Lumbar Interbody Fusion and Osteobiologics for Lumbar Fusion
Article information
Abstract
Lumbar interbody fusion (LIF) is an excellent treatment option for a number of lumbar diseases. LIF can be performed through posterior, transforaminal, anterior, and lateral or oblique approaches. Each technique has its own pearls and pitfalls. Through LIF, segmental stabilization, neural decompression, and deformity correction can be achieved. Minimally invasive surgery has recently gained popularity and each LIF procedure can be performed using minimally invasive techniques to reduce surgery-related complications and improve early postoperative recovery. Despite advances in surgical technology, surgery-related complications after LIF, such as pseudoarthrosis, have not yet been overcome. Although autogenous iliac crest bone graft is the gold standard for spinal fusion, other bone substitutes are available to enhance fusion rate and reduce complications associated with bone harvest. This article reviews the surgical procedures and characteristics of each LIF and the osteobiologics utilized in LIF based on the available evidence.
Introduction
Lumbar spinal disorders are among the most prevalent musculoskeletal disorders. Lumbar interbody fusion (LIF) is a treatment option that restores the intervertebral height, decompresses the spinal canal, stabilizes the instability, and resolves lordosis resulting from various lumbar pathologies [1]. It was introduced to overcome the low fusion rates seen after posterolateral fusion (PLF). Traditionally, LIF procedures were usually performed using anterior lumbar interbody fusion (ALIF) and posterior lumbar interbody fusion (PLIF) [1-3]. Recently, transforaminal lumbar interbody fusion (TLIF), lateral lumbar interbody fusion (LLIF), and oblique lumbar interbody fusion (OLIF) gained popularity over ALIF or PLIF because these procedures can be conducted using minimally invasive techniques to reduce surgery-related complications and improve early postoperative recovery [4-6]. To date, there is no clear high-level evidence on which of the approaches is clinically the best [7,8].
Lumbar fusion surgeries have increased over the past few decades owing to improvements in surgical technique and implants [9]. Despite these advances in surgical technology, pseudoarthrosis after LIF has not yet been overcome. Recent studies showed that nonunion rates after LIF still ranged from 5.5% to 20% [10,11]. Traditionally, autogenous iliac crest bone graft (ICBG) has been accepted as the “gold standard,” but harvesting of ICBG is restricted by donor site morbidities. Currently, several alternatives to ICBG are in use to enhance bony fusion.
This review presents the surgical technique of each LIF and provides their specific advantages, disadvantages, and indications/contraindications. In addition, the current options of osteobiologics for spinal fusion are reviewed.
Indications/Contraindications and Surgical Procedures
1. Posterior lumbar interbody fusion
PLIF was first recorded in 1944 [2], and Cloward [12] developed this procedure in the 1950s. PLIF is a traditional posterior approach and the most common lumbar approach. It allows access to the posterior and anterior columns in a single incision, and it allows for bilateral decompression with excellent visualization of neural structures [13,14]. This approach allows wide posterior visualization and circumferential decompression of nerve elements. Since the spine is already exposed for decompression, no separate incision is required. Accepted indications for PLIF include symptomatic spinal stenosis, segmental instability, spondylolisthesis, pseudoarthrosis, and recurrent disc herniation (Fig. 1) [15,16]. However, PLIF has its drawbacks. PLIF inevitably causes paravertebral muscle damage due to surgical access and prolonged muscle contraction [17]. PLIF also requires more retraction of the neural elements to access the intervertebral space [18-21]. Epidural bleeding and epidural fibrosis are inevitable, and sometimes neurological impairment occurs as a result. Additionally, retraction of the dura may entail an increased risk of durotomy, particularly during revision surgery [22]. Therefore, relative contraindications for PLIF surgery include extensive epidural scarring, arachnoiditis, and active infection [15]. The PLIF procedure consists of adequate laminectomy, medial facetectomy, annulotomy, discectomy, and insertion of cages or strut bone graft.
