|
 |
- Search
| Asian Spine J > Volume 16(5); 2022 > Article |
|
Abstract
In this systematic review and meta-analysis, we aim to thoroughly describe and objectively compare the efficacy of anterior cervical plate (ACP) and stand-alone cage (SAC). Although recognized as an effective procedure for cervical degenerative disease (CDD), a debate between the methods of anterior cervical discectomy and fusion exists. ACP provides stability to the fusion construct; however, some complications have been reported, such as dysphagia, adjacent disc disease, and soft tissue injury. To overcome these complications, a SAC was later introduced. A systematic search was conducted on the basis of PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines to identify relevant studies through PubMed, Google Scholar, and Cochrane database. A total of 14 studies (960 patients) were included in the meta-analysis. Twenty outcomes were clinically and radiologically compared between the two procedures. ACP and SAC were comparable in terms of dysphasia rate, loss of segmental angle, loss of disc height, the Odom criteria, Robinson’s criteria, hospital stay, Japanese Orthopaedic Association score, Neck Disability Index, Visual Analog Scale, and fusion time. However, SAC was superior in terms of shorter operation time, less blood loss, lower dysphagia rate, and lower rate of adjacent level disease, whereas ACP was advantageous in terms of lower subsidence rate, better maintenance of the cervical global and segmental angles and disc height, and higher fusion rate. Both procedures can be used in patients with CDD, although it might be more beneficial to choose ACP in patients with multi-level pathologies, wherein better mechanical stability is provided. However, SAC may be more beneficial to use in patients with comorbidities, anemia, or swelling problems because it offers lower complication rates.
First introduced in 1958 by Cloward [1] and by Smith and Robinson [2], anterior cervical operation has been known to be a safe and effective method for the treatment of degenerative cervical spondylosis. Although recognized as an effective procedure for cervical degenerative disease (CDD), a debate between the methods of anterior cervical discectomy and fusion (ACDF) exists [3]. As a routine traditional method of ACDF, anterior cervical plate (ACP) provides stability to the fusion construct [4,5]. However, some complications have been reported, such as dysphagia, adjacent disc disease, and soft tissue injury, especially in multi-level ACDF. To overcome these complications, a newer surgical technique was introduced in the 2000s, which was a stand-alone cage (SAC), also called a zero-profile implant or anchoring cage. It consists of a cage and an internal implant with a pair of locking screws, allowing the internal implant to be directly inserted into the intervertebral disc, with the screws inserted into the adjacent vertebral body as fixation. The major difference between ACP and SAC is that no additional plate is attached to the anterior surface of the vertebral body in SAC [6]. With this construct, SAC offers less soft tissue damage, resulting in fewer hardware-related complications. Conversely, some literature reported poor immediate stability, higher incidence of subsidence rate, and malalignment using SAC [5].
To date, there has been no consensus that thoroughly describes and objectively compares the efficacy between the two procedures in detail. Moreover, the results vary from one literature to another. Through this systematic review and meta-analysis, we aim to further clarify controversies regarding this matter by comparing the outcomes between ACP and SAC for CDD.
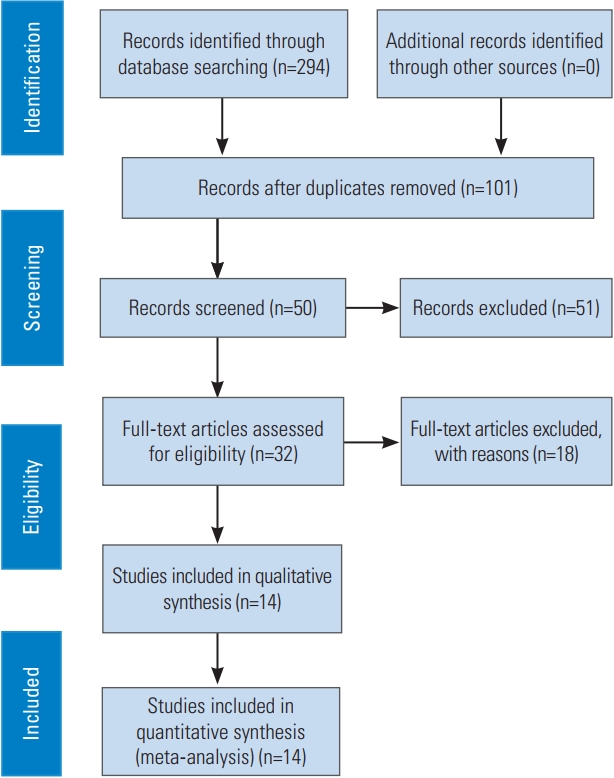
This study has been registered in the PROSPERO (international prospective register of systematic reviews) registry (registration no., CRD42021244236). The study design was a systematic review and meta-analysis of relevant randomized controlled trials and non-randomized comparative studies. A systematic search was conducted from October 2020 to January 2021 to identify relevant studies through PubMed, Google Scholar, and Cochrane database based on the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guideline (Fig. 1). The following were the keywords used: “Stand-alone Cage” AND “Anterior Cervical Plate” AND “Anterior Cervical Discectomy and Fusion” AND “Outcome”.
Titles and abstracts of the articles found were then manually scanned and reviewed by all authors. Full text of relevant articles was subsequently extracted and carefully selected according to the following inclusion criteria: (1) studies included a comparative design for SAC versus ACP in CDD, whether in retrospective, prospective as well as randomized controlled and observational studies; (2) studies reported a clinically and/or radiologically desirable outcome with either continuous or dichotomous variable; (3) studies with a follow-up period of at least 12 months; and (4) studies in English with any publication year. Exclusion criteria were patients with rheumatoid arthritis, congenital spine disorders, neoplasm or infection of the spine, traumatic cervical pathology, and history of previous cervical surgery and patients receiving other methods of treatment. Duplicate publications, noncomparative studies, nonhuman in vivo and in vitro studies, and articles in a language other than English were also excluded. Table 1 describes the population, intervention, control, and outcomes method for defining the inclusion and exclusion criteria. The Joanna Briggs Institute Scoring System will be used as a measure for eligibility assessment. Disagreements were solved by discussion among the three authors.
Data extraction was collected under basic characteristics and outcomes using designated tables in Microsoft Excel (Microsoft Corp., Redmond, WA, USA) for all identified and included studies. When the data were available, quantitative analysis was performed using Review Manager (RevMan, computer program ver. 5.3, the Cochrane Collaboration, 2014; The Nordic Cochrane Center, Copenhagen, Denmark). Outcomes were presented in the form of forest plots. In each study, the mean difference for continuous outcome and odds ratio for dichotomous outcome with a 95% confidence interval (CI) was calculated. A fixed-effects model was used when the heterogeneity (I2) was <50%, whereas a random-effects model was used when the heterogeneity was >50%.
A total of 14 studies (960 patients) were included, divided into 20 outcome analyses. Twelve studies were cohort retrospective design (level III evidence), whereas two articles were cohort prospective design (level II evidence) (Table 2). A total of 522 patients (54.38%) were treated with SAC, whereas 438 patients (45.62%) were treated with ACP. Critical appraisal of all included studies based on the Joanna Briggs Institute Scoring System showed that no study had more than three invalid parameters. Table 3 describes sample characteristics, whereas Tables 4 and 5 describe the outcome analysis of included studies. (Full tabulation of eligibility assessment and detailed study characteristics is available in the supplementary section.)
For the operation time, nine articles were included in the analysis, with a total sample of 520 patients; the mean operation time for SAC (n=278) and ACP (n=242) was 111.79 minutes and 130.73 minutes, respectively. Fig. 2 shows a significant difference in terms of operation time between the two procedures, wherein ACP required longer operation time than SAC (heterogeneity, I2=72%; weighted mean difference [WMD], −19.00; 95% CI, −25.06 to −12.94; p<0.00001).
