 |
 |
- Search
| Asian Spine J > Volume 17(3); 2023 > Article |
|
Abstract
Purpose
To investigate the radiological phenotype, patient and surgery-related risk factors influencing postoperative clinical outcome for cervical myelopathy caused by ossification of the posterior longitudinal ligament involving C2 following posterior instrumented laminectomy and fusion.
Overview of Literature
Ossified posterior longitudinal ligament (OPLL) is caused by ectopic ossification of the posterior longitudinal ligament. It can cause neurological impairment and severe disability. For multilevel cervical OPLL, studies have shown good neurological recovery following cord decompression via either an anterior or posterior approach. There is, however, a lacunae in the literature regarding the outcomes of patients with OPLL extending to C2 and above (C2 [+]).
Methods
We retrospectively studied 61 patients with C2 (+) OPLL who had posterior instrumented laminectomy and fusion at Ganga Hospital, Coimbatore between July 2011 and January 2021, with a minimum follow-up of 2 years. Data on demographics, clinical outcomes, radiology, and post-surgical outcomes were gathered.
Results
Among 61 patients, 56 were males and five were females. The OPLL pattern was mixed in 32 cases (52.5%), continuous in 26 cases (42.6%), segmental in two cases (3.3%), and circumscribed in one patient (1.6%). All of our patients showed signs of neurological improvement after a 24-month follow-up. The mean preoperative modified Japanese Orthopaedic Association (mJOA) score was 10.6 (range, 5–11) and the postoperative mJOA score was 15.8 (range, 12–18). The recovery rate was >75% in 27 patients (44.6%), >50% in 32 patients (52.5%), and >25% in two patients (3.3%). The average recovery rate was 71% (range, 33%–100%). The independent risk factor for predicting recovery rate is the preoperative mJOA score.
Ossified posterior longitudinal ligament (OPLL) is a type of degenerative cervical myelopathy caused by ectopic ossification in the posterior longitudinal ligament [1]. The subaxial cervical spine is the most common location for OPLL in the Asian population [2–5]. Spinal decompression procedures, either anterior or posterior, benefit symptomatic patients. However, due to complex anatomical structures, anterior decompression is risky in patients with OPLL extending above the C2 level (C2 [+] OPLL).
When approaching posteriorly, C2 is the key insertion point for the erector spinae muscle, which, along with the posterior tension band, aids in posture maintenance. Hence, concerns about muscle stripping at C2 leading to an increased risk of postoperative kyphosis and postoperative axial neck pain persist. In C2 (+) OPLL patients, surgical management with posterior surgeries like instrumented laminectomy [6], extended laminoplasty [7], and double dome laminoplasty [8] has been described. However, the literature on C2 (+) OPLL, is limited by a small number of patients. Furthermore, it is unclear whether C2 (+) OPLL differs from subaxial OPLL in terms of radiological characteristics and postoperative outcomes.
OPLL involving C2 and above is managed in our institute with posterior instrumented laminectomy and fusion when symptomatic, and in this study, we retrospectively analyzed the C2 (+) OPLL patients for radiological patterns, post-surgical outcomes, and complications with long-term follow-up.
From July 2011 to January 2018, we retrospectively obtained data on patients with symptomatic cervical myelopathy with C2 (+) OPLL who underwent posterior instrumented laminectomy with fusion at Ganga Hospital, Coimbatore. The endorsement from the institutional ethics review board of Ganga Hospital, Coimbatore was obtained (IRB approval no.,2019/06/02). The study excluded patients with cervical infection, cervical trauma, revision surgery, or incomplete radiological data. Informed consent was obtained from all individual participants included in the study.
A cervical spine computed tomography (CT) scan and cervical magnetic resonance imaging (MRI) with whole-spine screening were performed to confirm OPLL. OPLL was identified using CT-reconstructed axial and sagittal images. OPLL involving the cranial to the inferior endplate of C2 was defined as (C2 [+] OPLL). In the picture archiving and communication system, all radiological imaging was individually evaluated by two spine surgeons.
