. Furthermore, the distance between the centers of portals should be two-finger breath, approximately 2 cm. Therefore, there is only <1 cm of free space between the portal skin incision end margins. A scope and a certain instrument should be posed closer and a little parallel to be placed inside under the interlaminar space together, deeper to be closer to the contralateral foramen crossing the midline. Using a lower portal position, sublaminar access is permitted with minimal resection of the laminar edges (approximately 3 mm), permitting parallel sliding access under the sublaminar area. The lower portal position provides benefits for exposing bilateral exiting roots proximally and traversing roots distally, the so-called unilateral laminectomy and bilateral foraminotomy from levels 1–4 (Fig. 5). This portal position is very useful in decompression alone for central and foraminal stenosis while simultaneously preserving laminar continuity and facet integrity. This decompression-alone approach is a recommended alternative surgical strategy for multilevel LSS in elderly and senile patients with medical comorbidities.
Biportal ESS is performed in a water-based surgical environment. Continuous water input clearly washes away surgical debridement matters and potential contaminated debris. It decreases the surgical infection rate, which is beneficial for senile patients with medical comorbidities and lower immune responses or higher susceptibility to infection. In open fractures with high contamination, >10 L of saline irrigation is mandated. More than 10 L of saline (3,000 mL of saline×4 packs) is generally used for 1–2 hours of biportal ESS procedures, which seems to be enough irrigation to thoroughly wash away possible surgical debris. Preoperatively, only one dose of antibiotic injection is recommended in biportal ESS routine procedures. However, for its benefit, making a water output fluent is not always easy and requires some tricks. In full-endoscopic spine surgery, a scope is docked into Kambin’s triangle, which is called the “docking technique,” pushing water forcefully to wash away bleeding and keep the surgical field clear. The used water comes back through a tunnel through an endoscope. However, in biportal ESS, a scope is located at a certain distance from the target structures, which is the so-called “floating technique.” We can move the scope freely in any direction with panoramic views around the posterior lamina or foraminal area. Various instruments, such as Kerrison or Pituitary punch, generally used in open spine surgery, can be inserted through the gap between the scope and the structural surface. However, small bleeds enter the scoping view, making the surgical field turbid. Therefore, maintaining a continuous water flow is a key trick to provide a clear surgical field, which permits the differentiation of structural margins and decreases neural structural injury due to instrument mishandling under disorientation and mis-measuring depth in turbid water. Increasing the input water pressure to improve water output is not a good trick. If there was not good water output already, just putting water input in higher pressure will cause the cervical epidural pressure to increase, which may increase intracranial pressure [40–42]. Clinically sufficient water pressure can be achieved in a setting where two saline bags are placed 60–70 cm higher under natural gravity from the patient’s back level in the prone position on the surgical table. This height shows a similar pressure of 30–50 mm Hg for a water pump. It is not good to try too much water infusing in higher pressure, causing full dural compression for a longer time during surgery in a potential ischemic state [43,44]. Do not attempt to solve the output problem by increasing the input pressure. Water is generally trapped by the subcutaneous fascia or fat. On skin incision for making portals, basic preparation steps should be maintained, including subcutaneous fascia cross-cutting, subcutaneous fat-block removal, and control of the main bleeding foci on vascular geometry. Water output is mostly entrapped by the subcutaneous fascia rather than the deep muscles. A subcutaneous fascia cross-cut incision should be made in the transverse direction to the skin incision, and deeply scratching off the lateral margin of the spinous process (SP) bony surface is an essential trick for opening the subcutaneous fascia and keeping it wider to prevent water entrapment. Even in this trial, water did not come out fluently; therefore, subcutaneous fat blocks should be removed using a pituitary punch (Fig. 6). Another physics we should consider is that water is not running forward in one direction, as described in images from papers on scoping surgery. In summary, input water from a scope in a viewing portal will run forward and out through a working portal. This could occur if a skin retractor is used to open a working portal much wider than a scoping portal. Instrument manipulation at various angles can frequently squeeze and push down a working portal flat and closed. Therefore, the input water mostly escapes back through a viewing portal on instrument handling in a working portal. Therefore, the size of the skin incision for a viewing portal should be slightly larger than that for a working portal. This is a simple change; however, it is always helpful in heavily obese and thicker muscular cases.
As we start to perform upper laminectomy as the first step of decompression, a basecamp should be made to inspect bony margins to obtain an orientation and sufficient working space for accessing deeper areas of the spinal canal ipsilaterally or contralaterally. Upon placing the scope inside, we cannot obtain the right orientation immediately because the scope view is surrounded by deep back muscles. To obtain orientation in a dark room, we should first expand our hands to touch and lean on a wall. To obtain an orientation on scoping, the first thing we should do is to expose the bony surface of the interlaminar space from deep muscles. In real practice, this is difficult. A common reason for this is that the proximal portions of deep musculotendinous (MT) fibers remain attached to the basal area of the proximal SP, covering a scoping view at 10 to 1 o’clock. These MT fibers should be clearly detached to expose the laminar margins before placing the scope. To do this, we scratch the bony surface of the base area of the proximal SP using a muscle detacher (sharp-bladed Cobbs elevator) and detach the MT fibers attaching the proximal SP base approximately 1 cm to the dorsal area of the SP base from the dorsal lamina (approximately 1-cm area of spinolaminar junction between the SP base and upper laminar distal border) (Fig. 7). This MT fiber detaching procedure before scoping can help decrease the operating time of making a basecamp, clearly exposing the bony margin of the proximal SP base with easy orientation. Heavy bleeding on scoping should be controlled at three specific spots: 9 o’clock left, 7 o’clock under the muscle, and 5 o’clock at the facet corner. These bleeds come from the main branches of the segmental artery with high pressure; therefore, they must be completely coagulated using a radiofrequency (RF) wand. The corner bleeding at the distal area of the facet at 5 o’clock is divided into three branches. Its three heads should be thoroughly coagulated one by one (Fig. 8). If not, even a small missing bleed from one branch could make the surgical field thoroughly turbid during the entire surgical procedure.
A sufficient working space means that there should be an approximately 13–15-mm midline space at the interlaminar space. Through the middle of the interlaminar space, an 8-mm-diameter sheathed scope and a 5-m-width instrument were inserted together to access the contralateral side (Fig. 9). If not, the instrument is frequently jammed and trapped between the scope and bony structure, limiting its accessibility. In an insufficient working space, the instrument could not reach the targeted pathology around the foramen and contralateral distal area, resulting in incomplete decompression. Inappropriately forceful or wrongly directional handling in a narrow space could also result in dural or root injury. To create a sufficient working space, the proximal SP base should be resected before starting laminectomy or flavectomy. Proximal SP base resection provides a view of the contralateral side. The distal SP base should be resected before accessing the contralateral side. If instrument handling is uncomfortable to touch and resect the contralateral disk, SAP tip, or distal lamina, it mostly comes from an instrument pushed away proximally by the distal SP base. To overcome this, a little more resection of the distal SP base is mandatory, rather than wider laminectomy laterally.
The upper lamina is not mostly compressing pathologies that irritate the nerve roots at levels 3 and 4. Only in spondylolisthesis does a sublaminar thickened spur or ventral slip narrow the spinal canal at level 2. Therefore, the purpose of upper laminectomy is mostly to make a little more space for a scope to be placed inside for inspecting lower laminar areas at levels 3 and 4 and the SAP tip at level 2 in the sublaminar space. Approximately 3 mm of resection of the medial surface of the upper lamina using a straight chisel or burr is sufficient to create a space for placing a scope inside the interlaminar space. Sublaminar bony protrusion of the upper lamina at level 2 is also resected approximately 3 mm until the SAP tip is exposed using a pedicle chisel with an anteriorly 45° bent blade, preserving most of the upper lamina and facet surface (endpoint of upper laminectomy: distal 3-mm width of the lower lamina should be exposed at levels 3 and 4, proximally exposing the SAP tip at level 2) (Fig. 10). The sublaminar protruded portion should be resected at a thickness of approximately 3 mm to secure the spinal canal dimension at level 2 in spondylolisthesis. Ventral prolapsing in spondylolisthesis causes the loss of canal dimension in the sublaminar space, and sublaminar decompression is mandatory to compensate for and restore canal dimension in decompression alone. Therefore, if ventral prolapse is >1 cm, decompression alone cannot compensate for and restore canal dimension enough for dural expansion with the release of rootlets lying at the lateral portion of the dura, which are ready to bud out from the dura. Wider decompression with reduction using a cage and instrumentation is supposed to be a more reasonable trial in high-grade spondylolisthesis.
Contralateral upper laminectomy is performed in the oblique direction. It requires a measurement concept different from the vertical approach on the ipsilateral side because of its different accessing angles. The resection line for exposing the lower laminar distal area and SAP tip was calculated from the baseline of the contralateral upper lamina, rather than a high ridge on the dorsal surface of the ipsilateral upper lamina. Resection of the contralateral upper lamina at a high ridge should not be initiated. If not, nearly half of the upper lamina could be resected with loss of laminar and facet integrity. The purpose of upper laminectomy is only to expose the SAP tip covering the disk space at the proximal portion and a 3-mm area of the lower lamina at the distal portion beneath which the traversing root runs around the medial surface of the pedicle (Fig. 11).
The LF can be handled from the superficial layer to the deep layer rather than en bloc. The LF superficial layer covers the lower laminar joint margin and disturbs the surgical view to the contralateral side. The ventral margin of the upper lamina and the facet joint margin between the upper and lower lamina can be inspected after removal, facilitating safe and accurate laminectomy. The LF deep layer should be kept intact as long as possible until nearly finishing the lower laminectomy and foraminotomy by burring (Fig. 12). When the LF deep layer is resected before finishing lower laminectomy, a burr should not be used because of the higher risk of dural tear. In such situations, we should use a chisel and Kerrison punch for bony procedures. The LF deep layer serves as a dural protector from the scope and instrument and covers epidural vessels while maintaining a clear surgical view (Fig. 13). Most cases of incidental dural tears are reported as midline slit tears [27,45]. It would occur for a certain instrument, such as a Kerrison punch or dissector, to be inserted under the LF on crossing the midline under the LF. The saline input pressures the lateral side of the dura, and its central portion elevates like a thin band or folds. Without noticing it, careless insertion of an instrument beneath the epidural fat under LF can cause a dural tear. There is a key trick to avoiding dural tears when crossing the midline for flavectomy of the deep LF layer. Do not insert any instrument under the epidural fat. The safe way is between the LF and epidural fat. When the LF superficial layer is peeled and separated, thinning enough for capillaries to emerge on the thin LF deep layer in the central area of the spinal canal over the dura, with its full layer resection at the lateral recess overlying traversing roots, the dura can be fully decompressed and expanded, and radicular pain is subsided. This is an LF deep layer sparing technique with the intention of preventing technical risks, including dural tears, epidural haziness during surgery, postoperative epidural hematoma, and preventive effect on dural adhesion, which is one of the potential risk factors for dural tears in revision surgery. To maintain the tension of the LF deep layer, its distal attachment point to the distal SP basal surface should be protected. Resecting the proximal high and long peak of the distal SP base while preserving its very basal surface can preserve the tension of the LF dee payer while maintaining a sufficient working space.