For blood loss, eight articles were included in the analysis, with a total sample of 442 patients; the mean blood loss for SAC (n=222) and ACP (n=220) was 64.25 mL and 78.19 mL, respectively. Fig. 3 shows a significant difference in terms of blood loss between the two procedures, wherein SAC was beneficial with less intraoperative blood loss (heterogeneity: I2=96%; WMD, −12.84; 95% CI, −18.15 to −7.53; p<0.00001).
For dysphasia rate, three articles were included in the analysis, with a total sample of 282 patients; dysphasia was experienced in four of 141 patients (2.84%) treated with SAC and three of 141 patients (2.13%) treated with ACP, indicating no significant difference (heterogeneity: I2=0%, p=0.67) (Fig. 4).
For dysphagia rate, nine articles were included in the analysis, with a total sample of 602 patients; dysphagia was experienced in 12 of 320 patients (3.75%) treated with SAC and 51 of 282 patients (18.09%) treated with ACP, indicating a significant difference, wherein ACP resulted in higher dysphagia rate (heterogeneity: I2=0%, p<0.00001) (Fig. 5).
For the postoperative cervical angle, 10 articles were included in the analysis, with a total sample of 600 patients; the mean cervical angle for SAC (n=311) and ACP (n=289) was 12.78° and 14.99°, respectively. Fig. 6 presents a significant difference in terms of the cervical angle between the two procedures, wherein ACP resulted in a higher cervical angle than SAC (heterogeneity: I2=72%; WMD, −1.82; 95% CI, −2.98 to −0.66; p=0.002). As for the loss of cervical lordosis, two articles were included in the analysis, with a total sample of 116 patients; the mean loss of cervical lordosis for SAC (n=58) and ACP (n=58) was 3.9° and 1.9°, respectively. Fig. 7 shows a significant difference in terms of loss of cervical lordosis between the two procedures, wherein SAC was associated with a higher loss of cervical lordosis (heterogeneity: I2=0%; WMD, 2.04; 95% CI, 1.23 to 2.84; p<0.00001).
For the postoperative segmental angle, 8 articles were included in the analysis, with a total sample of 424 patients; the mean segmental angle for SAC (n=227) and ACP (n=197) was 3.22° and 5.06°, respectively. Fig. 8 shows a significant difference in terms of the cervical angle between the two procedures, wherein ACP resulted in a higher segmental angle than SAC (heterogeneity: I2=65%; WMD, −1.70; 95% CI, −3.04 to −0.35; p=0.01). As for the loss of segmental angle, three articles were included in the analysis, with a total sample of 168 patients; the mean loss of segmental angle for SAC (n=87) and ACP (n=81) was 1.84° and 1.2°, respectively. Fig. 9 reveals no significant difference in terms of loss of segmental angle between the two procedures (heterogeneity: I2=53%; WMD, 0.85; 95% CI, −0.02 to 1.72; p=0.06).
For the postoperative disc height, nine articles were included in the analysis, with a total sample of 152 patients; the mean postoperative disc height for SAC (n=90) and ACP (n=62) was 16.41 mm and 17.27 mm, respectively. Fig. 10 shows a significant difference in terms of disc height between the two procedures, wherein ACP resulted in a higher disc height postoperatively (heterogeneity: I2=71%; WMD, −0.56; 95% CI, −1.02 to −0.10; p=0.02). As for the loss of disc height, six articles were included in the analysis, with a total sample of 310 patients; the mean loss of disc height for SAC (n=159) and ACP (n=151) was 2.14 mm and 1.64 mm, respectively. Fig. 11 reveals no significant difference in terms of loss of disc height between the two procedures (heterogeneity: I2=82%; WMD, 0.40; 95% CI, −0.22 to 1.03; p=0.21).
For the subsidence rate, 13 articles were included in the analysis, with a total sample of 1.305 patients; subsidence was experienced in 200 of 692 patients (28.9%) treated with SAC and 82 of 613 patients (13.38%) treated with ACP, indicating a significant difference, wherein ACP may benefit with lower subsidence rate (heterogeneity: I2=32%, p<0.00001) (Fig. 12).
Functional outcomes were measured using the Odom criteria, Robinson’s criteria, Japanese Orthopaedic Association score (JOA), and Neck Disability Index (NDI). Four articles were included in the analysis of the Odom criteria. With a total sample of 254 patients, 116 of 129 patients (89.92%) treated with SAC and 113 of 125 patients (90.4%) treated with ACP had satisfying functional outcome, indicating no significant difference (heterogeneity: I2=0%, p=0.93) (Fig. 13). Four articles were included in the analysis of Robinson’s criteria. With a total sample of 216 patients, 110 of 128 patients (85.94%) treated with SAC and 79 of 88 patients (89.77%) treated with ACP had satisfying functional outcome, indicating no significant difference (heterogeneity: I2=11%, p=0.47) (Fig. 14). Five articles were included in the analysis of JOA, with a total sample of 388 patients; the mean JOA for SAC (n=194) and ACP (n=194) was 14.37 and 14.33, respectively. Fig. 15 shows no significant difference in terms of JOA between the two procedures (heterogeneity: I2=0%; WMD, 0.08; 95% CI, −0.10 to 0.27; p=0.39). Eight articles were included in the analysis of NDI, with a total sample of 570 patients; the mean NDI for SAC (n=303) and ACP (n=267) was 13.12 and 14.74, respectively. Fig. 16 displays no significant difference in terms of NDI between the two procedures (heterogeneity: I2=42%; WMD, −0.2; 95% CI, −0.55 to 0.15; p=0.26).
For the hospital stay, three articles were included in the analysis, with a total sample of 278 patients; the mean hospital stay for SAC (n=154) and ACP (n=124) was 6.2 days and 6.17 days, respectively. Fig. 17 shows no significant difference in terms of hospital stay between the two procedures (heterogeneity: I2=0%; WMD, −0.09; 95% CI, −0.29 to 0.12; p=0.40).
For adjacent level disease, seven articles were included in the analysis, with a total sample of 516 patients; adjacent level disease was experienced in nine of 255 patients (3.53%) treated with SAC and 29 of 261 patients (11.11%) treated with ACP, indicating a significant difference, wherein SAC was more advantageous with less adjacent level disease (heterogeneity: I2=0%, p=0.002) (Fig. 18).
For pain assessment as measured using Visual Analog Scale (VAS), five articles were included in the analysis, with a total sample of 336 patients; the mean VAS for SAC (n=163) and ACP (n=173) was 2.45 and 2.48, respectively. Fig. 19 shows no significant difference in terms of VAS between the two procedures (heterogeneity: I2=89%; WMD, 0.08; 95% CI, −0.77 to 0.93; p=0.85).
For fusion rate, 12 articles were included in the analysis, with a total sample of 912 patients; fusion was experienced in 419 of 478 patients (87.66%) treated with SAC and 409 of 434 patients (94.24%) treated with ACP, indicating a significant difference, wherein the fusion rate was higher in patients treated with ACP (heterogeneity: I2=0%, p=0.02) (Fig. 20).
For fusion time, three articles were included in the analysis, with a total sample of 290 patients; the mean fusion time for SAC (n=147) and ACP (n=143) was 8 months and 6.88 months, respectively. Fig. 21 reveals no significant difference in terms of fusion time between the two procedures (heterogeneity: I2=87%; WMD, 1.01; 95% CI, −0.68 to 2.7; p=0.24).
The decision to choose between ACP and SAC for patients with CDD is yet to be conclusive until now because the number of literatures thoroughly describing their comparison remains limited. Realizing the need for a thorough analysis comparing the two procedures in terms of intraoperative factors, radiological outcome, and functional outcome and putting in as many qualified studies as possible, we aim to give a wider image for treatment choice in patients with CDD undergoing anterior cervical procedure.
The main limitation of the previous meta-analysis is the limited outcomes and number of studies included in the final quantitative analysis. A study by Yang et al. [7] in 2019 compared ACP and SAC in terms of JOA score, NDI score, fusion rate, dysphagia rate, and adjacent segment degeneration. Another meta-analysis by Zhao et al. [8] in 2020 described clinical and radiological outcomes of the two procedures, stating that SAC is superior to ACP in terms of lower complication rates and comparable clinical outcomes. However, they only included 1–4 articles in each forest plot, causing the possibility of analysis bias [8]. In studies by Yin et al. [3] in 2016 and Nambiar et al. [9] in 2017, a detailed analysis was performed for several outcomes. However, the number of studies included was low. This study included 14 articles with a more comprehensive analysis of outcomes, which should be considered to perform decision making in surgery.