Age, gender, disease duration, body mass index, comorbidities, cervical lordosis, anteroposterior diameter of ossification, number of levels, the maximum level of ossification, occupancy ratio, and presence or absence of high intensity signal (HIS) in T2 weighted (T2W) MRI were all obtained. The relationship between the OPLL mass and to K line was discovered by Fujiyoshi et al. [9]. Surgical details including duration of the surgery, total blood loss, and complications were evaluated. According to the Japanese Ministry of Public Health and Welfare’s Investigational Committee on Ossification of Spinal Ligaments, OPLL is classified as continuous, segmental, mixed, and localized [10].
For pre- and postoperative neurological function assessment, the modified Japanese Orthopaedic Association (mJOA) scoring system was used. Neurological recovery rate (RR) calculated as=(final mJOA-preoperative mJOA)/18-preoperative mJOA. RR >75% was considered excellent, 50%–75% good, 25%–49% fair, and <25% poor. The Visual Analog Scale (VAS) was used to quantify the pre- and postoperative axial neck pain.
Using the standard posterior midline approach, all patients underwent posterior cervical laminectomy with instrumentation and fusion. Complete laminectomy was conducted at all engaged levels. C2 laminectomy was conducted in all our patients as there was substantial stenosis at C2 (Fig. 1). Significant stenosis at C2 was defined as the obliteration of the anterior or anterior with posterior subarachnoid space. A pedicle screw was used at C2, and lateral mass screws were used from C3 to C6. Pedicle screws were used in instances where instrumentation was extended to C7. For fusion, morselized autografts obtained locally were used. Postoperatively, patients were mobilized with a rigid cervical collar for about 6 weeks.
Mean and standard deviations were calculated for age, follow-up years, length of symptoms, preoperative mJOA score, postoperative mJOA score, RR, intraoperative blood loss, and surgical duration. Preoperative and postoperative parameter distinctions were decided by the independent-sample t-test. Univariate and multivariate linear regression analyses were conducted to assess the independent predictors of RR and mJOA score. Statistical analysis was conducted using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA) and p<0.05 was deemed statistically significant.
A total of 380 cervical OPLL patients were operated on at our institute during the study period. Seventy-one patients (18.7%) with C2 (+) OPLL were retrospectively identified. During the follow-up period, seven patients died from medical causes, and three patients were lost. The final study cohort included 61 patients (56 males and five females). The mean age was 58.2 years (range, 39–79 years). The OPLL was classified as a mixed pattern in 32 cases (52.5%), continuous type in 26 cases (42.6%), segmental type in two cases (3.3%), and circumscribed in one case (1.6%). The average number of involved vertebral bodies was 4.9 (range, 2–7). The average preoperative cervical lordosis (C2–C7 angle) was −15.9° (range, 3.2° to −21.3°). In 50 cases (82%), the distribution of maximum ossification was found between C2–C5 levels. The most stenotic level was the C3–C4 level in 26 cases (42.6%), the C4–C5 level in 13 cases (21.3%), and, C2–C3 in 11 cases (18%) (Fig. 2). The average occupancy ratio was 40.5% (range, 29%–62%). Within 61 patients, 53 patients were classified as K line (+), while eight patients have classified as K line (−) category. Preoperative T2W MRI revealed HIS within the cord (HIS [+]) in 44 patients (72.1%) and in 17 patients (27.9%) HIS absent (HIS [−]) (Table 1, Fig. 3).