Lower laminectomy is performed in separate steps, including resection of the distal SP base, including the distal lamina and SAP tip resection. When attempting to insert an instrument into the contralateral side, its accessibility is limited because of blocking by the higher-height distal SP. After flattening resection of the top, the ipsilateral and contralateral distal lamina can be resected much more easily. There must frequently be a lower laminar spur at the junction between the upper and lower lamina joint surfaces. Proximally and ventrally grown-up spurs are hidden and covered by the LF deep layer, compressing and irritating the traversing nerve root. Sometimes, without a grown-up spur, only its cortical thickening can cause stenotic compressing of the traversing root. Distal laminectomy of the lower lamina should always be sufficient distally at least 3–5 mm resection until a critical point is exposed where vessel bundles crossing the traversing root are coming out, the so-called “crossing vessel,” overlying the traversing root and touching the medial surface of the pedicle. Hard disk extrusion, distal laminar cortical bone thickening, collapsed disk height, or dynamic aggravation of disk space narrowing in the weight-bearing state can decrease the dimension where the traversing root runs under the distal lamina. Therefore, a little insufficiency of distal laminectomy could dynamically result in root compression with symptoms, including not being able to lift up the back straight for a long time due to early back fatigue and gradual buttock pain within an hour, frequently coming to be in need of rest in the sitting position for back stooping to increase the space of the distal lamina.
The SAP tip is partially resected to expose a disk space of approximately 3–5 mm or foraminal decompression. In the higher lumbar spine, the dura can expand wider until the overlying SAP margin during pulsation after flavectomy and remnant sharp-straight or medially growing SAP, which is located at the medial side of the spinal canal, could injure the dural lateral side with a risk of late-onset spontaneous dural tears (Fig. 14). Even in the lower lumbar region with a relatively wider spinal canal, discectomy without SAP tip resection could require additional retraction of the dura or root to create a working space on the disk space. In biportal ESS, a root retractor is generally not needed, even for central broad-type lumbar disk herniation (LDH). The disk space is located under the SAP tip, and SAP tip resection can provide a sufficient free disk space for discectomy.
Foraminotomy can be ipsilaterally or contralaterally available via the posterior approach in biportal ESS. The workflow of foraminotomy in the posterior approach includes SAP tip resection, subpedicular seal resection, foraminal flavectomy, and foraminal discectomy, if needed. The SAP tip is in contact with the undersurface of the upper lamina obliquely in the facet joint. A long time of surface stress brings about not only SAP tip hypertrophy but also sublaminar cortical hypertrophy just distal to the pedicle distal surface, the so-called “subpedicular seal,” named from a similar looking “window seal” (Fig. 15). An exiting root appears hidden under the subpedicular seal in the oblique-scoping view. Proximally wider upper laminectomy would be mandatory in vertical accessing skills in open or microscopic surgery, and even in biportal ESS using a higher portal position. Using the lower portal position in biportal ESS, however, the caudal to cranial directional scoping view can unveil an exiting root just after resecting the subpedicular seal. The SAP tip should be resected at its ventral portion, compressing the exiting root. The lateral portion of the SAP tip is located at the far lateral side and should be preserved as a functional structure that supports the transverse process–isthmus junction not to collapse further with decreasing foraminal height as disk degeneration progresses (Fig. 16). The seal is located under the sublaminar area, and it can be resected using a pedicle chisel with an anteriorly 45° blade rather than a straight chisel. Additional foraminal discectomy or endplate spur resection may be needed depending on the correlation between root mobility and restored foraminal dimension.
There is distinctive anatomical specificity of bony structures in the higher lumbar spine (L1–4) compared with that in the lower lumbar spine (L4–S1). Approach decisions for LDH are crucial for successful discectomy from this perspective. The higher lumbar spine has a narrow laminar width, and the lateral dura surface and axillary portion are located very close to the isthmus undersurface. To access foraminal or central broad disk rupture by oblique angled approach using biportal ESS, wider isthmus resection is unnecessary, which is common in vertical accessing tubular surgery that requires isthmus resection with higher potency of iatrogenic pars fracture due to overresection of the facet, particularly in the higher lumbar spine [46]. Furthermore, the disk space in the higher lumbar spine is approximately 10 mm apart proximally from the interlaminar space. If the ruptured disk fragment migrated up to the pedicle (up-migration LDH), a posterior approach requires proximally wider laminectomy, and even if possible, dura retraction in a narrow interlaminar space could cause a higher risk of conus medullaris syndrome (Fig. 17). In case of huge broad-type disk extrusion, safely removing it under direct visualization in a hazy and narrow surgical view in the posterior approach could be very challenging. The TFA in the higher lumbar spine is the best choice for reaching up-migration, central broad, or foraminal and extraforaminal LDH with no retraction of the dura and clear view of the axilla area and dural lateral margin. The posterior approach for discectomy in the higher lumbar spine is only useful in down-migrated LDH, which is located in front of the interlaminar space and does not require wider laminectomy and has very short distance from the interlaminar basecamp to a target point.
The disk is constructed using a soft nucleus and hard annulus. Most symptomatic disk rupture cases indicate that the root is harshly compressed by a hard and stiff annulus. Soft nucleus rupture with no annular fragment is generally tolerable within a few weeks and is easily resorbable under conservative management. Intolerable radicular pain is caused by irritation by a ruptured annulus fragment harder than the root or root impingement between the extruded annulus and bony margin of the lower lamina at the lateral recess. However, in open surgery under natural eye view, broad and radical discectomy is generally performed to remove pathological fragments, including surrounding innocent nuclei, not to miss and lose the targeted fragment. A wide range of radical discectomy, including innocent nuclei located deeper inside, could cause early collapsing of the disk space after surgery, which might be the same mechanical results of razor debridement of the disk space after tubular surgery for discectomy [47,48]. Revision surgery has been very harsh and a burden to spine surgeons who use open dissection for wider laminectomy, back muscle destruction, and consequent need of fusion for recurrent LDH. To avoid early revision using a larger surgical intervention, radical discectomy is believed to be one of the options. However, under a magnified endoscopic view, ruptured or extruded annular fragments can be inspected under the superficial layer of the annulus. After annular superficial layer incision, probing and eliminating the ruptured annular fragment is much more suitable for selective annulotomy of the torn portion in biportal ESS. Selective annulotomy in biportal ESS is a more precise elimination of pathologies to decrease the early recurrence of disk rupture or deterioration of the disk space due to secondary foraminal height collapse in radical nucleotomy in open surgery (Fig. 18). Selective annulotomy may become a reasonable concept in biportal ESS because revision surgery using biportal ESS is safer and less destructive than previous open spine surgeries in terms of overcoming safe scar release and less laminectomy with no need of conversion to instrumented fusion [49].
We could easily disorientate in the right-side posterior approach because our preconceptions and expectations in the learning curve depend primarily on our anatomical experiences under vertical views. On the right-side posterior approach, a scope is placed in the caudal to cranial direction very close to the midline of the interlaminar space. Therefore, do not expect posterior anatomical scenery in your imagination that is full of vertical views from open surgery or anatomic textbooks. They are composed of an oblique view in the proximal direction. We should accustom ourselves to them through several trials. The right-side posterior approach makes the medial surface of the upper lamina as wide and flat as possible and the dorsal surface as narrow and vertical as possible, in contrast to the left-sided approach, in which the medial surface is narrow and vertical and the dorsal surface is broad and flat (Fig. 19).
In addition to the difference in view, the proximal working space, which is accessed through a proximal working portal, is much narrower than that via a distal working portal in the left-side posterior approach. It is somewhat difficult to create a sufficiently wide working space because of the different anatomical configurations and narrow accessibility. To overcome these limitations, the posterior approach on the right side requires a lower portal position than the standard approach, in which a proximal working portal is located on the same level of the midportion of the interlaminar space just lateral to the SP, and a viewing portal should be 3 cm distally from the working portal. Somewhat distal movements of a working portal at the same level as the middle of the interlaminar space are made to avoid being blocked by the proximal lamina and proximal SP base with higher height. In the left-side posterior approach, an instrument is inserted from “the lower field” around a facet. However, in the right-side approach, an instrument should be inserted from “the higher mountain” of the proximal SP base and upper laminar distal border with a higher profile. If a higher portal position is used in the right-side posterior approach, instrument handling is frequently disturbed, and instruments cannot reach deeper areas inside the spinal canal without wider laminectomy. In particular, the ipsilateral SAP tip on the right side becomes inaccessible or untouchable. This is one of the common reasons for insufficient decompression of the right posterior side and incomplete discectomy with difficulty of SAP tip resection for securing safe discectomy and a sufficient working space, inaccessibility to the contralateral side with remnant stenotic pathology, and impossible ipsilateral foraminal access or foraminal decompression. On the right-side approach, surgeons in the learning curve could face the dilemma of whether wider upper laminectomy for safely accessing lower laminar areas with higher risk of laminar fracture or less upper laminectomy for preserving laminar continuity, but with higher risks of incomplete SAP tip resection, leaving sharp and irregular laminated ends, causing delayed dural tears by dural pulsation wider after flavectomy. To overcome this physical problem of portal positioning, most instruments designed with curved shapes should be used to preserve the upper laminar width and sufficiently resect the SAP tip (Fig. 20). The recommended indication for the right-side posterior approach is down-migration LDH on the right side. For decompressing bilateral-sided stenosis and up-migration LDH or at the foraminal area on the right, a left-sided posterior approach using a contralateral approach (CLA) with left-to-right access is much more reasonable, with a higher success rate and lower potency of technical complications.
To access a case with central stenosis on both sides with foraminal stenosis or foraminal LDH on the right side, we should stand on the left side and use the left-side posterior approach. The intentional choice of the opposite posterior portal to access the contralateral target obliquely through the sublaminar space with the purpose of bilateral central decompression and combined contralateral foraminal decompression is called the CLA. The left-side ipsilateral posterior approach for foraminal decompression allows only the ipsilateral foraminal area to be reached, but not the ipsilateral extraforaminal area (mostly extraforaminal LDH). In this case, an additional left-side TFA or right-side CLA for contralateral access to the left foramen for extraforaminal pathologies is required.