The operation time for SAC was significantly shorter than that for ACP in our analysis. This might be caused by the simple nature of the SAC implant with its self-locking structure, contributing to fewer required steps for the insertion of the anchoring clips compared with the conventional cage with a plate. Furthermore, a shorter duration of surgery and less manipulation also contribute to less soft tissue damage caused by surgery as well as blood loss, which minimizes the risk of some complications [5,10,11]. However, this might differ from one operator to another because of familiarity. In general, it is commendable to use SAC in high-risk cervical cases with significant comorbidities. Our study found a mean difference of 12.84 mL between the two procedures. Despite being statistically significant, we believe that it is clinically not significant. Increased complications and morbidity in anterior cervical surgery were found to be related to >300 mL blood loss and >5 hours.
As one of the most common complications after ACP, the pathogenesis of dysphagia is not fully understood. Fountas et al. [12] in 2007 reported that patients undergoing three-level fusion had a higher incidence of dysphagia than those undergoing single or two-level fusion, stating that intraoperative soft tissue irritation may contribute to this condition, considering SAC requires less surgical extent than ACP [9]. Furthermore, the design of SAC allows the whole implantation of the cage into the intervertebral space, avoiding implant contact with soft tissue anteriorly [5]. A study by Zhou et al. [10] in 2018 stated that dysphagia may be a result of tracheal intubation, soft tissue retraction, esophageal injury, postoperative hematoma, and/or adhesions. Furthermore, it was revealed that patients undergoing SAC had a faster resolution of dysphagia than those undergoing ACP [5]. Although most dysphagia is transient and disappears within 3 months postoperatively, chronic dysphagia-related symptoms are expected to be 3%–21% [13]. In contrast, Perrini et al. [14] in 2017 suggested that plate application did not induce significant esophageal irritation, contributing only to two out of 22 patients undergoing the ACP method. Conventionally, it was believed that a higher level of pathology correlates with a higher risk of dysphagia because more tissue retraction is required. However, recent studies found no difference between upper (C3–C4) and lower cervical levels (C5 and below) (Bazaz et al. [15] in 2002), and another study found lower levels (C4 and below) to be a risk factor (Kalb et al. [16] in 2012). Cervical graft material is another risk factor identified, wherein Bazaz et al. [15] in 2002 described one patient with dysphagia due to a prominent fibular graft, needing revision. In addition, plate thickness as a risk factor for dysphagia remains controversial, wherein a study by Lee et al. [17] in 2005 proved that a thicker plate correlated with a higher dysphagia rate and a study by Kalb et al. [16] in 2012 failed to prove implant type and thickness as risk factors for dysphagia. To minimize esophageal damage and reduce the risk of dysphagia, some studies advise the avoidance of large graft utilization, a reduction of endotracheal tube cuff pressure, the use of methylprednisolone as local irrigation, and minimizing pharynx as well as esophagus retraction intraoperatively [18,19].
Conversely, dysphasia/hoarseness has not been well described in the existing literature; however, it is believed to be a result of soft tissue damage and extensive exposure during surgery [10]. In our meta-analysis, the dysphasia rate was found to be not significantly different between the two procedures. Furthermore, Lu et al. [5] in 2018 stated that this complication resolved after conservative treatment and appropriate nursing. However, because both complications might affect a patient’s quality of life, it should be well informed to patients preoperatively, especially in patients receiving ACP.
Cervical and segmental angles are some radiological parameters that are postoperatively measured following ACDF. Our study proved that ACP had higher cervical and segmental angles than SAC postoperatively; however, the loss of segmental angle did not significantly differ between the two procedures. Cervical alignment and its maintenance are essential factors in cervical surgery because misalignment might lead to degenerative changes. Although still controversial, some studies stated that anterior plate is more effective in maintaining cervical alignment, especially for multi-level pathology, resulting in less loss of disc height and cervical malalignment [20–22]. Furthermore, sagittal malalignment will alter stress distribution along with the device and adjacent segments, increasing the risk of fixation failure and adjacent segment disease and causing chronic postoperative axial pain, neurological deficit, and worsening final functional outcome [13].
Restoring and maintaining intervertebral height and cervical segmental and global alignment as well as minimizing the risk of subsidence are significant elements of a successful anterior cervical surgery. Intervertebral foramen enlargement as a result of intervertebral space height restoration leads to nerve root decompression and symptom relief. Cage subsidence disturbs this by decreasing the intervertebral foramen space and causing the loss of cervical alignment [11]. Nemoto et al. [23] in 2015 defined subsidence as a decrease in total intervertebral disc height of >2 mm. In contrast, Lee et al. [24] in 2013 and Kim et al. [25] in 2013 set 3 mm as the cutoff point for subsidence. Our meta-analysis proved that SAC had a higher subsidence rate than ACP, possibly due to the better supportability of plate fixation in maintaining anterior disc height. The biomechanical properties of the cervical plate may contribute to this phenomenon, wherein axial loading is more evenly dispersed in the cervical plating group, whereas SACs may sink into vertebral bodies. Acting as a load-sharing device, the cervical plate reduces peak contact pressure; therefore, it is considered more stable in maintaining segmental height [14,24,26].
Furthermore, although subsidence may influence the segmental angle, the global cervical angle is not frequently affected because a single-level kyphosis can be compensated by the other cervical joints. This is supported by the study of Lee et al. [24] in 2013, stating that cervical plate application may preserve segmental angle, although not global cervical angle. However, this statement remains controversial, with some other literature stating that the significantly higher subsidence rate observed in SAC is inevitably correlated with the loss of cervical alignment, globally and segmentally, as well as the development of adjacent segment degeneration. To minimize these possible complications, well-understood surgical techniques in preserving bony endplate, appropriate selection of cage size and position, contact–surface ratio, and avoidance of over distraction should frequently be kept in mind, although patients’ bone density may also contribute to this condition [5,23].
Adjacent segment degeneration is defined as disc space narrowing, with or without osteophytes, ligament calcification, disc height loss of >30%, intervertebral herniation, and segmental instability. Although the results vary in diverse literature, our meta-analysis proved that ACP results in a higher incidence of adjacent level disease. Cervical fusion reduces segmental motion and further increases stress on adjacent discs below and above it, resulting in disc degeneration. In plate fixation, the higher fused segment rigidity is believed to cause greater stress in neighboring levels during motion postoperatively, thereby increasing the risk of adjacent segment degeneration [27,28]. In SAC, the device can be completely contained in the intervertebral space, contributing to less irritation to anterior cervical structures. However, to date, there has not been any literature sufficient enough to prove that adjacent level disease contributes to the clinical outcome of patients undergoing ACDF [29].
In terms of functional outcome, Odom and Robinson’s criteria have been used in some literature as an assessment method in patients with CDD undergoing cervical procedures. They both categorize functional outcomes as poor, fair, good, and excellent [30–32]. Although both ACDF methods could improve the clinical outcome in patients with CDD, our study failed to prove a significant difference between ACP and SAC in terms of postoperative functional outcomes as measured by these criteria. This symptom improvement is the result of compressive material removal and affected nerve decompression [10].
The use of JOA, NDI, and VAS is another means of assessing functional outcomes. A study by Zhang et al. [33] in 2018 stated that a lower preoperative JOA score is a risk factor for unsatisfactory outcome after ACDF and a higher occupying ratio contributed to this, wherein a patient with a higher occupying ratio had a higher risk for experiencing reperfusion postoperatively, causing permanent neurologic impairment and poor clinical outcome. Theologou et al. [34] in 2020 further suggested the assessment of possible coexistence of lumbar pathologies, wherein this condition may mask the symptoms of CDD, thereby resulting in a bias of clinical outcome. However, it has been said in various literature that the relationship between radiological and clinical outcomes is not yet established [26].