All patients had standard posterior instrumented laminectomy and fusion procedures. The average blood loss during surgery was 288.5 mL (range, 200–430 mL), and the average surgical time was 130.3 minutes (range, 88–195 minutes). Surgical complications, including dural tears and neurological deterioration, were observed in nine patients (14.8%) of the 61 successful procedures (Table 2). Four patients (6.6%) had an intra-operative dural tear repaired with 5-0 prolene and duragen. There were no further complications in three of these cases. A late-onset cerebrospinal fluid (CSF) pseudomeningocele was surgically repaired in one patient. Three patients (4.9%) experienced transient neurological worsening. All three patients had a Medical Research Council (MRC) grade 5/5 preoperative neurology. Two patients’ postoperative motor power was reduced to MRC grade 3/5, while the third patient’s neurology was reduced to MRC grade 1/5. The first two patients recovered completely within 48 hours, and the third recovered completely after 3 months of follow-up. Two patients (3.3%) developed postoperative C5 palsy.
The average follow-up period was 3.2 years (range, 2 –5 years). Neurology improved in all of our patients at the final follow-up, including those who experienced transient neurological worsening after surgery (Table 2). The average preoperative mJOA score was 10.6 (range, 5–11), and the average postoperative mJOA score was 15.8 (range, 12–18). The RR was >75% in 27 patients (45%), >50% in 32 patients (52%), and >25% in two patients (3%). The average RR was 71% (range, 33%–100%), with pre- and postoperative VAS of 7 (range, 5–8) and 3 (range, 2–4), respectively. The mean cervical lordosis was −12.2° (range, −2.8° to −20.8°) at follow-up.
Out of 61 patients, 53 were classified as K line (+) and eight patients were classified as K line (−). There was no statistically significant difference between the two groups in terms of preoperative mJOA scores. The K line (+) group had a mean preoperative mJOA of 10.6±2.4 and a postoperative mJOA of 15.8±1.5 (p<0.05). The mean mJOA score of the K line (−) group preoperatively was 10.3±3.2 and 15.9±1.1 at the final follow-up, which was statistically significant (p<0.05). The RR for the K line (+) group was 70.7%±15.1% and 69.5%±14% for the K line (−) group, with no statistical difference (p>0.05).
Among 61 patients, 44 patients were classified as HIS (+) and 17 as HIS (−) group. The HIS (+) group had a lower preoperative mJOA score than the HIS (−) group, but the difference was statistically not significant (p=0.42). The HIS (+) group had a mean preoperative mJOA score of 10.5±2.4 and a postoperative mJOA score of 15.6±1.5 (p<0.05). The mJOA score in the HIS (−) group was 10.7±2.5 preoperatively and 16.2±1.2 postoperatively (p<0.05). The RR in the HIS (+) group was 68.8%±14.3%, while it was 75.3%±15.7% in the HIS (−) group, which was statistically insignificant (p=0.06) (Table 3).
After adjusting for confounding factors, multivariate linear regression analysis revealed that the preoperative mJOA score (B=−3.187, p<0.001) and final mJOA score (B=11.246, p<0.001) were the independent predictors of RR (Table 4).
Early diagnosis and prompt surgical intervention for patients with cervical myelopathy have resulted in improved quality of life and longevity. Because the literature on C2 (+) OPLL is limited, the radiological specifics and postoperative clinical outcomes are still unknown. In our study, we reviewed data from 61 patients with C2 (+) OPLL who underwent standard posterior laminectomy with instrumented fusion and were followed for at least 24 months.
The primary goal of decompressive surgery for cervical OPLL is to adequately decompress the spinal cord from ongoing pressure to halt the progression and improve symptoms of spinal cord dysfunction. According to research, even after laminectomy and laminoplasty, there may be a slow increase in OPLL mass because these decompressive surgeries increase the range of motion in the cervical segments, causing biomechanical strain in the PLL [11–13]. Morphologically, continuous and mixed OPLL have been linked to an increased risk of OPLL progression [14]. Hence, several authors advocate for instrumented fusion surgeries to reduce the risk of progression [15,16]. Because cases majority (93.4%) in our study belonged to the continuous and mixed types, we performed instrumented laminectomy with fusion on all of our patients. No patients in our study group underwent C1 arch resection because the CSF signal was seen clearly in the MRI at the C1 level both anterior and posterior to the spinal cord. The following major findings concerning C2 (+) OPLL phenotyping and surgical outcomes are revealed in this study. First, in OPLL with C2 (+), the space available for the cord (SAC) from C2–C5 is minimal. Secondly, in approximately 97% of cases, posterior instrumented laminectomy provides excellent to a good recovery. Third, there was no difference in outcome between the K line (+) and K line (−) groups. Finally, preoperative mJOA score and final mJOA were discovered to be independent predictors of mJOA RR among all parameters.