There are some technical complications from a critical perspective that we should keep in mind on full knowledge before starting biportal ESS. It is the same situation in which “a seasoned fisher can read the sky before going fishing.” The learning curve period of ESS up to a certain level requires a longer time, rather than simply decreasing the operating time in a short period [50,51]. There are several issues, including RF and electrical brain-zap, burring and bone dust, incidental dural tear and patch method, postoperative hematoma and vascular geometry, and frequent neglected zone of decompression [27,45,52–54].
Using RF in higher-voltage ablation mode for debridement of deep muscles to expose bony surface and creating a basecamp is critical to brain neural tissues and can cause postictal seizure-like condition, the so-called “brain-zap” with stuporous mentality and hyperventilation and midsized fixed pupil for several hours. It should be used in the set of lower-voltage coagulation mode for coagulation of small bleeds and shrinkage of peripheral rugged margin of soft tissue for making a clear surgical view. Using a shaver for debridement of a larger amount of soft tissue in a wider area should be better, and additional short-term use of RF for shrinkage and cleansing of flappy ends of soft tissue on bony margins should be considered.
A burr is one of the most effective devices for laminectomy. However, in biportal ESS, the portal size is not wide enough for bone dust to be washed out. Bone dust is not well detected because the debris is collected and gathered under deep muscles behind a scope. After finishing surgery and scope removal, the inflated deep muscle comes down and covers closer to the interlaminar space, where remnant bone dust would be grafted over pluripotent laminar bone bleeding. In such conditions, hematomas would not be degraded and resolved in a few weeks but would change into hard fibrous tissue in a few weeks with consequent restenosis compressing the traversing root and dura, with corresponding neurological pain (Fig. 21). To overcome this situation, a soft tissue retractor or cannula can be used to open wider portals in procedures in higher portal positions with portals’ wide apart, or a chisel can be used only in lower portal positions with narrow portal distance.
Incidental dural tears or root injuries in endoscopic spine surgery have been reported in approximately 3.7% of all cases [55,56]. The patch method for sealing tear sites with expected natural healing should be recommended rather than direct suture after conversion to wider open laminectomy. It is effective in slits of <1 cm and longitudinal tears with a lower risk of rootlet incarceration. A case with >1 cm or flap-like tea should require suture with a vascular clip device or conversion to open repair. If cerebrospinal fluid (CSF) continuously leaks from the surgical wound postoperatively within a week, immediate endoscopic revision for repatch and subcutaneous tight suture is mandatory. Even in such an endeavor or insufficient trial, there could be a palpable soft mass, a peudomeniningocele, on the back at 3–5 weeks postoperatively. All the better in pesudomeningocele, a drain way for CSF in muscle layers is sealed and isolated by pseudomembrane-like bursa with partially healed and closing of a smaller tear site. Biportal ESS revision over the cyst leads you to the tear site through the pseudomembrane tunnel. Repatch over there well and perform debridement of cyst-like pseudomembrane in the muscle layer. In any condition of a dural tear, do not leave a drain. Iatrogenic muscular hematoma is necessary to compress the dura to prevent dural pulsation and to avoid torn site diversion and rootlet incarceration [45,52,57].
Senile and elderly patients have a relatively higher thromboembolic risk (pulmonary embolism or deep vein thrombosis) and commonly take preventive medications (anticoagulants). Therefore, any surgical intervention is associated a higher risk of postoperative hematoma. It can always happen, and you have your own strategy to decide the exact timing of endoscopic revision irrigation and hematoma evacuation. To prevent it as much as possible during surgery, bone and soft tissue bleeding should be well controlled with thrombin-mixed materials or bone waxing and selective focal control of bleeding on muscle by RF. A drain could not thoroughly prevent the collection of blood in the interlaminar space because its canal diameter is not sufficiently wide. There should be an attempt to coagulate small bleeds as much as possible on closing the water input at the last stage of the operation [58]. During waking up from anesthesia, keeping not to elevate systolic blood pressure is a reasonable trick [59].
After surgery, postoperative magnetic resonance imaging checking within 1–3 days must be the exact examination to differentiate postoperative hematoma from other reasons, such as remnant or incomplete decompression. Intolerable back and buttock pain even with eliminating radicular pain or cauda equina syndrome in the immediate postoperative period must be considered a red flag, which requires early revision for hematoma evacuation [53]. Remnant stenotic pathology is one of the common reasons for patient dissatisfaction [35,60,61]. In biportal ESS, the frequently neglected zone of decompression in the learning curve period is the sublaminar space in level 2, which is hidden in the vertical scoping view, and the foraminal area for unskillful accessibility or mismatching view on scoping to radiographic examination (Fig. 22). Central decompression alone, leaving foraminal stenosis neglected, is also a common mistake. A patient who has central and foraminal stenosis simultaneously is generally in a stooping state to widen the central canal dimension to relieve buttock and radicular pain. Therefore, there could be little complaint of foraminal stenosis due to dynamic foraminal widening in the stooping position. If there is no detailed medical interview after MR interpretation, there could be a lack of foraminal decompression for a simple excuse, “no pain, no pathology,” even under common knowledge that foraminal stenotic symptoms dynamically occur (Fig. 23).
A drain is inserted through a portal, and its tip should be located at the dorsal surface of the lamina. Its deeper location could irritate or tear the dura when it inflates wider on pulsation with no protection from the LF. A small sticky blood clot is not sucked out through such a narrow diameter. Postoperative hematomas should be prevented intraoperatively using antithrombin-mixed materials. It might not be a reasonable choice to depend on a longer time of residence of drain in the operating site. It is mainly for draining irrigation saline left in the spinal canal upward and downward. The drain is removed 1 or 2 days after surgery. If the drain amount abruptly increases after ambulation and the drained blood-mixed saline becomes much clearer, an insensible dural tear should be suspected first, and the drain should be removed immediately. Do not hesitate to remove a drain for fear of potential hematoma. A drain with a narrow canal does not prevent postoperative hematoma.
After full recovery of the patient’s mentality and sensory and motor functions of the distal lower extremities, free ambulation can be permitted as soon as possible. It may take 4–6 hours after returning to a ward and resting for a while, not dependent on the methods of anesthesia. Patients could feel back heaviness or buttock pain on the 3rd or 4th postoperative day. It might be due to back and buttock muscle strain due to longer surgical time in the prone position. Caudal block can help solve this type of muscle fatigue and tolerable discomfort on the back and buttocks.
Another reason for back and buttock pain in the early postoperative period could be postoperative hematoma, which generates mild to severe pain, sometimes with radicular pain. Resting in the supine position could aggravate the pain at night, and walking with a slightly stooping posture decreases the pain. Therefore, walking well with some back heaviness and buttock pain is not a serious complicated state. Generally, hematomas are spontaneously resorbed 3 or 4 weeks postoperatively. Ambulation and back stretching under analgesics or 2–3 root block sessions every 3 or 4 days are effective in conservatively managing saline infusion into the spinal canal to promote resorption and dilution for its early degradation. Postoperative hematoma with a larger amount that encompasses >50% of the spinal canal and corresponds to moderate to severe pain should be immediately irrigated and removed using biportal ESS. Conversion to open debridement is not required. Endoscopic debridement is effective in thoroughly removing it from the distal interlaminar to the proximal sublaminar area.
Driving a short distance should not be prohibited. Walking for 1 hour as an aerobic exercise is recommended for better functional recovery. After 1 month postoperatively, gym exercise, swimming, climbing, and golfing could also be permitted. Longer-time sitting, such as an office job, might cause more frequent back heaviness and pain. Back muscle exercise and stretching education should be mandatory for patients before discharge.
There could be remnant neural symptoms with somewhat intolerable or early aggravated radicular pain with a painful limping gait. If there was no direct neural tissue damage, remnant symptoms could result from neural dysfunctional neuralgia for longer exposure to a severely compressive state. Even with sufficient decompression, there could be weakness in motor power or paresthesia in the lower extremities. A longer duration of moderate to severe neural compression could decrease thigh or calf muscle volume with sensory decrease and/or coldness of the foot. Such discomfort is usually prolonged for more than 6 months or is permanent. However, more aggravated symptoms with a painful limping gait could result from incomplete decompression, particularly remnant disk fragment under the root, insufficient distal laminectomy with less free space for the traversing root, or neglected foraminal stenosis. If there is a painful limping gait, early revision using biportal ESS could solve the problem under the suspicion of incomplete decompression. Early conversion to wider redecompression and instrumented fusion in the name of instability may not be required. According to many reports on postoperative spinal pain syndrome, incomplete decompression occurs much more frequently than postoperative surgical instability [31,34,35].
The purpose of decompression using an open wider, microscopic, or endoscopic approach is the same as that for the surgical removal of pathologies that compress neural structures. However, accompanied surgical damage during dissection, accessibility while preserving segmental integrity, and the success of eliminating pathologies with less damage to surrounding tissues are quietly different from each other, which is the reason why they are called their own surgical names and generally considered as different techniques. Decompression alone can be performed via biportal ESS and shows outstanding benefits in terms of preserving healthy structures and accessibility to various pathologies located deeper inside the spinal canal and foramen. Remnant neurological pain should be believed to result from incomplete decompression, which is an endeavor to overcome technical challenges rather than the impetuous decision of instrumented fusion for the concept of observational radiographic hypermotion or instability in the early degenerative stage.
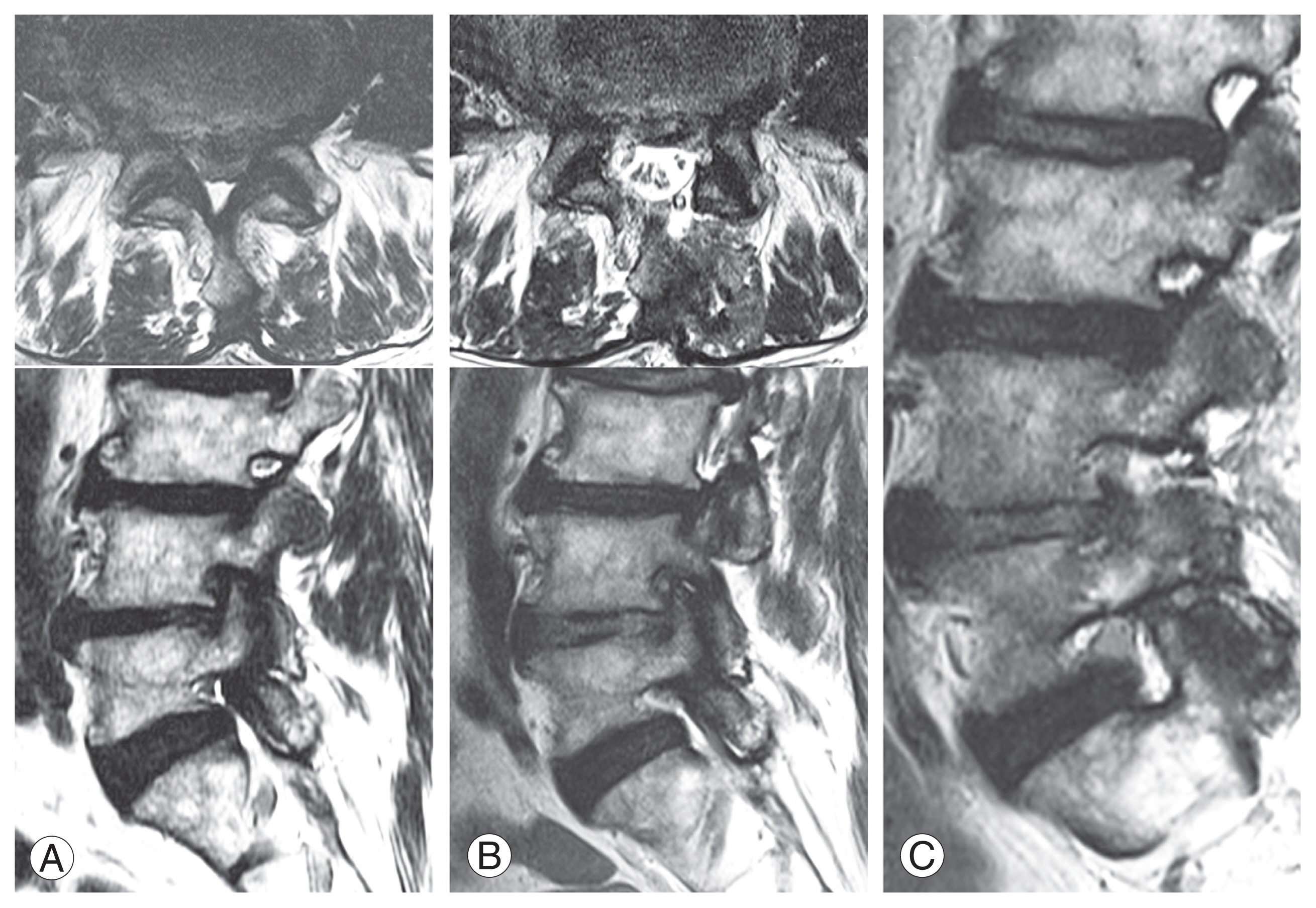
Fig. 1
Clinical symptomatic instability. Creeping subsidence of endplate fracture happens if instability is on-going even at the end stage of segment degeneration and stabilization. Decompression-alone for these cases showed early recurrence of foraminal stenosis or foraminal disc rupture due to creeping subsidence of endplate and further collapsing of foramen. (A) Kypholisthesis, it comes from nearly total collapsing of disc space, segmental anterior-wedging with loss of holding power of facet and maceration of upper endplate. (B) Scoliolisthesis, lateral wedging due to total collapsing of disc space with subtle fracture of endplate, meaning this area is under continuous axial stress even in last stage of segment degeneration.
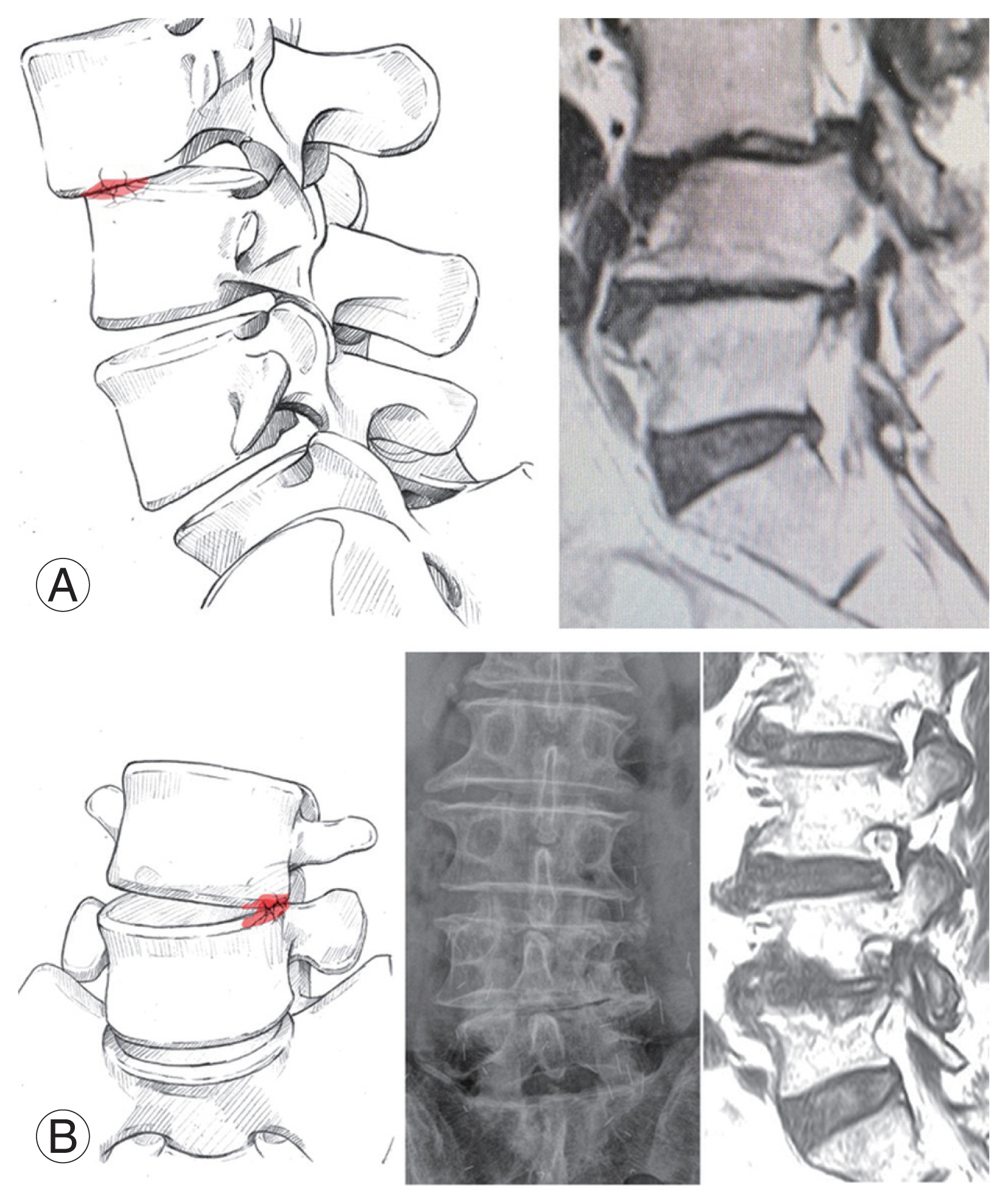
Fig. 2
Four-level stenosis protocol. Serial axial magnetic resonance (MR) images cut and divided as four levels, foraminal, sublaminar, lower laminar, and distal laminar areas (A) and matched endoscopic view (B). Axial MR views at a segment are evaluated at 3 mm-interval cuts from foramen to just proximal to distal pedicle. Disc space is covered by superior articular process (SAP) tip at level 2. A traversing root starts to be budded (axillar point of traversing root) from dural stalk at level 4 in higher lumbar spine (L1–2–3–4), and at level 3 in L4–5 and L5–S1 segments. Lv., level.