Bony fusion has been the aim of ACDF, and our study proved that ACP results in a significantly higher fusion rate than SAC. Joo et al. [27] in 2010 stated that this might be the result of an insufficient fixation power of cage alone, causing a higher rate of subsidence and pseudoarthrosis in the SAC group. This lack of stable fixation further causes constant micromotions and prevents bone fusion [27]. However, some studies proved that the addition of an anterior plate may enhance stability and rigidity, although not the fusion rate and time [24]. The study of Zhou et al. [10] in 2018 further stated that SAC has comparable stability, fusion rate, and fusion time with ACP, due to its unique structure that fits the adjacent vertebral body and endplate.
The current systematic review and meta-analysis suggest that ACP and SAC are comparable in terms of dysphasia rate, loss of segmental angle, loss of disc height, the Odom criteria, Robinson’s criteria, hospital stay, JOA, NDI, VAS, and fusion time. However, SAC is superior in terms of the shorter operation time, less blood loss, lower dysphagia rate, and a lower rate of adjacent level disease, whereas ACP is advantageous in terms of lower subsidence rate, better maintenance of the cervical global and segmental angles and disc height, and higher fusion rate. Therefore, both procedures are comparable in terms of functional outcomes, but SAC is superior in terms of less complication rate, and ACP is superior in terms of radiological outcomes.
This study has several limitations. First, most of the studies included are of level III evidence. Second, the heterogeneity of some analyses is high (>50%). Third, because of the scarcity of studies, all single and multi-level pathologies were included in the analysis, which may cause bias in the overall analysis. However, to our knowledge, this study is the first to formulate a thorough systematic review and meta-analysis on this matter from as many aspects as possible preoperatively, intraoperatively, and postoperatively, wherein we succeeded to cover as much as 20 aspects out of 14 included studies, both from clinical and radiological points of view. It is anticipated that this study might be beneficial as a guideline in choosing the appropriate method of treatment for patients with CDD, with the consideration of each patient’s characteristics, comorbidities, and pathology, and further inspire other researchers to conduct well-designed trials with a bigger number of samples and perform subgroup analysis.
Both procedures can be used in patients with CDD, although it might be more beneficial to choose ACP in patients with multi-level pathologies, wherein better mechanical stability is provided. However, in patients with comorbidities, anemia, or swelling problems, SAC may be more beneficial to use because it offers lower complication rates.
Fig. 2
Forest plot analysis for operation time. SD, standard deviation; IV, intravenous; CI, confidence interval; df, degrees of freedom.

Fig. 3
Forest plot analysis for blood loss. SD, standard deviation; IV, intravenous; CI, confidence interval; df, degrees of freedom.

Fig. 4
Forest plot analysis for dysphasia. M-H, Mantel-Haenszel; CI, confidence interval; df, degrees of freedom.
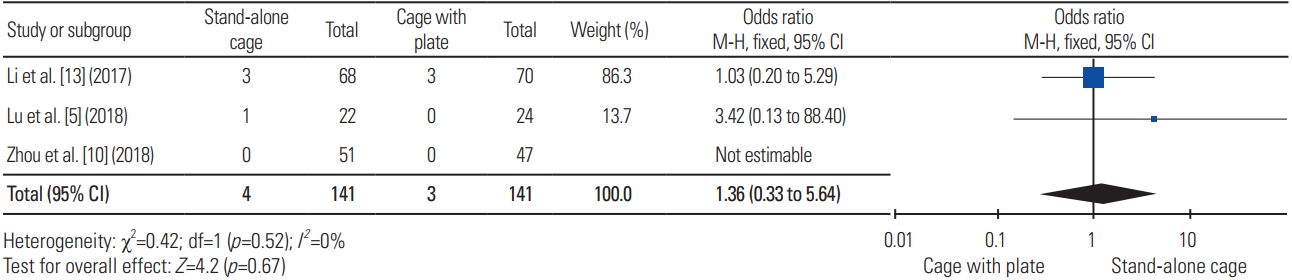
Fig. 5
Forest plot analysis for dysphagia. M-H, Mantel-Haenszel; CI, confidence interval; df, degrees of freedom.
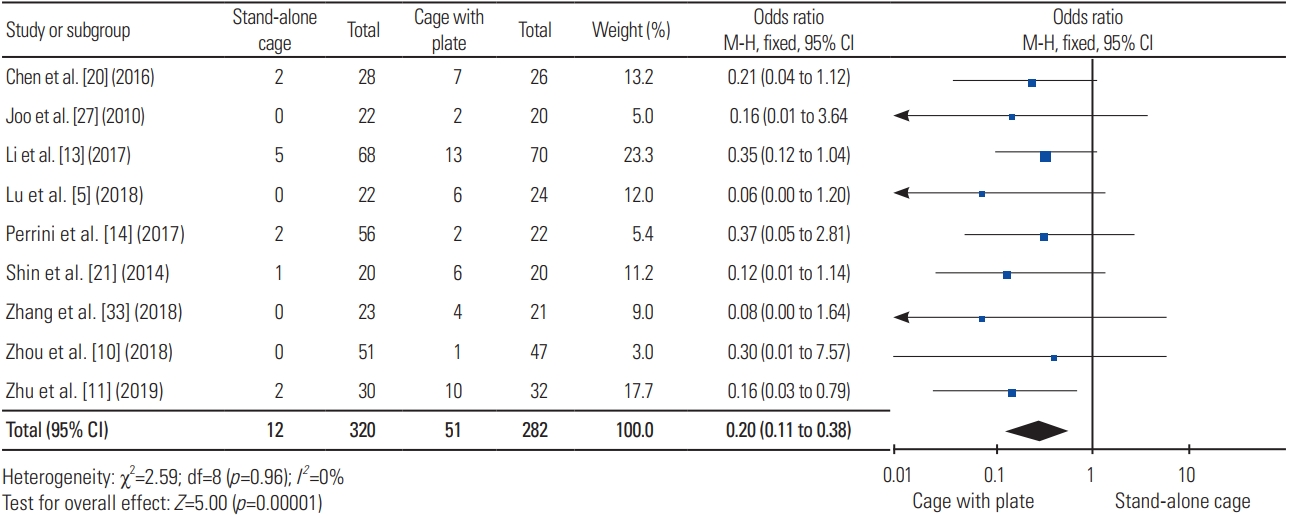
Fig. 6
Forest plot analysis for cervical angle. SD, standard deviation; IV, intravenous; CI, confidence interval; df, degrees of freedom.

Fig. 7
Forest plot analysis for loss of cervical lordosis. SD, standard deviation; IV, intravenous; CI, confidence interval; df, degrees of freedom.
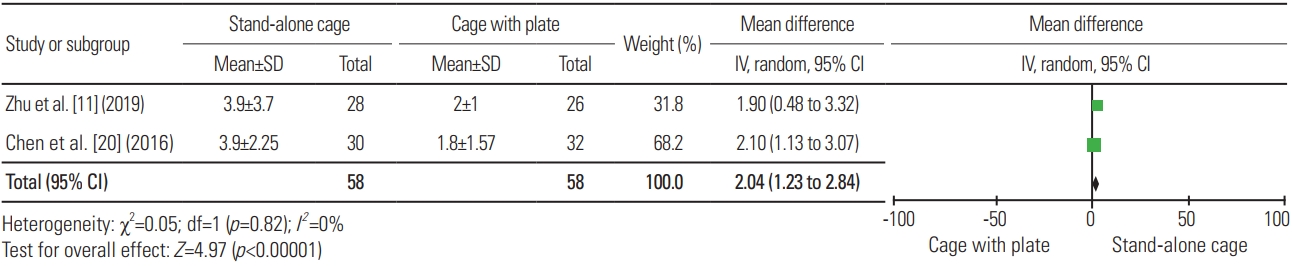
Fig. 8
Forest plot analysis for segmental angle. SD, standard deviation; IV, intravenous; CI, confidence interval; df, degrees of freedom.