2. Transforaminal lumbar interbody fusion
TLIF was popularized by Harms and Rolinger as an alternative to PLIF [4,5]. The idea behind TLIF is to access the intervertebral disc space from a more lateral trajectory compared to PLIF [23]. Resection of the superior and inferior facets at the intended level of fusion is required to access the intervertebral disc [24]. Subsequent steps including discectomy, endplate preparation, and cage insertion are similar to PLIF; however, the major advantage of TLIF over PLIF is that it provides lateral access to the disc, reducing retraction of nerve elements. Therefore, TLIF may be associated with a decreased risk of incidental dural tear and neural complications when compared with PLIF [25]. TLIF can also be safely performed in the upper lumbar segment. TLIF may be a good option in revision cases because a lateral approach avoids the postoperative scar tissue of a posterior approach [26-28]. Also, TLIF preserves the interlaminar surface and facet joint on the contralateral side, allowing them to be used as an additional surface area for fusion. Compared to PLIF, TLIF can maintain biomechanical stability due to less damage to the posterior ligament complex. TLIF appears to have similar results to PLIF in biomechanical studies [29,30].
Since Foley et al. [31,32] introduced TLIF using minimally invasive surgery (MIS) to reduce approach-related complications, the MIS technique has gained popularity as it has led to better outcomes due to reduced blood loss and muscle damage, shorter operation time, and earlier recovery after surgery compared to the conventional open technique (Fig. 2) [25,33-39]. Patient selection for TLIF is similar to that for PLIF. Indications for TLIF include spinal stenosis, segmental instability, spondylolisthesis, pseudoarthrosis, and recurrent disc herniation. Contraindications are arachnoiditis, epidural scarring, active infection, and severe osteoporosis. Furthermore, conjoined nerve roots are a relative contraindication as they may preclude access to the intervertebral disc.

A 67-year-old female patient underwent minimally invasive surgery-transforaminal lumbar interbody fusion for degenerative lumbar spondylolisthesis and right foraminal stenosis at L4–L5. Preoperative magnetic resonance imaging (MRI) (A) and X-ray (B). Postoperative MRI (C) and X-ray (D) show foraminal decompression and placement of cages and pedicle screws/rods.
Recently, endoscopic LIF has been attempted [40], and the basic principles of endoscopic LIF are the same as TLIF [41]. TLIF can be conducted using biportal endoscopic systems with two channels: one working and one endoscopic channel. The next step is similar to TLIF in that discectomy and endplate preparation are conducted through the endoscopic system. Although evidence of the pros and cons relating to surgical outcomes and complications is still lacking, published studies have shown promising postoperative outcomes [42-44]. There are, however, unavoidable obstacles such as a learning curve to overcome [42,45-47].
3. Anterior lumbar interbody fusion
ALIF allows a less traumatic approach compared to the posterior approach, resulting in less pain and a shorter hospital stay [48-50]. The direct visualization of the intervertebral disc allows complete discectomy for a larger interbody cage. Large interbody devices offer significant biomechanical advantages over other LIFs [51]. Placing more lordotic cages in the lower lumbar spine may increase the chances of achieving sagittal alignment [52-56]. Also, ALIF can yield higher fusion rates and preserve the posterior structures, including back muscles and ligaments [57,58].