In the current study, 50.8% of C2 (+) OPLL had a mixed pattern, 42.6% had a continuous pattern, 3.3% had a segmental pattern, and one had a circumscribed pattern. The distribution of maximum ossification was found between C2–C5 in 82% of cases. The highest stenotic level was C3–C4, followed by C4–C5 and C2–C3, with an average of 4.9 vertebral bodies. The average occupancy ratio was 40.47% overall. Our findings are consistent with previous research on C2 (+) OPLL by Lee et al. [17] and Wang et al. [6]. In the study by Lee et al. [17], SAC was narrowest from C2–C4 with an average of 4.4 segment involvement in 40 cases with C2 (+) OPLL. The mixed type of OPLL predominated in their series, with the most common level being C4–C5. Wang et al. [6] studied 45 cases of C2 (+) OPLL, mixed type OPLL being the recurring pattern with mean segments involvement 5.1, and the thinnest spinal canal was found at C3–C4 level followed by C3 (Table 5).
We discovered that 53 patients (86.9%) were K line (+) and, 8 (13.1%) were K line (−). In K line (+) patients, posterior decompression and stabilization are universally accepted. However, several studies have found conflicts between the anterior and posterior approaches still exist for K line (−) cases. According to Fujiyoshi et al. [9] and Koda et al. [18], adequate posterior shift of the spinal cord and neurological improvement were not obtained following posterior decompression surgery in K line (−) patients. However, Koda et al. [18] discovered no significant difference in JOA score between anterior decompression with fusion (ADF) and posterior instrumented decompression and fusion (PDF). They discovered a significant difference in the RR between the ADF and PDF groups. In K line (−) cases, posterior decompression outperformed laminoplasty in terms of JOA score improvement. However, neither study was limited to C2(+) OPLL patients. The mJOA score improved statistically significantly in both the K line (+) and (−) groups. The RR was 71%±15% and 69%±14% in the K line (+) and K line (−) groups, respectively, without statistical variation (p>0.05). In a study by Wang et al. [6], the RR was 74±9 in the K line (+) group and, 70±17 among the K line (−) without significance (p=0.59). Saito et al. [19], also reported that in K line (−) patients, PDF provide moderate JOA score recovery of 44%.
Intramedullary HIS on T2 weighted image (T2WI) was associated with myelopathic symptoms in cervical OPLL cases. Choi and Hum [20] discovered a link between HIS and occupancy ratio. It has already been demonstrated that the presence of signal intensity change in T1WI is associated with poor surgical outcomes [21]. In T2W images, we found 44 cases with HIS (+) (72.1%) and 17 cases with HIS (−). Wang et al. [6] discovered a HIS (+) incidence of 73.3% in T2WI-MRI. They discovered a RR of 69%±16% in the HIS (+) group and 74%±16% in the HIS (−) group (p=0.44), which was comparable to our findings.
In our study, four patients had dural tears repaired during surgery using 5-0 prolene and duragen. However, at 6 months of follow-up, one of these developed pseudomeningocele and required surgical repair. It is critical to avoid dural tears because they increase the likelihood of patient-related adverse outcomes and impede postoperative care and rehabilitation. According to previous reports, the most common step leading to a dural tear is using the Kerrison punch for decompression. When we perform a laminectomy for cervical OPLL, we can only barely retract the cord, which can result in spinal cord injury. It is even more difficult with thick OPLL and narrow SAC, where the dura tightly adheres to the lamina. Therefore, it is difficult to identify the footplate of the Kerrison punch when it is inserted beneath the lamina, and the chances of a dural tear are increased during this step. Dural tears, in our experience, mostly occurred during our initial learning phase of using an ultrasonic bone scalpel. Particularly in thick eccentric OPLL with limited space between the lamina and OPLL mass.