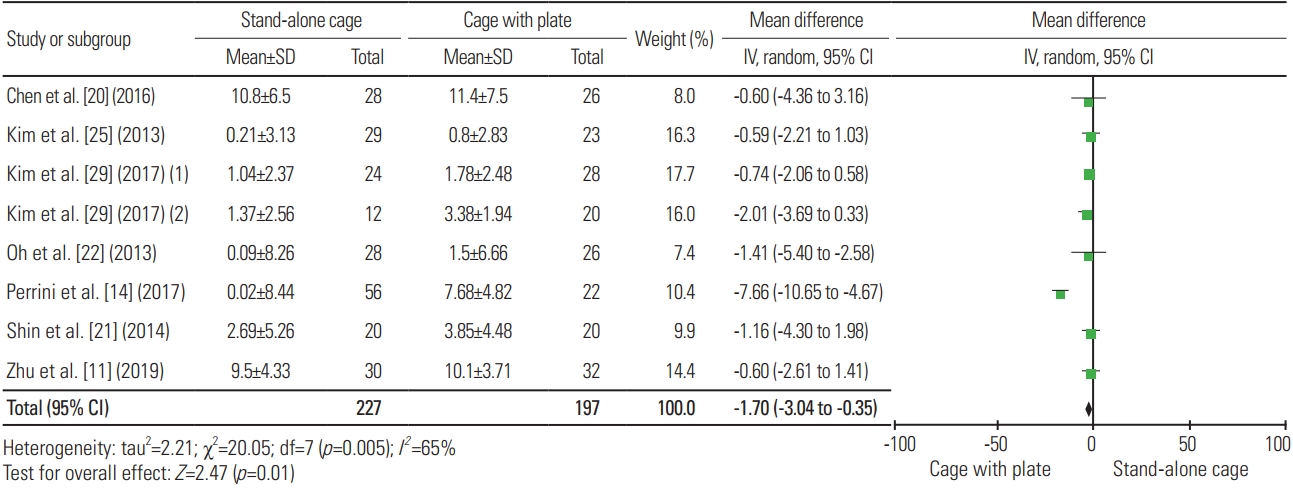
Fig. 9
Forest plot analysis for loss of segmental angle. SD, standard deviation; IV, intravenous; CI, confidence interval; df, degrees of freedom.
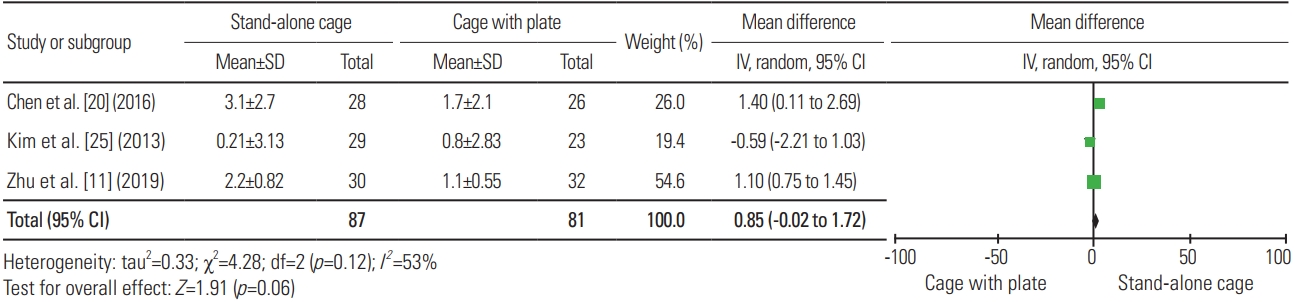
Fig. 10
Forest plot analysis for disc height. SD, standard deviation; IV, intravenous; CI, confidence interval; df, degrees of freedom.

Fig. 11
Forest plot analysis for loss of disc height. SD, standard deviation; IV, intravenous; CI, confidence interval; df, degrees of freedom.
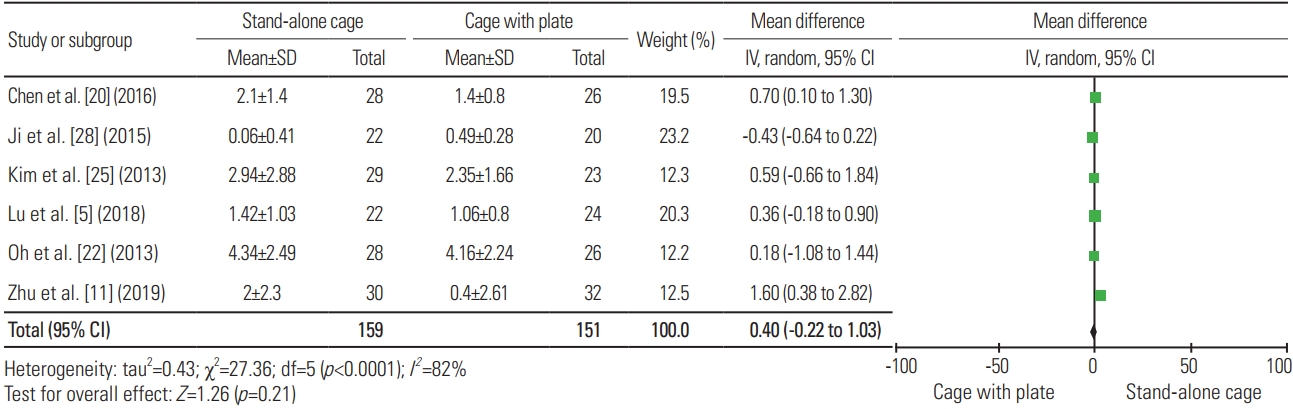
Fig. 12
Forest plot analysis for subsidence rate. M-H, Mantel-Haenszel; CI, confidence interval; df, degrees of freedom.

Fig. 13
Forest plot analysis for odom’s criteria. M-H, Mantel-Haenszel; CI, confidence interval; df, degrees of freedom.

Fig. 14
Forest plot analysis for robinson’s criteria. M-H, Mantel-Haenszel; CI, confidence interval; df, degrees of freedom.
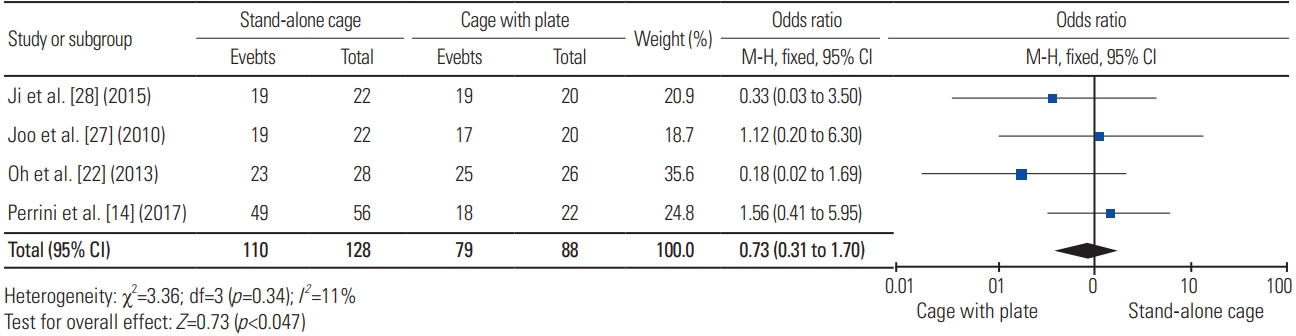
Fig. 15
Forest plot analysis for the Japanese Orthopaedic Association score. SD, standard deviation; IV, intravenous; CI, confidence interval; df, degrees of freedom.