Fig. 3
(A, B) Portal position and assembly angle of a scope and an instrument. Higher portal position with 4–5 cm wide apart (red circles) needs triangulation of a scope and an instrument to focus a target locating deeper after wider laminectomy. Lower portal position with 1 cm narrow apart (blue circles) uses parallel sliding to access sublaminar approach.
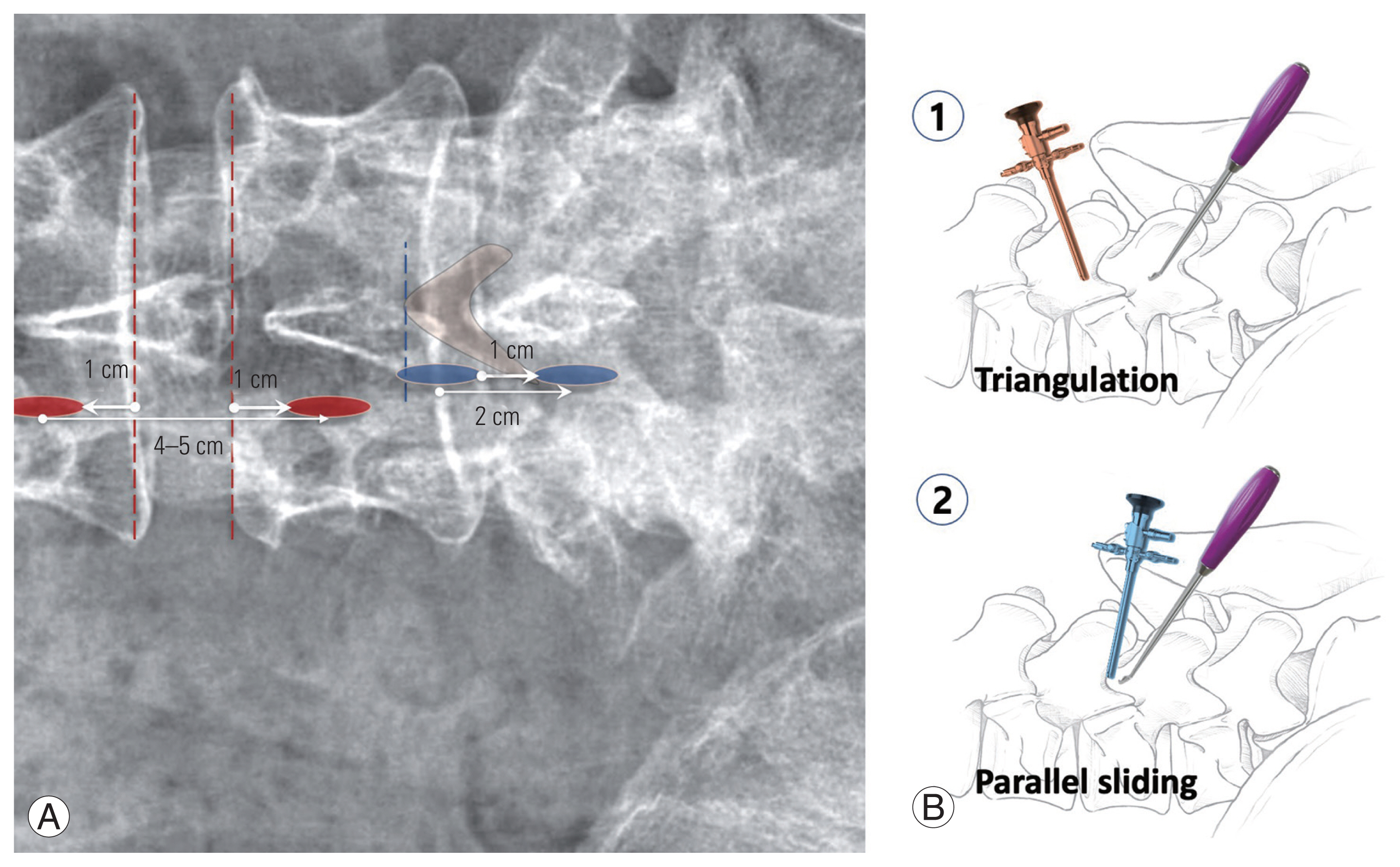
Fig. 4
Different amounts of laminectomy dependent on portal positions. Lower portal position with facet preservation. (A) Postoperative (PO) magnetic resonance axial view showed larger amount preservation of facet contact surface (#) due to about 3 mm-laminectomy. (B) Higher portal position with “vacant facet” for judicious subtotal laminectomy. Disorientation during laminectomy resulted in subtotal laminectomy with total loss of facet contact surface (*).
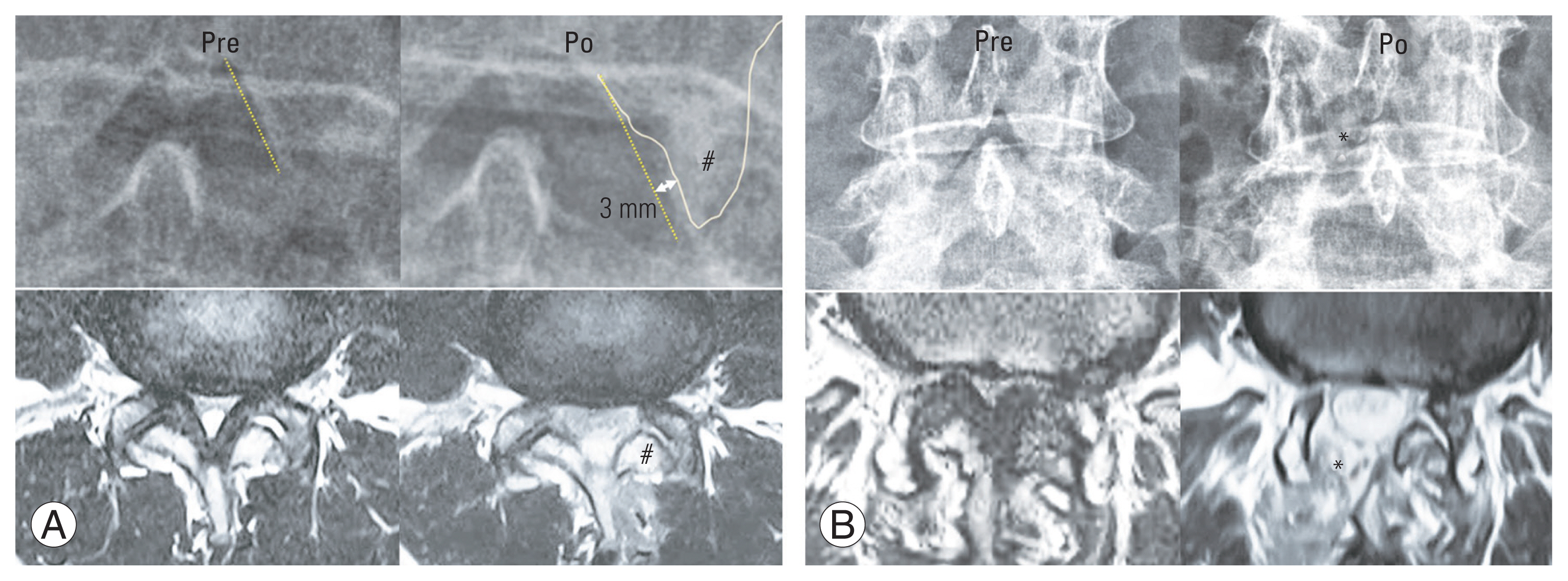
Fig. 5
(A, B) Unilateral laminectomy and bilateral foraminotomy. Both sided proximal foramens and distal lateral recesses can be decompressed using parallel sliding under sublaminar area. Lv., level; Rt, right; Lt, left.