There is currently agreement in the literature that multilevel cervical OPLL is best treated via a posterior approach. Lee et al. [8] recently reported positive results with the double-door laminoplasty technique in patients with C2 (+) OPLL. Wu et al. [22], discovered in a meta-analysis that overall surgical interventions (anterior or posterior) significantly (p<0.00001) improved the JOA score at the latest follow-up without affecting cervical lordosis. However due to the complex anterior neck anatomy of the upper cervical spine, C2 (+) OPLL is quite difficult to approach and requires meticulous surgical skills. Hence, in C2 (+) OPLL patients, the posterior approach is preferred [6,17]. Axial neck pain is a symptom of degenerative multilevel cervical cord compression. Hence, performing decompression with fusion is recommended. The addition of instrumentation improves biomechanical stability, thereby increasing fusion rates. Long-segment fusions, however, have a few long-term complications such as adjacent segment disease (ASD) and junctional kyphosis. The long-term incidence of ASD after long-segment cervical fusions has been estimated to be as high as 20%–30% [23,24]. However, no patients developed ASD or junctional kyphosis in our study, which had a mean follow-up of 3.2 years. ASD and junctional kyphosis have been linked to a variety of causes, including poor bone quality, excessive paraspinal muscle dissection, and instrumentation failure. According to research, the length of the fusion construct itself is not consistently associated with the occurrence of adjacent segment pathology or junctional kyphosis [25,26].
Our study has the hereunder limitations. First, it was a retrospective study with a small number of cases recruited. For all cases, only one standard surgical technique was conducted, and no comparisons between various surgical techniques could be made. Our center did not routinely perform postoperative CT/MRI scans unless the patient developed new symptoms, so the progression of OPLL in our patients after surgery could not be documented. A prospective study comparing clinical outcomes for C2 (+) and C2 (−) OPLL groups would aid in the pathology’s understanding.
C2 (+) OPLL are typically mixed or continuous types with thicker diameters and long segments. The narrowest SAC is located between C2–C5 with the most stenotic area as C3–C4. The most significant independent risk factor for predicting RR is the preoperative mJOA score, and posterior instrumented decompression with fusion is a relatively safe approach with satisfactory functional outcomes.
Notes
Author Contributions
Conception or design of the work: APS, NAS, GPK, JM, RMK, SR; the acquisition, analysis, or interpretation of data for the work: APS, NAS, GPK, JM, RMK, SR; drafting the work or revising it critically for important intellectual content: APS, NAS, GPK, JM, RMK, SR; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: APS, NAS, GPK, JM, RMK, SR; and final approval of the version to be published: APS, NAS, GPK, JM, RMK, SR.
Fig. 1
(A, B) Anteroposterior and lateral cervical spine radiographs showing ossification behind the upper cervical vertebral bodies. (C, D) Midsagittal and axial computed tomography scan images showing ossified posterior longitudinal ligament mass C2 to C5 with canal narrowing. (E, F) T2 weighted midsagittal and axial magnetic resonance imaging sections showing significant cord compression at C2 level. (G, H) Postoperative cervical spine radiographs C2–C6 posterior instrumented laminectomy.
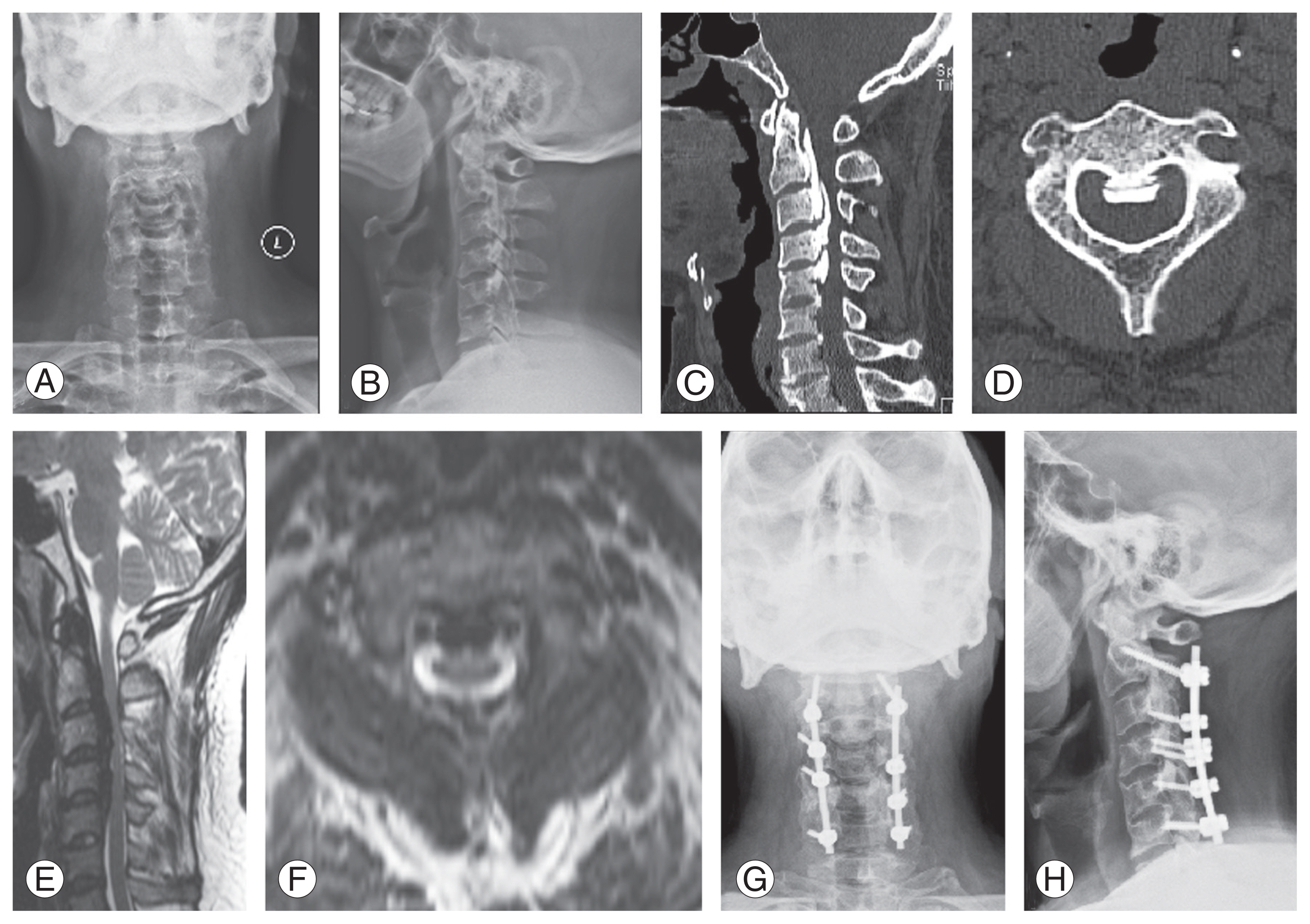
Fig. 2
(A, B) Lateral cervical radiograph and midsagittal computed tomography (CT) scan showing C2 (+) ossified posterior longitudinal ligament mass extending till C4 level. (C) T2 weighted midsagittal magnetic resonance imaging (MRI) showing significant spinalcord compression. (D, E) Axial CT and MRI scan images showing severe stenosis of the spinal canal at C3–C4 level.