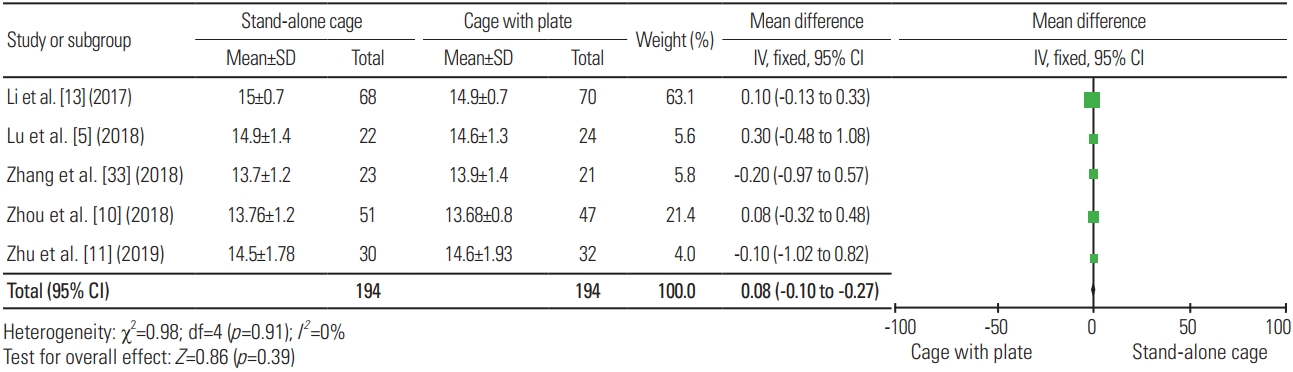
Fig. 16
Forest plot analysis for Neck Disability Index. SD, standard deviation; IV, intravenous; CI, confidence interval; df, degrees of freedom.
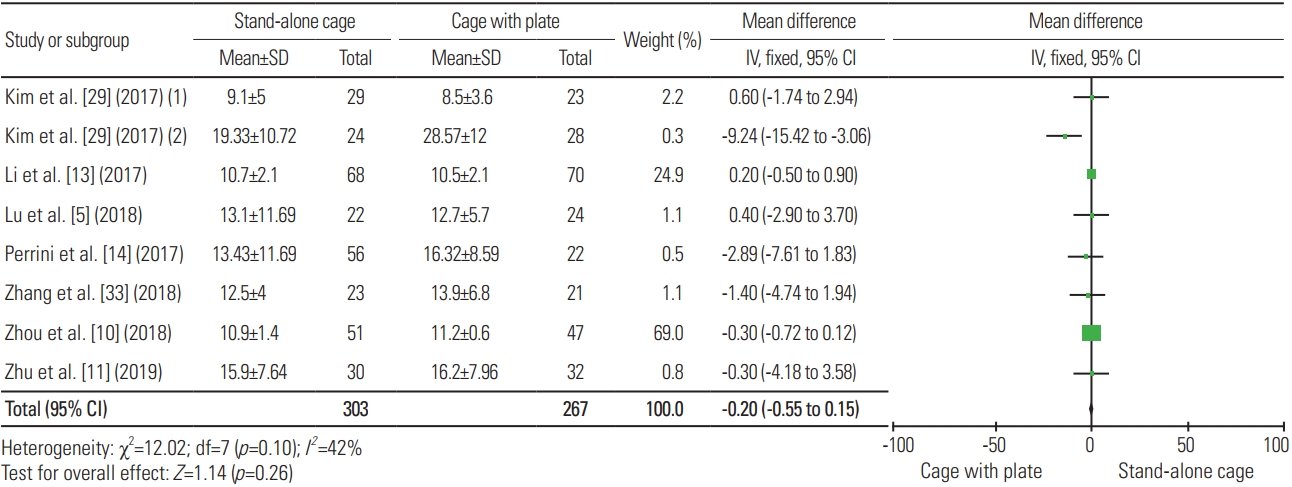
Fig. 17
Forest plot analysis for hospital stay. SD, standard deviation; IV, intravenous; CI, confidence interval; df, degrees of freedom.
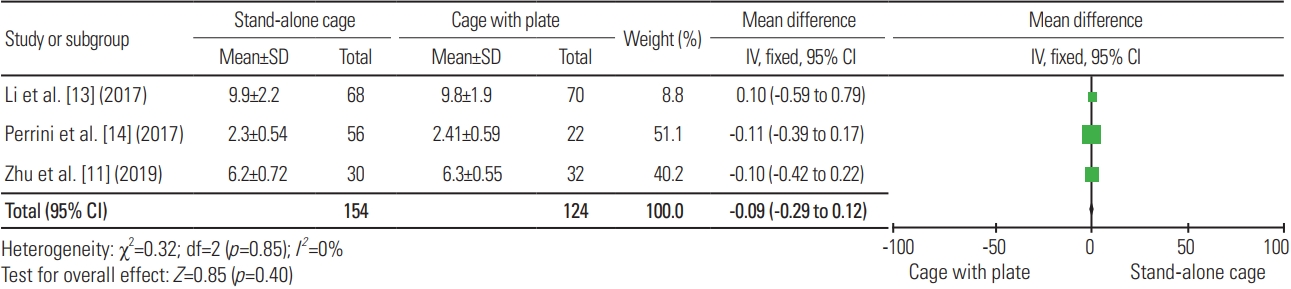
Fig. 18
Forest plot analysis for adjacent level disease. M-H, Mantel-Haenszel; CI, confidence interval; df, degrees of freedom.
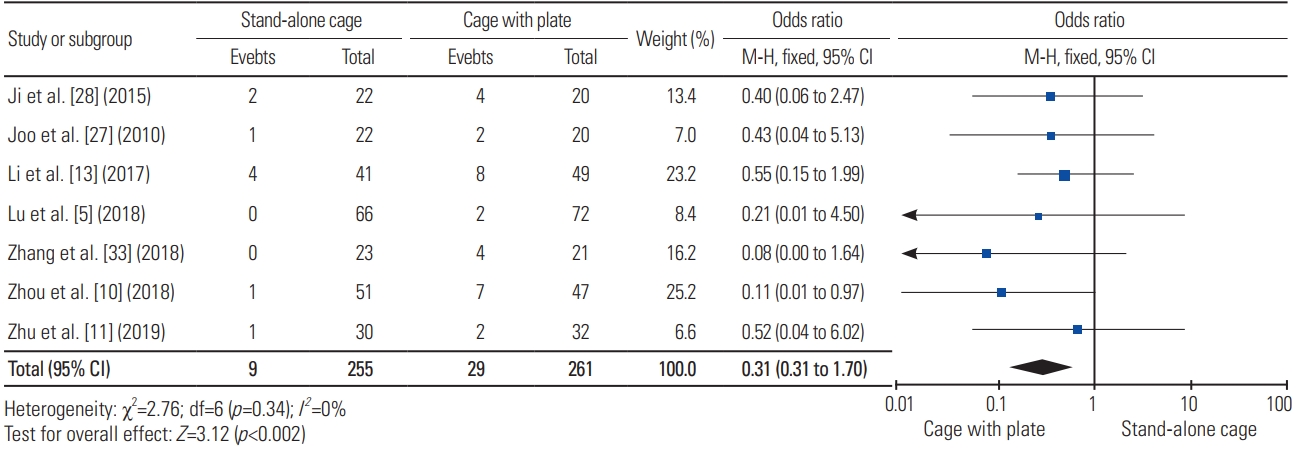
Fig. 19
Forest plot analysis for Visual Analog Scale. SD, standard deviation; IV, intravenous; CI, confidence interval; df, degrees of freedom.