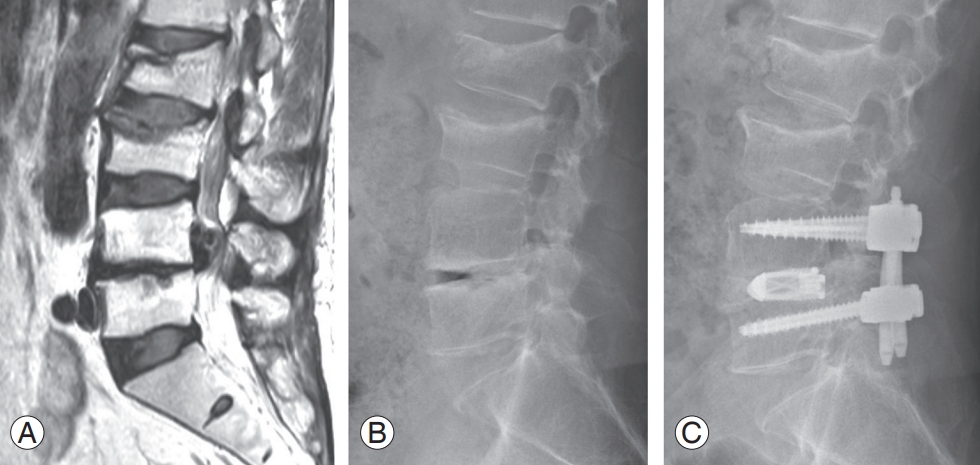
The anterior approach is an excellent approach for the L5–S1 level. A thorough preoperative evaluation is required before considering ALIF. A careful review of the vascular structures along with the lumbar spine by preoperative magnetic resonance imaging (MRI) and/or computed tomography scan is recommended to assess the region of interest (Fig. 3) [59-61]. Generally, the retroperitoneal approach is preferred, because transperitoneal access is associated with high rates of ileus, internal organ injury, and retrograde ejaculation and is restricted in the L5–S1 segment. Because the vena cava is more vulnerable than the aorta, the usual access is from the patient’s left side. By careful examination with gentle finger dissection, the arcuate line and the transverse fascia are seen and separated from the abdominal wall. After the transverse fascia is incised, all the peritoneal components are swept anteriorly to access the retroperitoneal space through a gentle dissection. The reference points are usually the round psoas muscle and the left common iliac artery. The next landmark is the sacral promontory. The superior hypogastric plexus exits in front of the L5–S1 disc and can be damaged by unipolar electrocautery, resulting in autonomic dysfunction. Injury to the superior hypogastric plexus may develop, leading to a high rate of retrograde ejaculation in men (up to 45%) [62-64]. There is a risk of damage to the abdominal organs and ureters, and postoperative hernias may occur [65]. Large vessel manipulation may result in deep vein thrombosis and/or direct injury [65]. Surgical indications for ALIF include sagittal plane deformities, degenerative disc disease, pseudarthrosis, and postoperative spondylodiscitis. Contraindications include severe obesity, previous abdominal surgery or radiation therapy, and severe aortic disease.
4. Lateral lumbar interbody fusion
First described by Ozgur et al. [66] in 2006, LLIF is considered a safe and effective alternative to ALIF or PLIF. LLIF can minimize approach-related morbidity such as soft tissue damage, blood loss, and length of hospital stay, with better clinical and radiological results compared to a traditional open approach [67-69]. LLIF begins with the patient in either a left or right lateral decubitus position. After a lateral incision is made, the abdominal muscles are bluntly dissected. After reaching the retroperitoneum, care must be taken not to injure important nerves, including the intercostal nerve, the subiliac nerve, the iliac inguinal nerve, and the lateral femoral cutaneous nerve. Intraoperative nerve monitoring is helpful in preventing damage to the lumbar plexus within the psoas [70]. After the placement of sequential dilators and a tubular retractor on the psoas muscle, a classic discectomy and cage insertion are performed with fluoroscopy.
Indications for LLIF include degenerative disc disease, spondylolisthesis, and scoliosis. LLIF is especially effective in revision cases where there is adjacent segment pathology or postlaminectomy syndrome [71,72]. Correction of sagittal as well as coronal deformities can be achieved using lordotic and big cages [72-76]. Furthermore, LLIF has been demonstrated to restore foraminal height and central canal surface through indirect decompression [76-79]. However, LLIF has disadvantages, including damage to the psoas, internal organs, or lumbar plexus [72,80]. Postoperative groin or thigh pain, hip flexion weakness, and paresthesia may also occur on the approach side [72,80]. Contraindications are adhesive retroperitoneal situations that inhibit safe access, including a history of previous retroperitoneal surgery, infection, or radiation therapy. This approach is contraindicated on L5–S1 discs because the iliac wing obstructs the true lateral accessibility.