Fig. 3
(A, B) Lateral cervical radiograph and midsagittal computed tomography scan showing ossified posterior longitudinal ligament (OPLL) extending from C2 to C4 level. (C, D) T2 weighted image and short-tau inversion recovery magnetic resonance imaging (MRI) images showing high intensity signal (HIS) at C2–C3 level (HIS [+] type). (E) Axial MRI images showing severe cord compression by the OPLL mass. (F, G) Anteroposterior and lateral postoperative radiographs following C2–C6 posterior instrumented laminectomy.
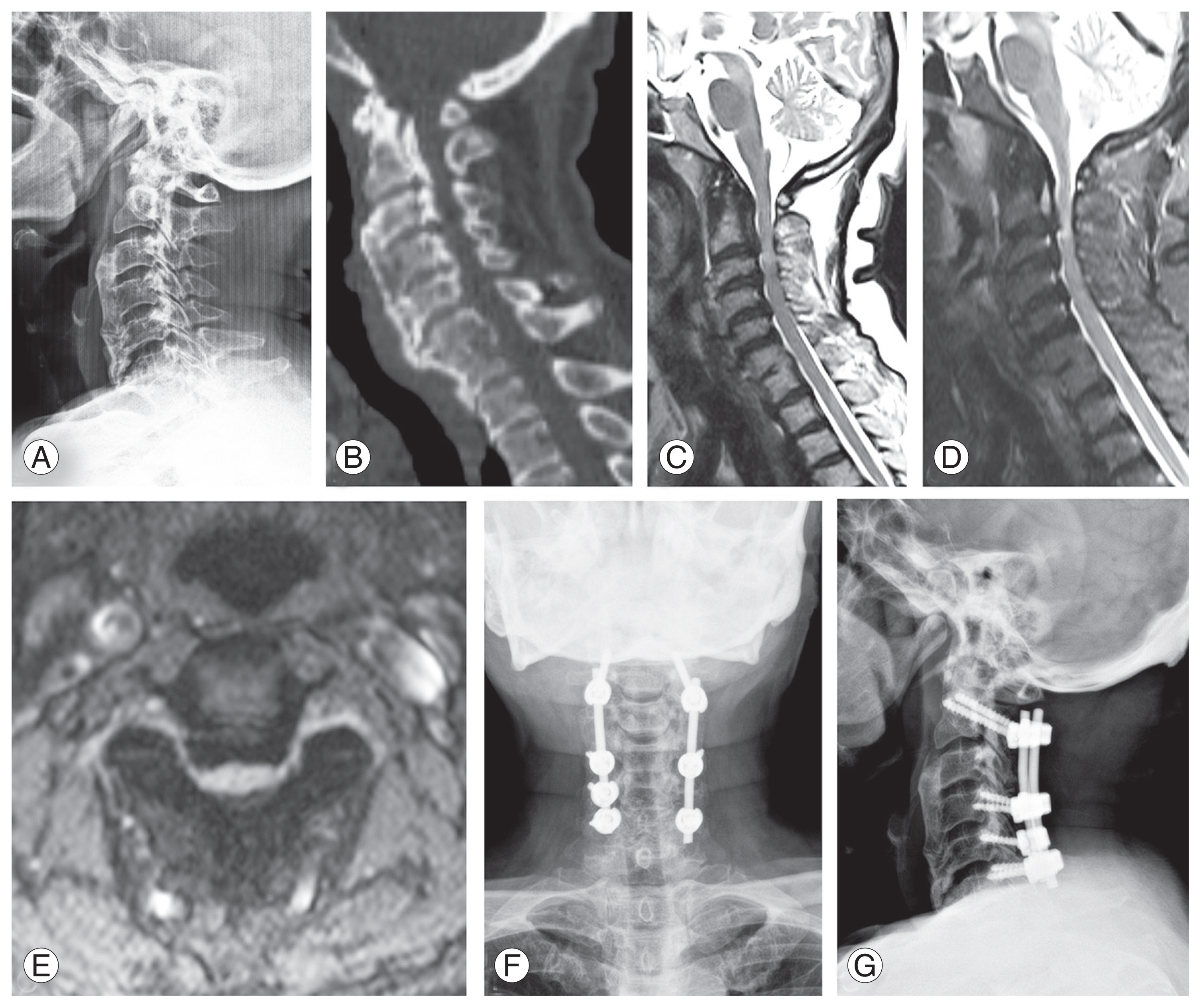
Table 1
Demographic and clinical data of patients with C2 (+) OPLL
Table 2
Surgical outcomes in the included patient cohort
Table 3
Postoperative clinical outcomes in the different subgroups
Table 4
Multivariate linear regression analysis of mJOA score recovery rate
Table 5
Review of literature
| Variable | Lee et al. [17] (2016) | Wang et al. [6] (2018) | Our study (2022) |
|---|---|---|---|
| No. of C2 (+) OPLL patients | 40 | 45 | 61 |
| Type: mixed (%) | 77.5 | 73.3 | 52.5 |
| No. of segments involved | 4.41±1.53 | 5.1 | 4.9 |
| Occupancy ratio (%) | 55.19±10.74 | 65.28 (47.6–87.2) | 40.5±7.6 |
| HIS (+) (%) | 45 | 73.3 | 72.1 |
| K line (−) (%) | - | 11.1 | 13.1 |
| Preoperative mJOA | 12.83±2.71 | 10.3±1.6 | 10.6±2.4 |
| Postoperative mJOA | 15.65±1.44 | 15.0±1.3 | 15.8±1.4 |
| Recovery rate (%) | 67.58±32.68 | 70.7±16.0 | 70.6±14.9 |
| Surgery performed (%) | Posterior (72.5) (procedure not mentioned) | Posterior open door laminoplasty and instrumented fusion | Posterior instrumented laminectomy (100.0) |
References
1. Mori K, Yoshii T, Hirai T, et al. Prevalence and distribution of ossification of the supra/interspinous ligaments in symptomatic patients with cervical ossification of the posterior longitudinal ligament of the spine: a CT-based multicenter cross-sectional study. BMC Musculoskelet Disord 2016;17:492.