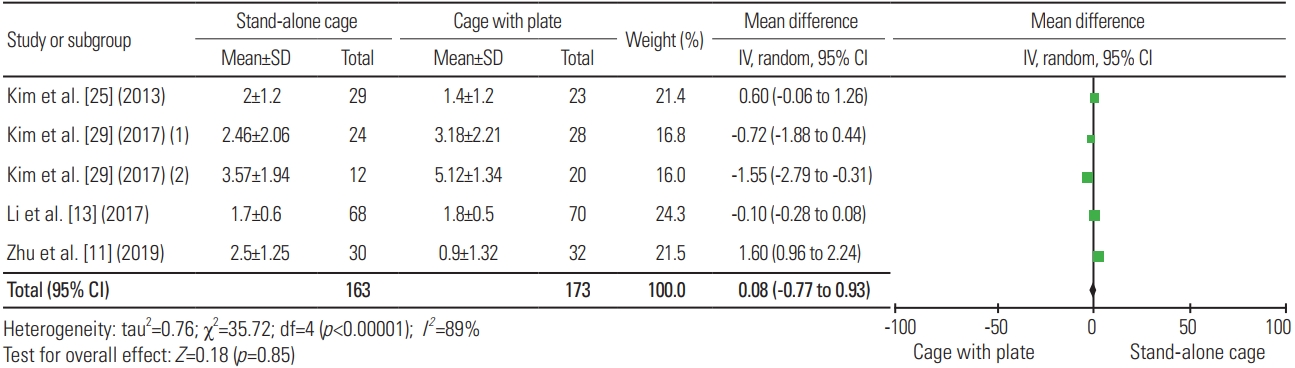
Fig. 20
Forest plot analysis for fusion rate. M-H, Mantel-Haenszel; CI, confidence interval; df, degrees of freedom.
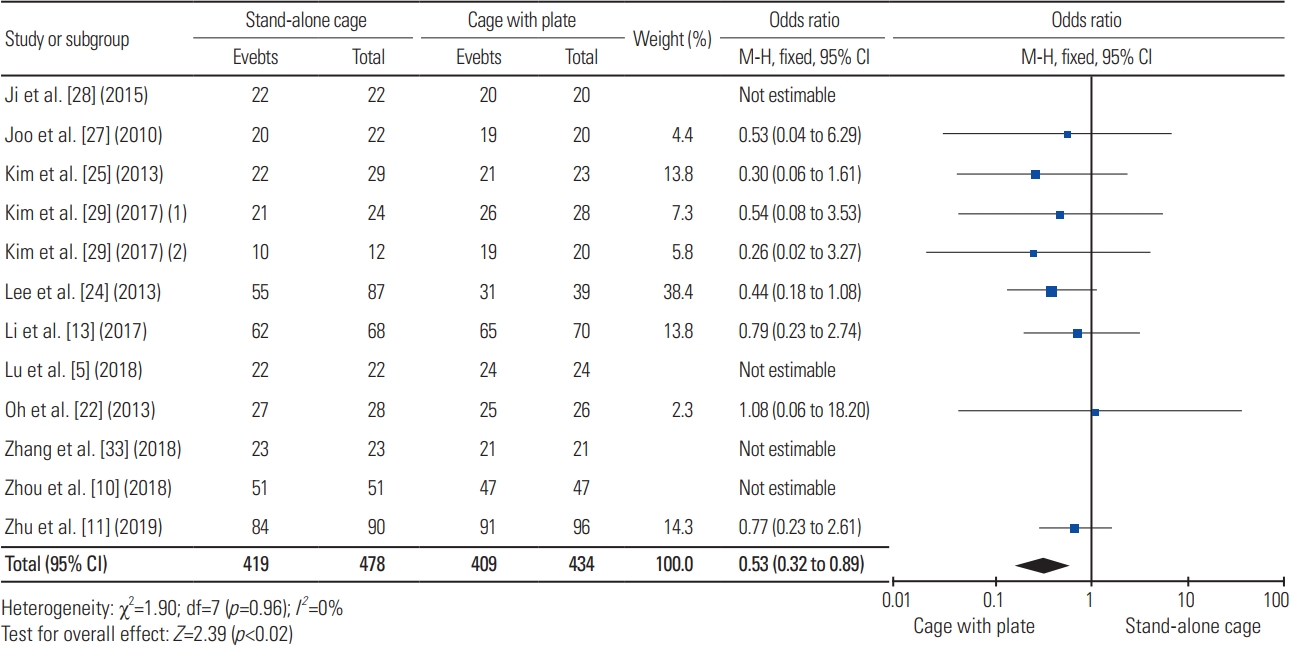
Fig. 21
Forest plot analysis for fusion time. SD, standard deviation; IV, intravenous; CI, confidence interval; df, degrees of freedom.
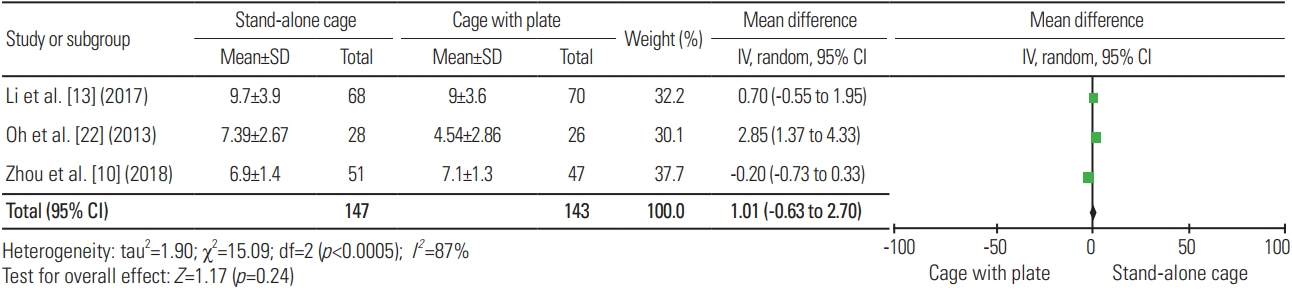
Table 1
Population-Intervention-Comparison-Outcome table describing inclusion and exclusion criteria
Table 2
Studies included in the analysis
| Reference | Journal | Study design | Level of evidence |
|---|---|---|---|
| Chen et al. [20] (2016) | Eur Spine J | Cohort retrospective | Level III |
| Ji et al. [28] (2015) | J Spinal Disord Tech | Cohort retrospective | Level III |
| Joo et al. [27] (2010) | J Korean Neurosurg Soc | Cohort retrospective | Level III |
| Kim et al. [25] (2013) | Neurosurgery | Cohort prospective | Level II |
| Kim et al. [29] (2017) | J Korean Neurosurg Soc | Cohort prospective | Level II |
| Lee et al. [24] (2013) | J Spinal Disord Tech | Cohort retrospective | Level III |
| Li et al. [13] (2017) | Eur Spine J | Cohort retrospective | Level III |
| Lu et al. [5] (2018) | Medicine (Baltimore) | Cohort retrospective | Level III |
| Oh et al. [22] (2013) | J Spinal Disord Tech | Cohort retrospective | Level III |
| Perrini et al. [14] (2017) | Clin Neurol Neurosurg | Cohort retrospective | Level III |
| Shin et al. [21] (2014) | Korean J Spine | Cohort retrospective | Level III |
| Zhang et al. [33] (2018) | BMC Musculoskelet Disord | Cohort retrospective | Level III |
| Zhou et al. [10] (2018) | Clin Neurol Neurosurg | Cohort retrospective | Level III |
| Zhu et al. [11] (2019) | Med Sci Monit | Cohort retrospective | Level III |
Table 3
Sample characteristics of included studies
| Reference | Sample size | Age (yr) | Sex | Procedure | Level | Follow-up (mo) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| SAC | ACP | SAC | ACP | SAC | ACP | SAC | ACP | SAC | ACP | ||
| Chen et al. [20] (2016) | 54 | 54.1±8.8 | 54.7±12.1 | M=18 (64.29%); F=10 (35.71%) | M=15 (57.69%); F=11 (42.31%) | 28 | 26 | 3 levels: C3–C6=17; C4–C7=11 | 3 levels: C3–C6=14; C4–C7=12 | 28.8±9.7 | 29.6±8.3 |
|
|
|||||||||||
| Ji et al. [28] (2015) | 42 | 57.7 (49–72) | 56.1 (44–68) | M=10 (45.45%); F=12 (54.55%) | M=14 (70%); F=6 (30%) | 22 | 20 | 2 levels: C3–C5=2; C4–C6=9; C5–C7=11 | 2 levels: C3–C5=1; C4–C6=9; C5–C7=10 | 26.9 (24–50) | 27.8 (24–48) |
|
|
|||||||||||
| Joo et al. [27] (2010) | 42 | 59.09 (38–75) | 54.33 (33–73) | M=15 (68.18%); F=7 (31.82%) | M=13 (65%); F=7 (35%) | 22 | 20 | 2 levels: C3–C5=1; C4–C6=7; C5–C7=14 | 2 levels: C3–C5=2; C4–C6=6; C5–C7=12 | 15.7 (6–30) | 16.2 (6–47) |
|
|
|||||||||||
| Kim et al. [14] (2013) | 52 | 49.48±12.75 | 55.17±12.40 | M=17 (58.62%); F=12 (41.38%) | M=14 (60.87%); F=9 (39.13%) | 29 | 23 | 1 level: C3–C4=4; C4–C5=3; C5–C6=19; C6–C7=3 | 1 level: C3–C4=1; C4–C5=6; C5–C6=14; C6–C7=2 | 12 | |
|
|
|||||||||||
| Kim et al. [29] (2017) | 84 | 1 level: 52.5±10.3 (36–73); 2 levels: 60.7±8.6 (50–72) | 1 level: 50.4±12.1 (29–75); 2 levels: 53.1±12.5 (44–74) | M=25 (69.44%); F=11 (30.56%) | M=30 (62.5%); F=18 (37.5%) | 36 | 48 |
1 level: C3–C4=4; C4–C5=2; C5–C6=9; C6–C7=9; C7–T1=0 2 levels: C3–C5=2; C4–C6=2; C5–C7=8 |
1 level: C3–C4=2; C4–C5=5; C5–C6=12; C6–C7=8; C7–T1=1; 2 levels: C3–C5=4; C4–C6=10; C5–C7=6 | 24 | |
|
|
|||||||||||
| Lee et al. [24] (2013) | 126 | 52.3 (24–82) | M=72 (56.96%); F=54 (43.04%) | 87 | 39 | 1 level: C5–C6=60; C6–C7=34; C4–C5=24; C3–C4=8 | 12 | ||||