Fig. 6
Control methods of water-output fluent. After skin incision for portal, subcutaneous fascia should be cross-cut till scratching spinous process (SP) bony surface to be open wider. Subcutaneous fat-blocks could also interfere water-output flow so that removing fat-blocks beneath portals is mandatory.

Fig. 7
(A, B) Making a basecamp. Before scoping after skin incision for portals, scratching on 1 cm-around area from spinolaminar junction should be scratched by a muscle dilator device to scratch off basal musculotendinous (MT) fibers from bony surface of spinous process and upper laminar distal border. Scoping without this step of detaching deep MT fiber shows only muscle fibers with no exposing bony surface, leading disorientation and lengthening operating time for making a basecamp with a nice orientation on laminar bony margins.

Fig. 8
Standard anatomic references in posterior approach on the left. Getting a correct orientation or not, is evaluated whether this standard view with sufficiently exposing these reference structures. (A) Six-reference landmarks include 1. Musculotendinous bundle of deep rotator muscles, 2. Upper lamina (distal margin), 3. Proximal spinous process (SP) base, 4. Distal SP base, 5. Interspinous ligament, 6. Distal lamina (proximal margin). (B) Main bleeding foci at posterior interlaminar approach. (B) Main bleeding foci on posterior approach. At left-9 O’clock closed to proximal SP base, at 7 O’clock under deep rotator muscle, and at 5 O’clock around the facet, having three branches.
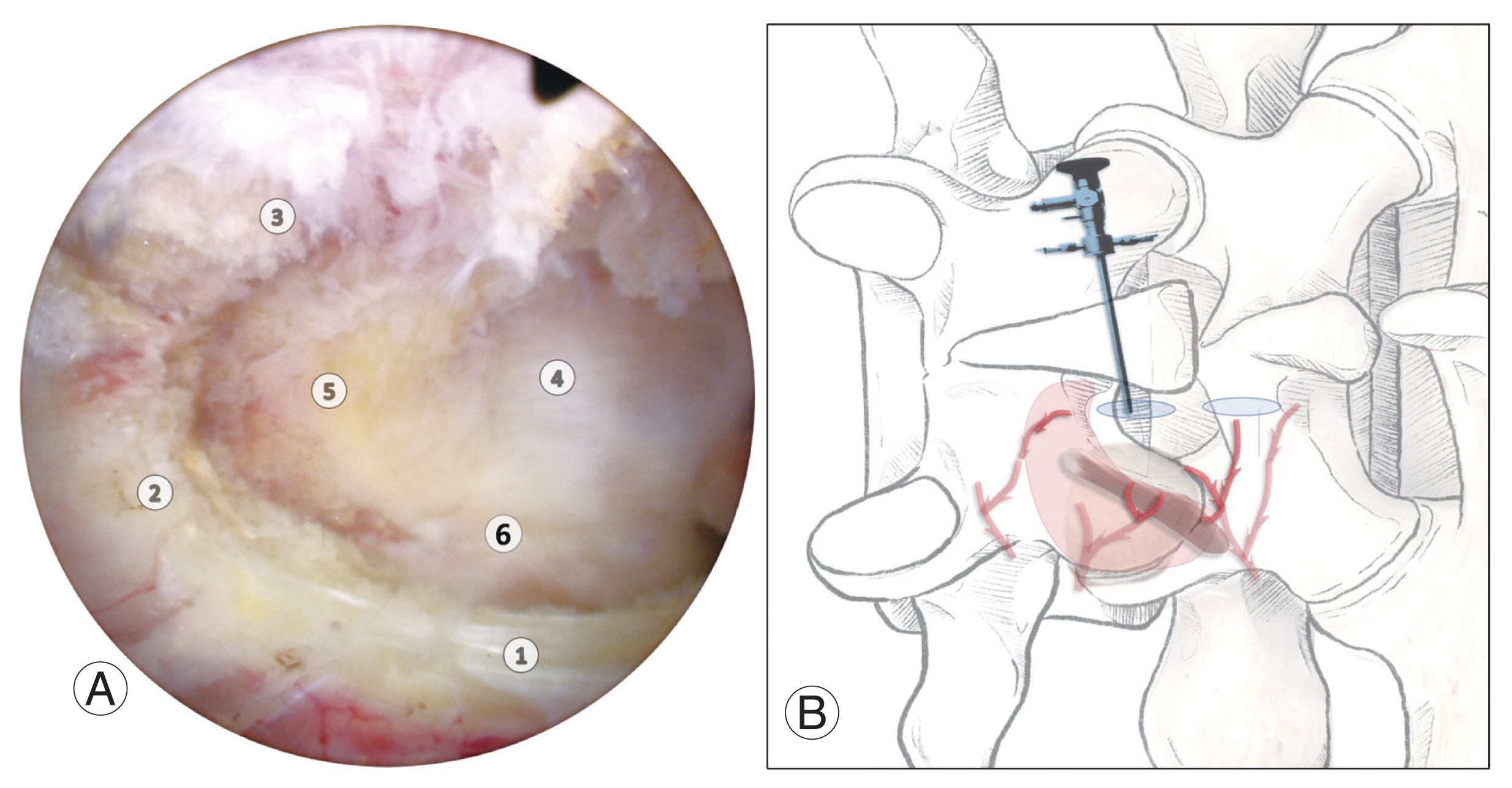
Fig. 9
Enough size of working space. (A) Interlaminar space in spinal stenosis is too narrow for a scope and an instrument to be inserted together. (B, C) To make working space enough, partial resection of proximal and distal spinous process (SP) bases is mandatory for securing enough working space.
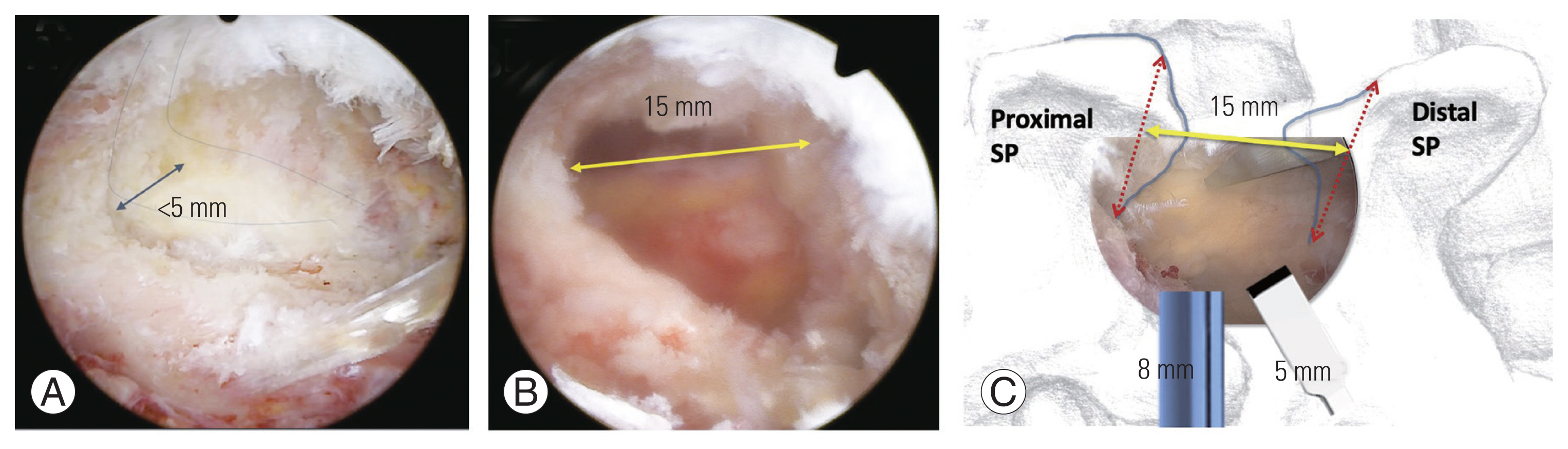
Fig. 10
Ipsilateral upper laminectomy. (A) Upper lamina has dorsal surface (d) and medial surface (m). High ridge (h) line is a meeting line of them. (B) Upper laminectomy should be done minimally, but superior articular process tip (*) and distal laminar area (#) of lower lamina should be exposed about 3–5 mm width. When a scope can be inserted below the level of upper lamina, no wider upper laminectomy is needed.

Fig. 11
(A, B) Resection line of upper laminectomy. At ipsilateral side (left), laminectomy starts at high ridge line (black solid line) between dorsal and medial surfaces. At contralateral side (right) from left side portals, it should start at 2 mm-above from ventral margin (blue solid line). If it started at high ridge on the contralateral side (red dotted line) by oblique accessing angled approach from left side, nearly half of upper lamina could be sacrificed to expose superior articular process (SAP) tip. The left ipsilateral resection line is different from the right contralateral resection line for upper laminectomy. IPA, ipsilateral posterior approach; CLA, contralateral approach.
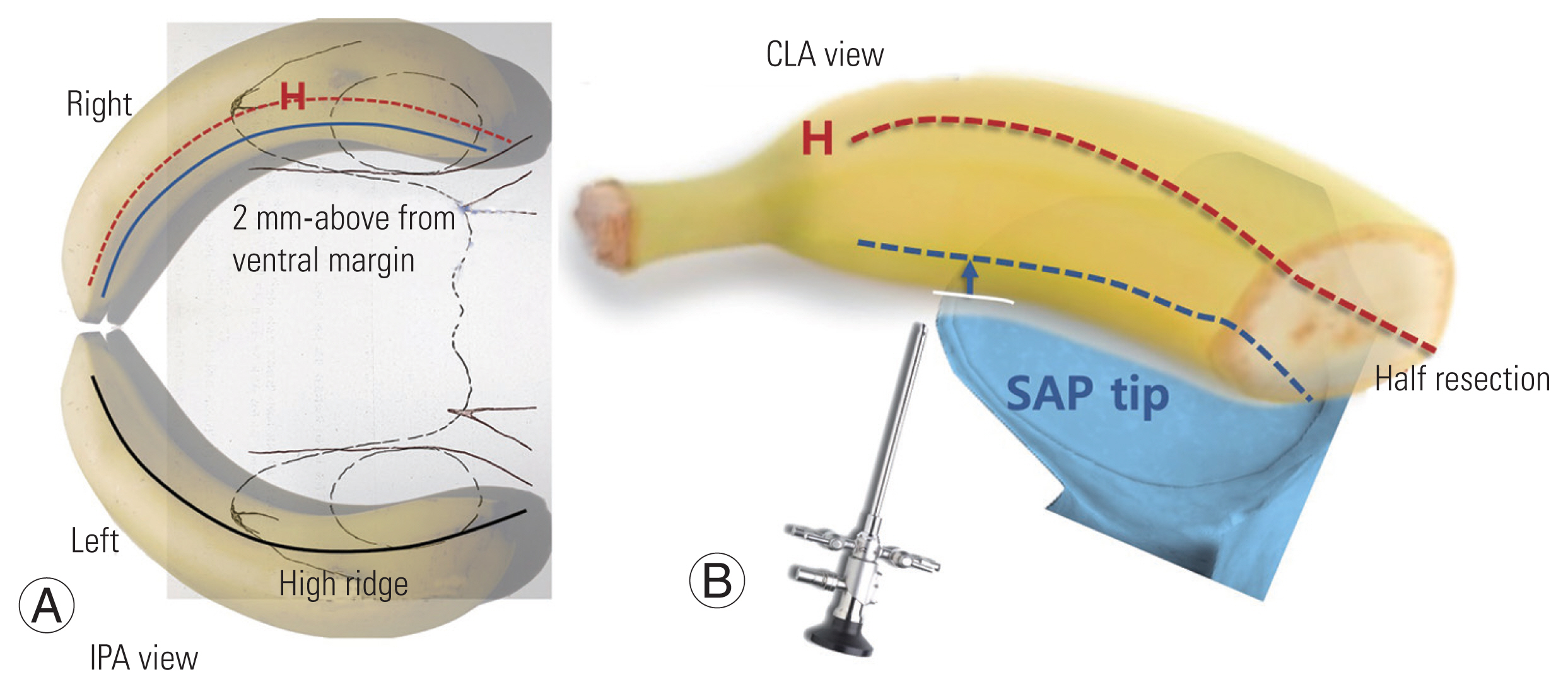
Fig. 12
(A–E) Ligamentum flavum (LF) deep layer preservation technique. At first view, LF superficial layer covers contralateral side of upper laminar bony surface (B). Using a freer dissector, LF is divided superficial layer over the level of lower lamina and deep layer (C). Superficial layer is gotten rid of from medial surface of contralateral upper lamina by shavering (D). Contralateral side can be inspected after only removing LF superficial layer and preserved LF deep layer works as protector of dura with no bothering of upper and lower laminectomy (E).
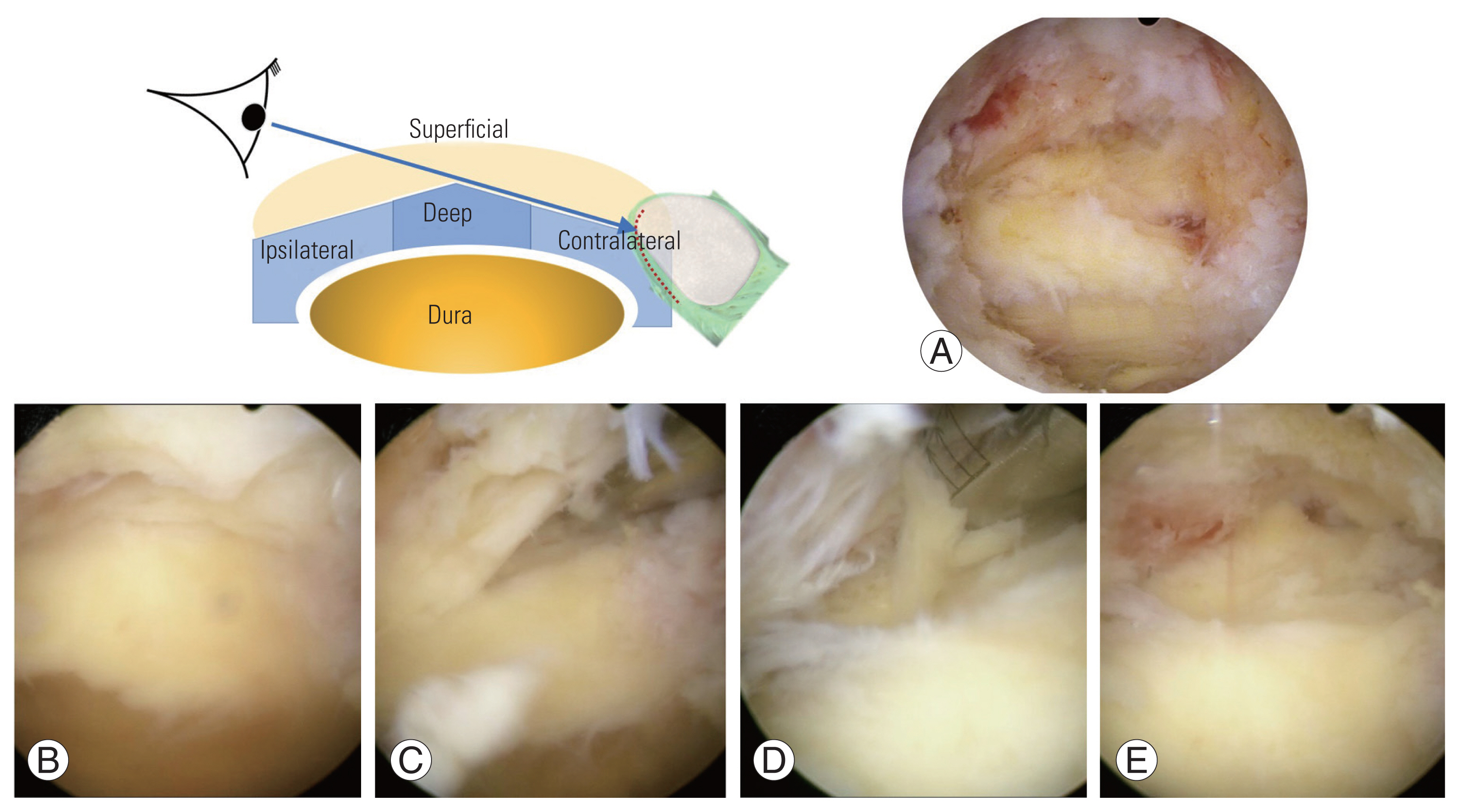
Fig. 13
(A–C) Surgical meaning of ligamentum flavum (LF) deep layer sparing. LF superficial layer should be peeled until exposing capillaries on deep layer. It can protect dura during bony procedures under less bleeding from epidural space with lower risks of dural tear or possible epidural hematoma. It is also thin enough for dura to permit dural pulsation and expansion for restoring spinal canal dimension. Pre, preoperative; PO, postoperative.