5. Oblique lumbar interbody fusion
OLIF, also called anterior-to-psoas or prepsoas, had been developed to overcome LLIF’s limitations. Access to discs between the psoas muscle and the major abdominal vessels reduces the risk of injury to the muscles and lumbar plexus (Fig. 4). Thus, postoperative groin or thigh pain can be avoided and neuromonitoring is not required [81]. Meta-analysis found that the risk of anterior thigh pain, ipsilateral hip flexor weakness, and lumbar plexus injury were decreased with OLIF compared to LLIF [80,82,83]. However, the risk of large vessel damage may increase due to its proximity [80,83]. Another disadvantage of OLIF is the risk of sympathetic damage due to the presence of the sympathetic chain in the working window [80,83].

A 70-year-old female patient underwent minimally invasive surgery-oblique lumbar interbody fusion for foraminal stenosis at L3–L4 and L4–L5. (A) On preoperative magnetic resonance imaging, the bare window (red arrow) between the aorta and the psoas muscle should be confirmed. (B) Preoperative X-ray. (C) Postoperative X-ray shows restoration of disc heights and placement of cages and pedicle screws/rods.
Indications and contraindications of OLIF are the same as for LLIF. The L5–S1 space cannot be accessed by LLIF, but OLIF can target L5–S1; however, access above L2–L3 using OLIF is difficult due to the ribs. The surgeon’s view is much more advantageous in OLIF; while LLIF only allows the surgeon to see the surgical field vertically. Typical indications for OLIF include degenerative disc disease, spondylolisthesis, discitis, and pseudarthrosis from L2–L3 to L5–S1. Contraindications include a history of previous retroperitoneal surgery, radiation therapy, or infection. The surgical procedure is similar to LLIF. A skin incision is made around the anterior edge of the disc. After the placement of sequential dilators and a tubular retractor in the bare area between the aorta and the psoas muscle, a classic discectomy and cage insertion are performed with the orthogonal maneuver.
Osteobiologics for Lumbar Interbody Fusion
Autogenous and allogeneic bone grafts can be used for spinal fusion, but autogenous ICBG is considered the gold standard due to its osteogenic, osteoinductive, and osteoconductive properties.
Autograft is the gold standard, but has limitations, as the amount of bone obtained through decompression is limited and additional incisions for ICBG harvesting due to donor site morbidity increase complications such as donor site pain, increased blood loss, and increased operation time [84-86]. These drawbacks have created a need to reduce the use of ICBG and find alternative sources of graft material. Consequently, the use of ICBG has declined consistently and efforts have been made to find cost-effective alternatives to ICBG. Osteobiologics are bone substitutes that promote bone healing and are increasingly being used in spinal surgery. The use of osteobiologics is believed to reduce complications and improve fusion rates [87]. Currently, common options for osteobiologics include allografts, bone morphogenetic protein (BMP), and stem cell-based therapy. In this review, we will briefly discuss the properties, efficacy, and future applications of various osteobiologics.
1. Allogeneic bone graft
Allograft bone is sourced from cadavers and is readily available. Generally, an allograft can act as an osteoconductive and weakly osteoinductive scaffold; however, it has no osteogenic potential because it has no viable cells. Nevertheless, previous studies have shown that allograft is a reasonable alternative to autograft. A recent systematic review by Liao et al. [88] showed that fusion rates between allograft and autograft in patients who underwent lumbar fusion were not significantly different. Although several studies have demonstrated similar fusion rates between the two, a randomized clinical trial (RCT) found autograft showed a relatively shorter time to complete fusion [89]. Gao et al. [90] reported that allograft alone yielded a lower fusion rate compared to allograft with autogenous bone marrow and allograft with BMP in their LLIF series. The use of allografts to supplement local autogenous bone can produce similar results to ICBG. For this reason, allografts generally act as autograft extenders rather than alone in posterior spinal fusion.
2. Demineralized bone matrix
Demineralized bone matrices (DBMs) are acid-extractive allogeneic bone grafts, leaving behind the organic matrix of type 1 collagen, non-collagenous proteins, and growth factors. DBMs are commercially available in various forms, from putty or paste to injectable gel. They are less osteoconductive and non-osteogenic than mineralized allografts but retain more osteoinductivity and are also used as autograft extenders. In an RCT by Kang et al. [91], fusion rates at 2-year follow-up were 92% and 86% for autograft and DBM, respectively. Kim et al. [92] reported fusion rates using autograft and DBM were 62.2% and 52% at the second year, respectively. However, previous studies supported that fusion rate with DBM increased when supplemented with autografts [93,94]. DBM is a reasonable substitute as an autograft extender in spinal fusion, but there is currently little or no evidence that DBM is a good stand-alone osteobiologic.