2. Fujimori T, Watabe T, Iwamoto Y, Hamada S, Iwasaki M, Oda T. Prevalence, concomitance, and distribution of ossification of the spinal ligaments: results of whole spine CT scans in 1500 Japanese patients. Spine (Phila Pa 1976) 2016;41:1668–76.

3. Sohn S, Chung CK, Yun TJ, Sohn CH. Epidemiological survey of ossification of the posterior longitudinal ligament in an adult Korean population: three-dimensional computed tomographic observation of 3,240 cases. Calcif Tissue Int 2014;94:613–20.



4. Fujimori T, Le H, Hu SS, et al. Ossification of the posterior longitudinal ligament of the cervical spine in 3161 patients: a CT-based study. Spine (Phila Pa 1976) 2015;40:E394–403.

5. Singh NA, Shetty AP, Jakkepally S, Kumarasamy D, Kanna RM, Rajasekaran S. Ossification of posterior longitudinal ligament in cervical spine and its association with ossified lesions in the whole spine: a cross-sectional study of 2500 CT scans. Global Spine J 2023;13:122–32.




6. Wang L, Jiang Y, Li M, Si H, Li L, Qi L. Radiological characteristics and clinical outcome of ossification of posterior longitudinal ligament involving C2 after posterior laminoplasty and instrumented fusion surgery. Spine (Phila Pa 1976) 2019;44:E150–6.