Fig. 14
Lower laminectomy. (A) Contralateral lower laminectomy. Superior articular process (SAP) tip is resected by a hockey chisel after making a notch for safe approach on chiseling by 2 mm-Kerrison punch. (B) Ipsilateral lower laminectomy. SAP is partially covered by upper lamina. It can be resected with wider upper laminectomy using a hockey chisel. (C) Insufficient SAP tip could bring about delayed dural tear due to restoring dural pulsation with no covering of ligamentum flavum. (D) Hockey chisel, left and right bladed.
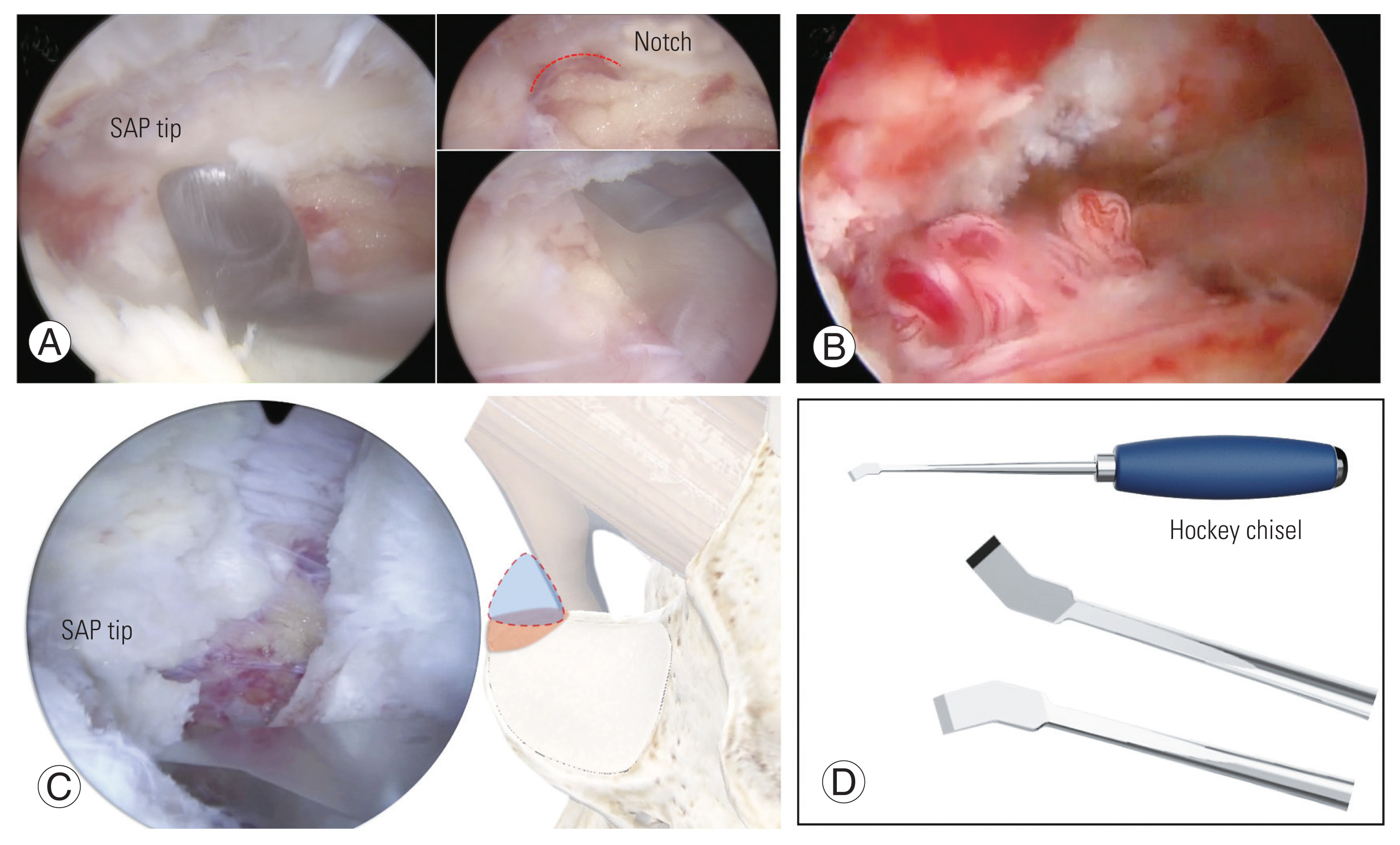
Fig. 15
(A–D) Foraminotomy. Longer time of stress between superior articular process (SAP) tip and sublaminar space on disc space collapsing, subpedicular cortical thickening is progressed as a seal, covering an exiting root. To decompress an exiting root, SAP tip, subpedicular seal, and foraminal ligament flavum should be eliminated.
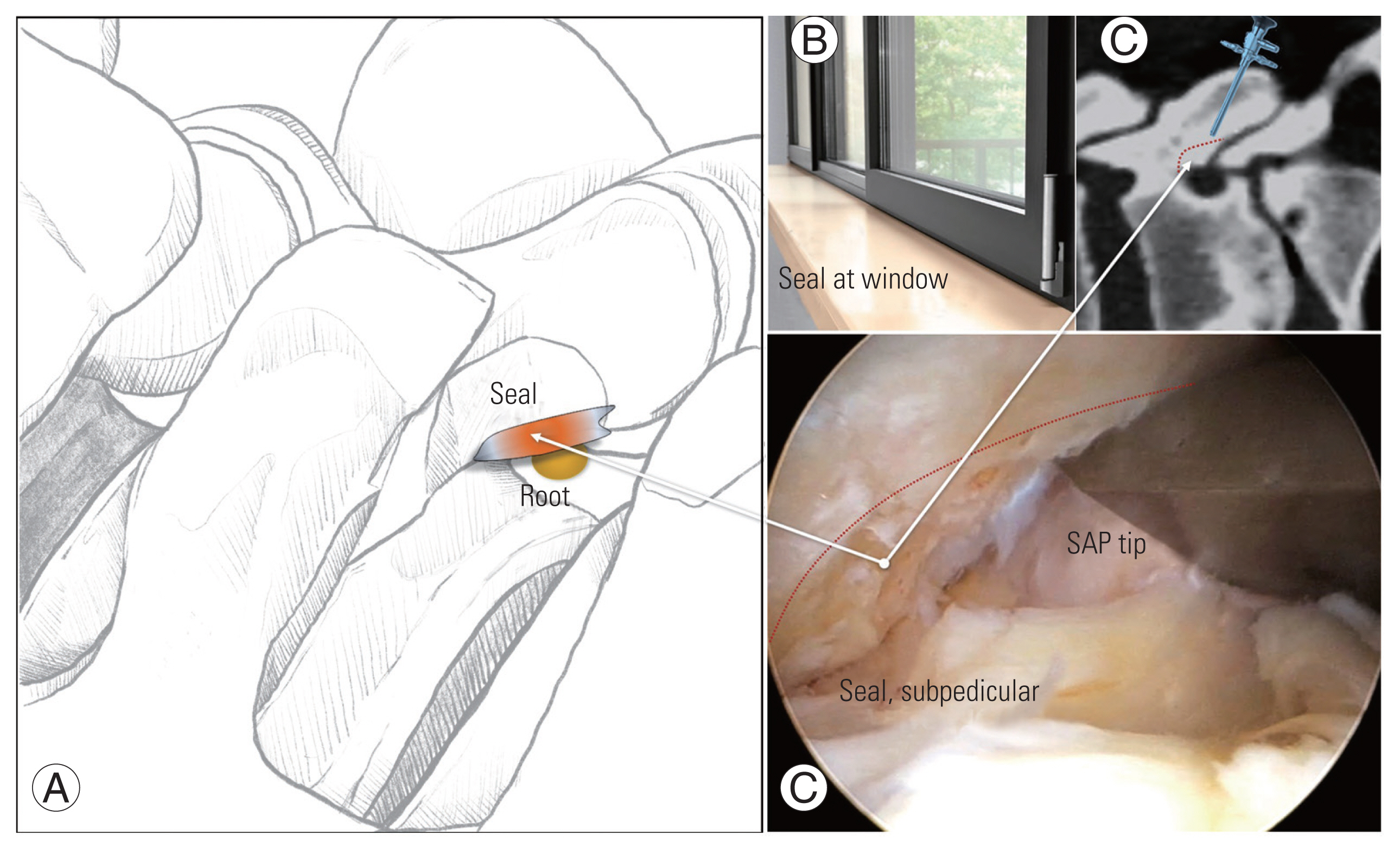
Fig. 16
Propping mechanism of superior articular process (SAP). When the disc is degenerated and collapsed with loss of free space (*) between transverse process (TP)–isthmus junction and SAP lateral portion, SAP lateral plane works as propping against TP–isthmus junction (#), so-called propping mechanism of SAP lateral portion for secondary facet stabilization.
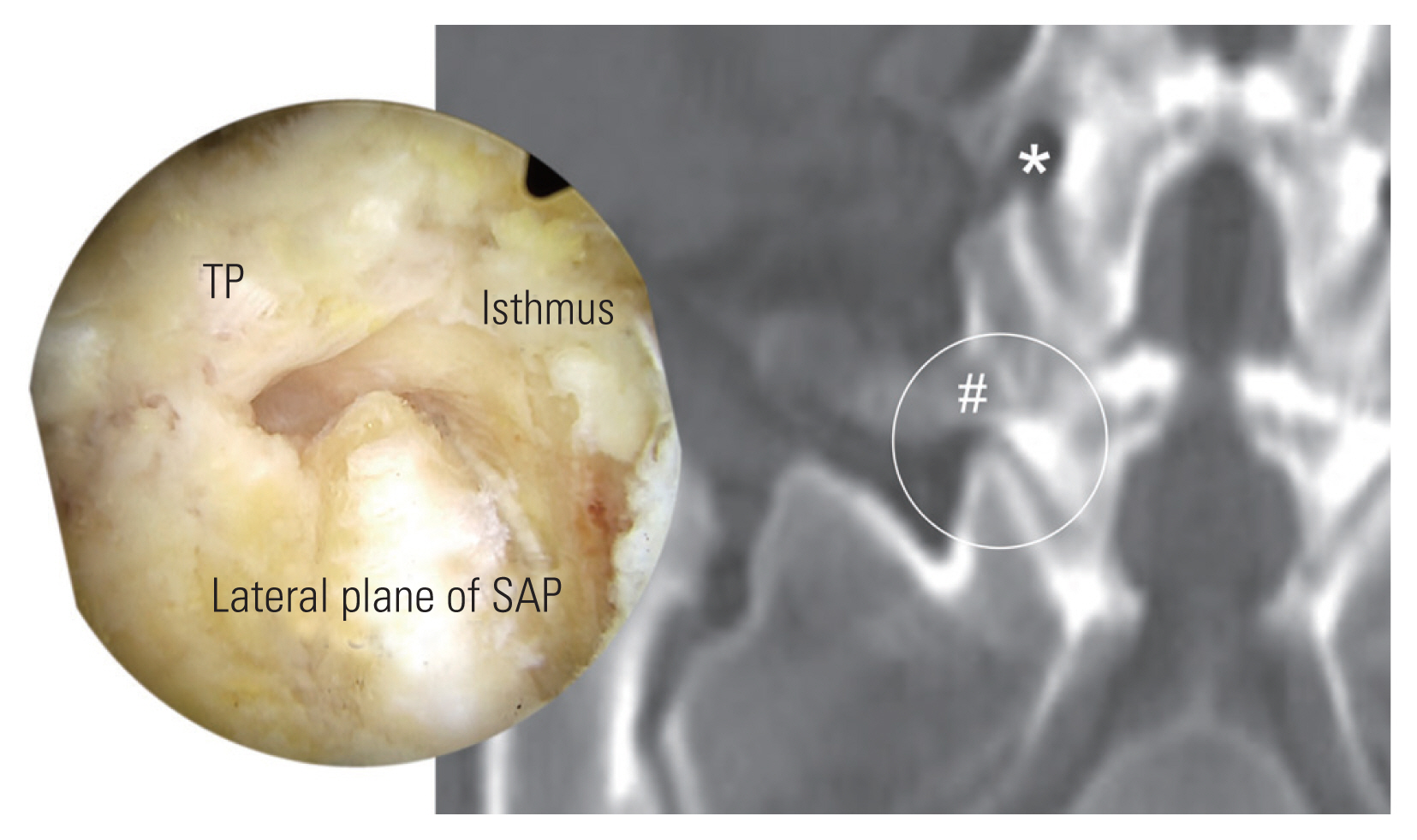
Fig. 17
(A–C) Conus medullaris in higher lumbar spine. There is anatomic variance in position of Conus medullaris from L1 to L3. Forceful and longer time of dural retraction could cause Conus medullaris syndrome.