3. Bone morphogenetic protein
BMPs, first isolated by Urist [95] in 1965, are growth factors of the transforming growth factor-β family with osteogenic capabilities. So far, more than 20 subtypes of BMPs are known but BMP-2, BMP-4, and BMP-7 are the most studied. BMPs are widely used in various medical areas, and recombinant human BMP-2 (rhBMP-2) is the one most widely used for spinal fusion. Since the US Food and Drug Administration (FDA) approval of ALIF in 2002, improved fusion rates have been demonstrated in many studies [96,97]. Early RCTs even showed an increased fusion rate and decreased complications compared to ICBG [96,97]. Recently, the off-label use of rhBMP-2 has widely expanded into posterior lumbar fusion and cervical fusion, as well as ALIF. Numerus studies have shown successful fusion rates with rhBMP-2 in PLIF, TLIF, and PLF. In an RCT of 463 patients who underwent PLF, the rhBMP-2 group showed a significantly higher fusion rate at 2 years and a less reoperation rate compared to the ICBG group [98]. A systematic review found that fusion rates with rhBMP-2 use are statistically significantly improved in ALIF and PLF, but not in PLIF or TLIF [99]. However, the same authors also cautiously concluded that the results could be biased due to the heterogeneity of dosing and surgical procedures.
Potentially serious adverse outcomes have been reported with rhBMP-2 use. In cervical fusion, serious complications such as airway edema and dysphagia have led to an FDA-issued warning [100,101]. Other concerns in lumbar fusion include retrograde ejaculation, seroma formation, radiculitis, ectopic ossification, and vertebral osteolysis [101]. Crandall et al. [102] in a 5-year follow-up of 509 TLIF patients with rhBMP-2, however, reported that BMP-related complication rates were rare (seroma in 0.4% and ectopic bone growth in 0.6%). Another critical concern is the possible association between rhBMP-2 and tumorigenesis [103], although insufficient conclusions were drawn on meta-analysis [104,105]. Further research is needed to find safe and effective doses of rhBMP-2.
4. Stem cell-based therapy
The use of stem cells in tissue regeneration is rapidly expanding and osteogenic stem cells are thought to have the potential to bring about bony fusion. Among various stem cells, mesenchymal stem cells (MSC), which can differentiate into osteoprogenitor cells and osteoblasts, are the most likely candidate substitute for bone. Recent preclinical and clinical trials using MSC-based therapy for spinal fusion demonstrated that MSC could be a viable option for utilization in spinal fusion [106,107]. One study using autogenous MSC from the iliac crest reported a fusion rate of 95.1% when used together with porous tricalcium phosphate for posterior spinal fusion [108]. A recent RCT of 24 patients who underwent 1-to-3 lumbar posterior fusions compared the fusion rates between ICBG and allografts with bone marrow aspirates containing autogenous stem cells. This study showed no significant difference in the fusion rate between the two groups [109]. However, bone marrow aspirates yield only 0.001% to 0.01% MSC [110]. While stem cell use is promising in spinal fusion, there are still limitations to overcome.
Conclusions
LIF has already proven to be an excellent surgical technique for a variety of lumbar diseases. With the continuous development of surgical techniques and cage design, several LIF procedures are now available, and each has its own characteristics. The optimal surgical procedure and implant should be selected according to the anatomical, pathological, and surgical conditions. The most important surgical goal is bony fusion and avoidance of complications; however, successful fusion remains a major challenge to spinal surgeons. Future studies are needed to improve fusion rates and reduce adverse effects with the development of more effective osteogenic factors.
Notes
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: YHK, KYH, KWK; data curation: HYP, JK, SIK; formal analysis: YSK, JHS; methodology: YSK, KWR, JBP, JHS; project administration: YSK, YHK; writing–original draft: YHK, SIK; writing–review & editing: YYK, JSL, SJL; and final approval of the manuscript: all authors.