7. Liu X, Li T, Shi L, et al. Extended laminoplasty for ossification of posterior longitudinal ligament involving the C2 segment. World Neurosurg 2019;130:317–23.


8. Lee DH, Dadufalza GK, Baik JM, et al. Double dome laminoplasty: a novel technique for C2 decompression. Neurospine 2021;18:882–8.




9. Fujiyoshi T, Yamazaki M, Kawabe J, et al. A new concept for making decisions regarding the surgical approach for cervical ossification of the posterior longitudinal ligament: the K-line. Spine (Phila Pa 1976) 2008;33:E990–3.

10. The ossification of the posterior longitudinal ligament of the spine (OPLL): the investigation committee on OPLL of the Japanese Ministry of Public Health and Welfare. Nihon Seikeigeka Gakkai Zasshi 1981;55:425–40.
11. Riew KD. Double dome laminoplasty: works well but there are exceptions. Neurospine 2021;18:889–90.




12. Kawaguchi Y, Kanamori M, Ishihara H, et al. Progression of ossification of the posterior longitudinal ligament following en bloc cervical laminoplasty. J Bone Joint Surg Am 2001;83:1798–802.


13. Takatsu T, Ishida Y, Suzuki K, Inoue H. Radiological study of cervical ossification of the posterior longitudinal ligament. J Spinal Disord 1999;12:271–3.


14. Chiba K, Yamamoto I, Hirabayashi H, et al. Multicenter study investigating the postoperative progression of ossification of the posterior longitudinal ligament in the cervical spine: a new computer-assisted measurement. J Neurosurg Spine 2005;3:17–23.


15. Ota M, Furuya T, Maki S, et al. Addition of instrumented fusion after posterior decompression surgery suppresses thickening of ossification of the posterior longitudinal ligament of the cervical spine. J Clin Neurosci 2016;34:162–5.


16. Katsumi K, Izumi T, Ito T, Hirano T, Watanabe K, Ohashi M. Posterior instrumented fusion suppresses the progression of ossification of the posterior longitudinal ligament: a comparison of laminoplasty with and without instrumented fusion by three-dimensional analysis. Eur Spine J 2016;25:1634–40.



17. Lee SE, Jahng TA, Kim HJ. Surgical outcomes of the ossification of the posterior longitudinal ligament according to the involvement of the C2 segment. World Neurosurg 2016;90:51–7.


18. Koda M, Mochizuki M, Konishi H, et al. Comparison of clinical outcomes between laminoplasty, posterior decompression with instrumented fusion, and anterior decompression with fusion for K-line (−) cervical ossification of the posterior longitudinal ligament. Eur Spine J 2016;25:2294–301.



19. Saito J, Maki S, Kamiya K, et al. Outcome of posterior decompression with instrumented fusion surgery for K-line (−) cervical ossification of the longitudinal ligament. J Clin Neurosci 2016;32:57–60.


20. Choi BW, Hum TW. Significance of intramedullary high signal intensity on magnetic resonance imaging in patients with cervical ossification of the posterior longitudinal ligament. Clin Orthop Surg 2015;7:465–9.



21. Shakya A, Sharma A, Singh V, Jaiswal A, Marathe N, Garje V. Preoperative T1 magnetic resonance imaging changes carry a poor postoperative prognosis in cervical myelopathy: a retrospective study of 182 patients. Surg Neurol Int 2021;12:629.



22. Wu D, Liu CZ, Yang H, Li H, Chen N. Surgical interventions for cervical spondylosis due to ossification of posterior longitudinal ligament: a meta-analysis. Medicine (Baltimore) 2017;96:e7590.


23. Lee JC, Lee SH, Peters C, Riew KD. Risk-factor analysis of adjacent-segment pathology requiring surgery following anterior, posterior, fusion, and nonfusion cervical spine operations: survivorship analysis of 1358 patients. J Bone Joint Surg Am 2014;96:1761–7.

24. Schroeder GD, Kepler CK, Kurd MF, et al. Is it necessary to extend a multilevel posterior cervical decompression and fusion to the upper thoracic spine? Spine (Phila Pa 1976) 2016;41:1845–9.