Fig. 18
Radical nucleotomy and selective annulectomy. (A) Technical concept of open surgery under natural eyed view is radical nucleotomy, during which larger amount of innocent soft nucleus must be eliminated and possible remnant extruded annulus. Posterior decompression could affect decreasing radicular pain, but due to dura looks compressed by still extruded hard annulus. (B) Annular layer can be inspected under magnified endoscopic view, permitting selective annulectomy, eliminating ruptured hard annular mass with preserving deep layer of annulus and nucleus.

Fig. 19
(A, B) Right-side posterior view. It serves medial surface of the upper lamina rather than dorsal surface. Medial surface looks flat and broad, in addition to vertical-and narrow-looking dorsal surface. A straight chisel is very parallel to medial surface, and it is difficult to resect partially upper lamina in adequate amount for accessing lower lamina.
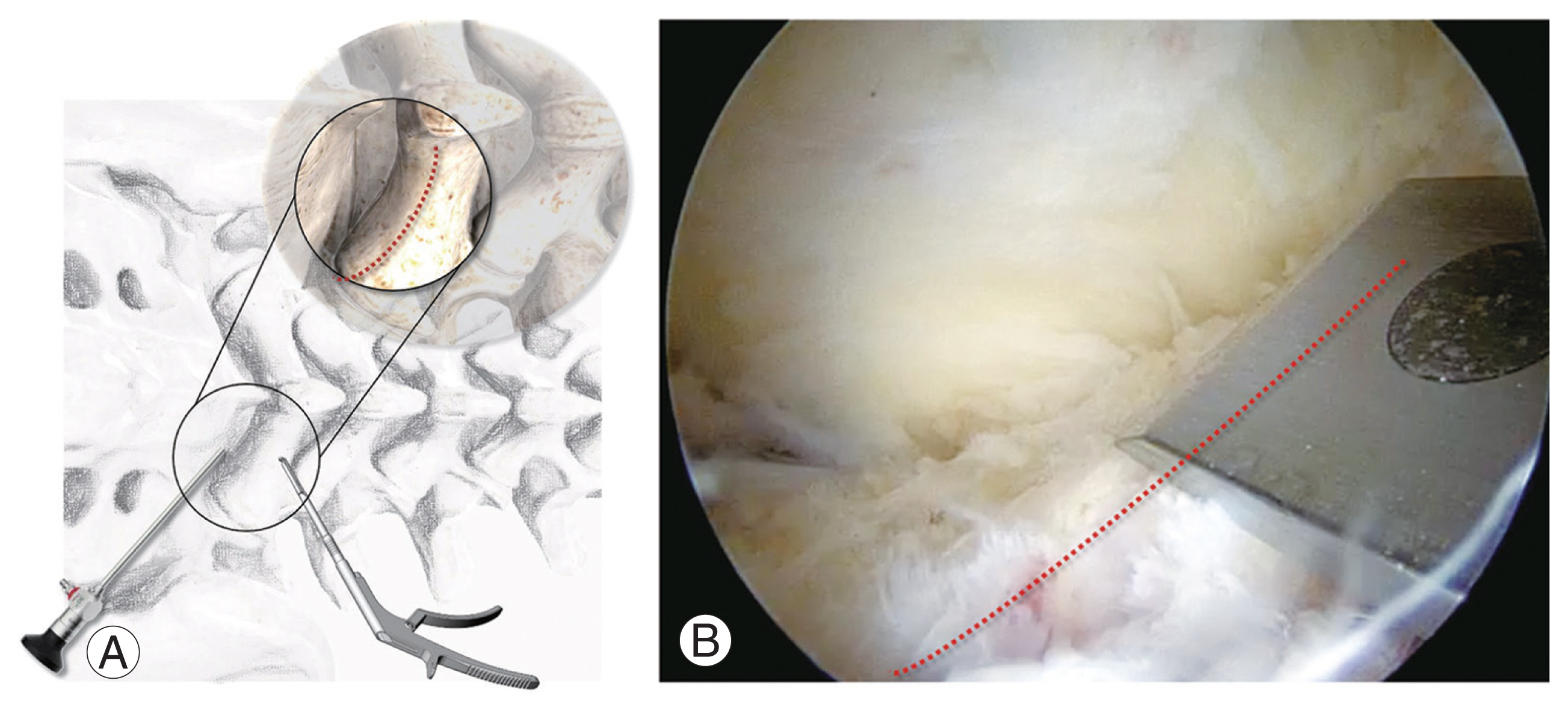
Fig. 20
Technical tricks on right-side posterior approach. On right-side approach, there is higher risk of technical complication including root injury, incomplete flavectomy, laminar fracture by forceful leverage on using a straight instrument. Using a curved instrument is more reasonable on right-side posterior approach to access in condition of preservation of upper lamina. LF, ligamentum flavum.

Fig. 21
Early restenosis after decompression. Radicular pain on the right side recurred after postoperative (PO) 2 weeks. Immediate postoperative magnetic resonance (MR) axial view (A) showed sufficient decompression. MR axial view at 2-week PO (B) and 4-week PO (C). Early fibrosis and restenosis were progressing in inflammatory period. It could be supposed to fibrosis of hematoma due to bone graft effect of bone dust generated from burring of lamina—(E) endoscopic view and (F) hematoma with bone dust grafted down in surgical field—which was strongly suspected by HE staining with thick fibrous change containing dead bone materials (arrow) (D).

Fig. 22
(A–E) Incomplete decompression on central canal. Central incomplete decompression of right side and proximal central canal on level 1 at foraminal level and level 2, sublaminar level. It happened for inappropriate making of working space in learning curve. Without 15 mm-interlaminar mid-space, a sheathed scope and a certain instrument cannot be inserted into contralateral side and proximal sublaminar space. Lv., level.
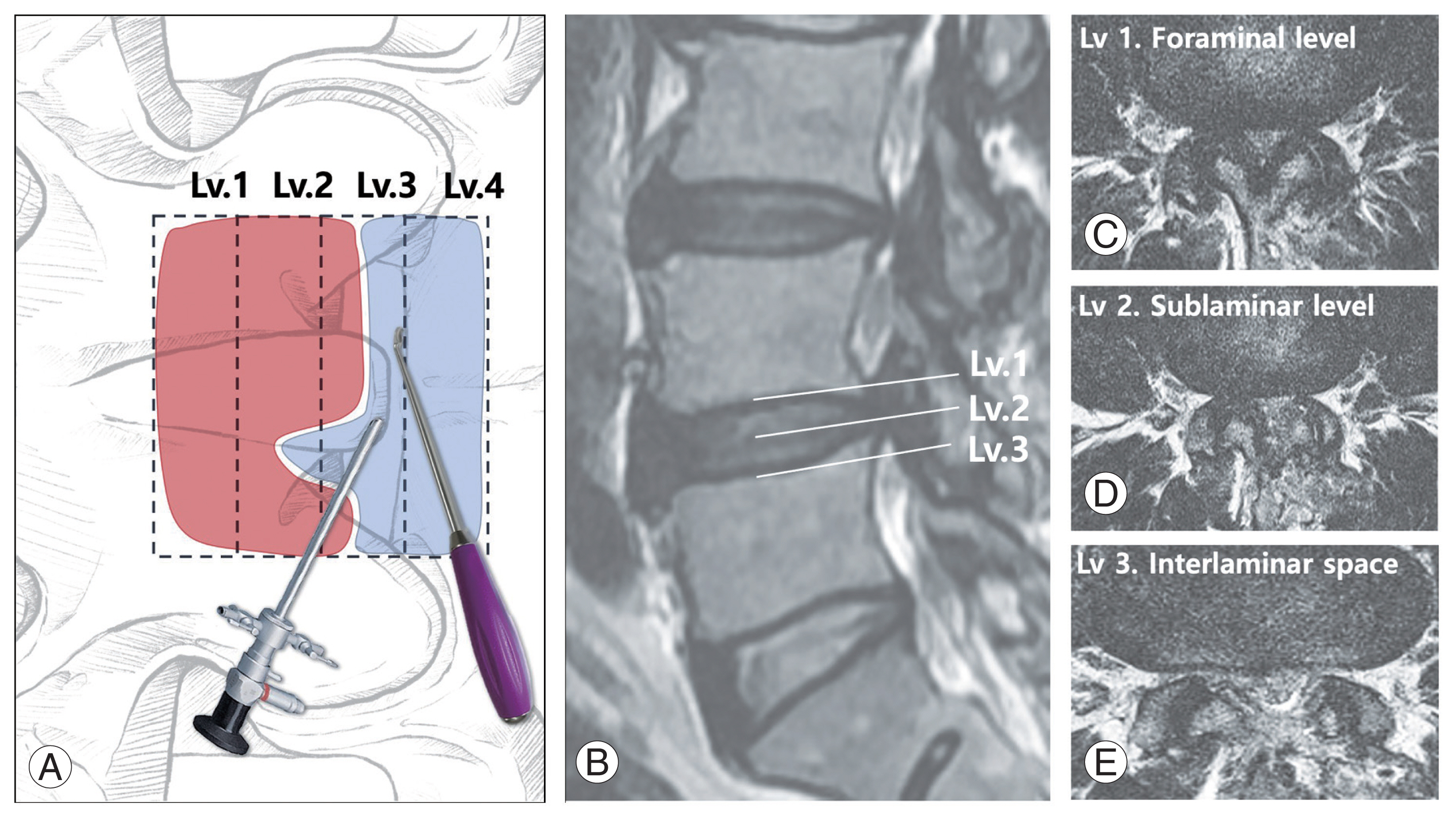
Fig. 23
Neglected foraminal stenosis. (A) Preoperative magnetic resonance views, central stenosis at L4–5, and foraminal stenosis on the right. (B) Postoperatively, sufficient central decompression with neglected foraminal stenosis. (C) Additional foraminal decompression was performed in 4 weeks after first central decompression.
References
1. Kwon JW, Moon SH, Park SY, et al. Lumbar spinal stenosis: review update 2022. Asian Spine J 2022;16:789–98.




2. Choi JY, Park SM, Kim HJ, Yeom JS. Recent updates on minimally invasive spine surgery: techniques, technologies, and indications. Asian Spine J 2022;16:1013–21.




3. Wu PH, Kim HS, An JW, et al. Prospective cohort study with a 2-year follow-up of clinical results, fusion rate, and muscle bulk for uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion. Asian Spine J 2023;17:373–81.




4. Junjie L, Jiheng Y, Jun L, Haixiong L, Haifeng Y. Comparison of unilateral biportal endoscopy decompression and microscopic decompression effectiveness in lumbar spinal stenosis treatment: a systematic review and meta-analysis. Asian Spine J 2023;17:418–30.




5. Caputy AJ, Luessenhop AJ. Long-term evaluation of decompressive surgery for degenerative lumbar stenosis. J Neurosurg 1992;77:669–76.


6. Javid MJ, Hadar EJ. Long-term follow-up review of patients who underwent laminectomy for lumbar stenosis: a prospective study. J Neurosurg 1998;89:1–7.


7. Omidi-Kashani F, Hasankhani EG, Ashjazadeh A. Lumbar spinal stenosis: who should be fused?: an updated review. Asian Spine J 2014;8:521–30.



8. Schaeren S, Broger I, Jeanneret B. Minimum four-year follow-up of spinal stenosis with degenerative spondylolisthesis treated with decompression and dynamic stabilization. Spine (Phila Pa 1976) 2008;33:E636–42.


9. Bridwell KH, Sedgewick TA, O’Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord 1993;6:461–72.


10. Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb 1982;120:343–7.


11. Liao JC, Chen WJ. Revision surgery for postoperative spondylodiscitis at cage level after posterior instrumented fusion in the lumbar spine-anterior approach is not absolutely indicated. J Clin Med 2020;9:3833.



12. Zhong ZM, Deviren V, Tay B, Burch S, Berven SH. Adjacent segment disease after instrumented fusion for adult lumbar spondylolisthesis: incidence and risk factors. Clin Neurol Neurosurg 2017;156:29–34.


13. Maragkos GA, Motiei-Langroudi R, Filippidis AS, Glazer PA, Papavassiliou E. Factors predictive of adjacent segment disease after lumbar spinal fusion. World Neurosurg 2020;133:e690–4.


14. Changoor S, Faloon MJ, Dunn CJ, et al. Does percutaneous lumbosacral pedicle screw instrumentation prevent long-term adjacent segment disease after lumbar fusion? Asian Spine J 2021;15:301–7.



15. Okuda S, Nagamoto Y, Matsumoto T, Sugiura T, Takahashi Y, Iwasaki M. Adjacent segment disease after single segment posterior lumbar interbody fusion for degenerative spondylolisthesis: minimum 10 years follow-up. Spine (Phila Pa 1976) 2018;43:E1384–8.

16. Hu ZJ, Fang XQ, Zhou ZJ, Wang JY, Zhao FD, Fan SW. Effect and possible mechanism of muscle-splitting approach on multifidus muscle injury and atrophy after posterior lumbar spine surgery. J Bone Joint Surg Am 2013;95:e192(1–9):

17. Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech 2005;18(Suppl): S1–6.

18. Satake K, Kanemura T, Nakashima H, Yamaguchi H, Segi N, Ouchida J. Cage subsidence in lateral interbody fusion with transpsoas approach: intraoperative endplate injury or late-onset settling. Spine Surg Relat Res 2017;1:203–10.



19. Peng L, Guo J, Lu JP, Jin S, Wang P, Shen HY. Risk factors and scoring system of cage retropulsion after posterior lumbar interbody fusion: a retrospective observational study. Orthop Surg 2021;13:855–62.




20. Parisien A, Wai EK, ElSayed MS, Frei H. Subsidence of spinal fusion cages: a systematic review. Int J Spine Surg 2022;16:1103–18.



21. Hasan S, Hartl R, Hofstetter CP. The benefit zone of full-endoscopic spine surgery. J Spine Surg 2019;5(Suppl 1): S41–56.



22. Yeung YK, Park CW, Jun SG, Park JH, Tse AC. Comparative cohort study for expansion of lateral recess and facet joint injury after biportal endoscopic ipsilateral decompression and contralateral decompression. Asian Spine J 2022;16:560–6.



23. Choi DJ, Kim JE, Jung JT, et al. Biportal endoscopic spine surgery for various foraminal lesions at the lumbosacral lesion. Asian Spine J 2018;12:569–73.




24. Iii WS, Orias AA, Shifflett GD, et al. Image-based markers predict dynamic instability in lumbar degenerative spondylolisthesis. Neurospine 2020;17:221–7.




25. Resnick DK, Watters WC 3rd, Sharan A, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: lumbar fusion for stenosis with spondylolisthesis. J Neurosurg Spine 2014;21:54–61.


26. Sugiura T, Okuda S, Matsumoto T, et al. Surgical outcomes and limitations of decompression surgery for degenerative spondylolisthesis. Global Spine J 2018;8:733–8.




27. Choi DJ, Choi CM, Jung JT, Lee SJ, Kim YS. Learning curve associated with complications in biportal endoscopic spinal surgery: challenges and strategies. Asian Spine J 2016;10:624–9.



28. Kong CB, Jeon DW, Chang BS, Lee JH, Suk KS, Park JB. Outcome of spinal fusion for lumbar degenerative disease: a cross-sectional study in Korea. Spine (Phila Pa 1976) 2010;35:1489–94.

29. Menendez JY, Omar NB, Chagoya G, et al. Patient satisfaction in spine surgery: a systematic review of the literature. Asian Spine J 2019;13:1047–57.




30. Christelis N, Simpson B, Russo M, et al. Persistent spinal pain syndrome: a proposal for failed back surgery syndrome and ICD-11. Pain Med 2021;22:807–18.




32. North RB, Campbell JN, James CS, et al. Failed back surgery syndrome: 5-year follow-up in 102 patients undergoing repeated operation. Neurosurgery 1991;28:685–91.



33. Nachemson AL. Evaluation of results in lumbar spine surgery. Acta Orthop Scand Suppl 1993;251:130–3.


34. Sebaaly A, Lahoud MJ, Rizkallah M, Kreichati G, Kharrat K. Etiology, evaluation, and treatment of failed back surgery syndrome. Asian Spine J 2018;12:574–85.




35. Hong X, Liu L, Bao J, Shi R, Fan Y, Wu X. Characterization and risk factor analysis for reoperation after microendoscopic diskectomy. Orthopedics 2015;38:e490–6.


36. Slipman C. Posterior joints of the lumbar spine as a potential cause of low back pain. Pain Med 2004;5:287–8.


37. Kirkaldy-Willis WH, Wedge JH, Yong-Hing K, Reilly J. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine (Phila Pa 1976) 1978;3:319–28.


38. Pao JL, Lin SM, Chen WC, Chang CH. Unilateral biportal endoscopic decompression for degenerative lumbar canal stenosis. J Spine Surg 2020;6:438–46.



39. Kim JE, Choi DJ, Park EJ, et al. Biportal endoscopic spinal surgery for lumbar spinal stenosis. Asian Spine J 2019;13:334–42.




40. Joh JY, Choi G, Kong BJ, Park HS, Lee SH, Chang SH. Comparative study of neck pain in relation to increase of cervical epidural pressure during percutaneous endoscopic lumbar discectomy. Spine (Phila Pa 1976) 2009;34:2033–8.


41. Kang MS, Park HJ, Hwang JH, Kim JE, Choi DJ, Chung HJ. Safety evaluation of biportal endoscopic lumbar discectomy: assessment of cervical epidural pressure during surgery. Spine (Phila Pa 1976) 2020;45:E1349–56.

42. Vargas RA, Moscatelli M, Vaz de Lima M, et al. Clinical consequences of incidental durotomy during full-endoscopic lumbar decompression surgery in relation to intraoperative epidural pressure measurements. J Pers Med 2023;13:381.



43. Soliman HM. Irrigation endoscopic discectomy: a novel percutaneous approach for lumbar disc prolapse. Eur Spine J 2013;22:1037–44.




44. Soliman HM. Irrigation endoscopic decompressive laminotomy: a new endoscopic approach for spinal stenosis decompression. Spine J 2015;15:2282–9.


45. Kim JE, Choi DJ, Park EJ. Risk factors and options of management for an incidental dural tear in biportal endoscopic spine surgery. Asian Spine J 2020;14:790–800.




46. Ikuta K, Tono O, Tanaka T, et al. Surgical complications of microendoscopic procedures for lumbar spinal stenosis. Minim Invasive Neurosurg 2007;50:145–9.


47. Fountas KN, Kapsalaki EZ, Feltes CH, et al. Correlation of the amount of disc removed in a lumbar microdiscectomy with long-term outcome. Spine (Phila Pa 1976) 2004;29:2521–6.


48. McGirt MJ, Eustacchio S, Varga P, et al. A prospective cohort study of close interval computed tomography and magnetic resonance imaging after primary lumbar discectomy: factors associated with recurrent disc herniation and disc height loss. Spine (Phila Pa 1976) 2009;34:2044–51.

49. Choi DJ, Jung JT, Lee SJ, Kim YS, Jang HJ, Yoo B. Biportal endoscopic spinal surgery for recurrent lumbar disc herniations. Clin Orthop Surg 2016;8:325–9.




50. Morgenstern R, Morgenstern C, Yeung AT. The learning curve in foraminal endoscopic discectomy: experience needed to achieve a 90% success rate. SAS J 2007;1:100–7.



51. Kim JE, Yoo HS, Choi DJ, Hwang JH, Park EJ, Chung S. Learning curve and clinical outcome of biportal endoscopic-assisted lumbar interbody fusion. Biomed Res Int 2020;2020:8815432.




52. Hu HS, Xue M, Xu R, Wang XJ, Chen MY, Yan SH. Sudden asystole during radiofrequency ablation: a case report and literature review. BMC Res Notes 2014;7:351.




53. Ahn DK, Lee JS, Shin WS, Kim S, Jung J. Postoperative spinal epidural hematoma in a biportal endoscopic spine surgery. Medicine (Baltimore) 2021;100:e24685.



54. Wu TM, Choi DJ, Chang WS, Hwang JH, Kim MC, Kim DG. Exploring physical lumbar microvascular geometry through endoscopy and illustrations: implications for clinical interpretation. Global Spine J 2023 Nov 28 [Epub]. https://doi.org/10.1177/21925682231218729

55. Xu WB, Kotheeranurak V, Zhang HL, et al. Is biportal endoscopic spine surgery more advantageous than uniportal for the treatment of lumbar degenerative disease?: a meta-analysis. Medicina (Kaunas) 2022;58:1523.



56. Kang SS, Kim JE, Choi DJ, Park EJ. Pseudomeningocele after biportal endoscopic spine surgery: a case report. J Orthop 2019;18:1–4.



57. Chun YM, Lee SH, Moon KS, Chang MC. Treatment of dural tear with nerve root herniation after unilateral biportal endoscopic decompression using an epidural blood patch: a case report. J Int Med Res 2022;50:3000605221144147.



58. Ahn DK, Kim YH, Ko YR, Jang SJ, Jung JS. The influence of systolic blood pressure at the time of extubation on the development of postoperative spinal epidural hematoma. Clin Orthop Surg 2023;15:265–71.




59. Ahn DK, Shin WS, Kim JW, Yi SM. Why cannot suction drains prevent postoperative spinal epidural hematoma? Clin Orthop Surg 2016;8:407–11.