|
|
|||||||||||
| Li et al. [13] (2017) | 138 | 50.6±7.5 | 51.3±7.9 | M=41 (60.29%); F=27 (39.71%) | M=45 (64.29%); F=25 (35.71%) | 68 | 70 | 1 level: 32; 2 levels: 21; 3 levels: 11; 3 levels: 4 | 1 level: 34; 2 levels: 23; 3 levels: 10; 4 levels: 3 | 29.7±6.5 | 30.8±6.6 |
|
|
|||||||||||
| Lu et al. [5] (2018) | 46 | 56.6±6.4 | 58.6±7.2 | M=13 (59.09%); F=9 (40.91%) | M=15 (62.5%); F=9 (37.5%) | 22 | 24 | 2 levels: C3–C4 + C5–C6=15; C4–C5 + C6–C7=7 | 2 levels: C3–C4 + C5–C6=19; C4–C5 + C6–C7=5 | 30.5±5.2 | 32.1±6.5 |
|
|
|||||||||||
| Oh et al. [22] (2013) | 54 | 57.9 (43–72) | 54.3 (31–71) | M= 13 (46.43%); F=15 (53.57%) | M=20 (76.92%); F=6 (23.08%) | 28 | 26 | 2 levels: C3–C5=2; C4–C6=15; C5–C7=11 | 2 levels: C3–C5=3; C4–C6=9; C5–C7=14 | 23.4 (12–46) | 20.6 (12–36) |
|
|
|||||||||||
| Perrini et al. [14] (2017) | 78 | 51.07±10.40 | 55.56±7.76 | M=31 (55.36%); F=25 (44.64%) | M=11 (50%); F=11 (50%) | 56 | 22 | 2 levels: C3–C5=4; C4–C6=15; C5–C7=37 | 2 levels: C3–C5=3; C4–C6=12; C5–C7=7 | 12 | |
|
|
|||||||||||
| Shin et al. [21] (2014) | 40 | 49.0±11.0 | 44.3±9.7 | M=12 (60%); F=8 (40%) | M=13 (65%); F=7 (35%) | 20 | 20 | 1 level: C3–C4=2; C4–C5=3; C5–C6=8; C6–C7=7 | 1 level: C3–C4=4; C4–C5=3; C5–C6=8; C6–C7=5 | 13.1±1.2 | 13.7±1.1 |
|
|
|||||||||||
| Zhang et al. [33] (2018) | 44 | 53.3±8.8 (40–68) | 57.8±9.2 (39–70) | M=15 (65.22%); F=8 (34.78%) | M=9 (42.86%); F=12 (57.14%) | 23 | 21 | 2 levels: C3–C4 + C5–C6=13; C4–C5 + C6–C7=10 | 2 levels: C3–C4 + C5–C6=8; C4–C5 + C6–C7=13 | 34.7±7.6 (24–48) | 36.2±5.2 (26–48) |
|
|
|||||||||||
| Zhou et al. [10] (2018) | 98 | 62.3±6.7 (40–75) | 64.4±3.2 (38–70) | M=23 (45.1%); F=28 (54.9%) | M=22 (46.8%); F=25 (53.2%) | 51 | 47 | 1 level: 20; 2 levels: 18; 3 levels: 13 (C3–C4=18; C4–C5=23; C5–C6=29; C6–C7=25) | 1 level: 21; 2 levels: 14; 3 levels: 12 (C3–C4=16; C4–C5=20; C5–C6=27; C6–C7=22) | 39.7±3.2 (37–48) | 42.2±4.1 (39–50) |
|
|
|||||||||||
| Zhu et al. [11] (2019) | 62 | 56.6±12.6 | 55.3±13.1 | M=16 (53.33%); F=14 (46.67%) | M=18 (56.25%); F=14 (43.75%) | 30 | 32 | 3 levels: C3–C6=19; C4–C7=11 | 3 levels: C3–C6=17; C4–C7=15 | >36 | |
Table 4
Outcomes discussed in included studies (1)
| Reference | Operation time (min) | Blood loss (mL) | Dysphagia | Cervical angle (°) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| SAC | ACP | SAC | SAC | ACP | ACP | SAC | ACP | |
| Chen et al. [20] (2016) | 113.7±31.5 | 138.2±39.1 | 159.9±38.1 | 16.0±6.7 (24-mo postop) | 19.1±7.9 (24-mo postop) | 187.4±42.8 | 2 (7.14%) | 7 (26.92%) |
|
|
||||||||
| Ji et al. [28] (2015) | NA | NA | NA | NA | NA | NA | NA | NA |
|
|
||||||||
| Joo et al. [27] (2010) | NA | NA | NA | −4.71 (−9 to −2) | −4.63 (−8 to −2) | NA | 0 | 2 (10%) |
|
|
||||||||
| Kim et al. [25] (2013) | NA | NA | NA | NA | NA | NA | NA | NA |
|
|
||||||||
| Kim et al. [29] (2017) | NA | NA | NA | 1 level: 11.70±10.31; 2 level: 8.53±7.78 | 1 level: 11.16±9.55; 2 levels: 10.78±7.93 | NA | NA | NA |
|
|
||||||||
| Lee et al. [24] (2013) | NA | NA | NA | −13.05 (12 mo) | −14.78 (12 mo) | NA | NA | NA |
|
|
||||||||
| Li et al. [13] (2017) | 73.2±22.3 | 81.2±19.5 | 54.6±33.3 | 14.01±1.47 | 14.49±1.70 | 75.7±46.8 | 5 (7.4%) | 13 (18.6%) |
|
|
||||||||
| Lu et al. [5] (2018) | 121.1±14.7 | 154.4±12.3 | 79.1±12.0 | 15.9±3.7 (24 mo) | 16.3±3.9 (24 mo) | 86.3±15.8 | 0 | 6 (25%) |
|
|
||||||||
| Oh et al. [22] (2013) | NA | NA | NA | 8.74±11.19 | 9.61±8.91 | NA | NA | NA |
|
|
||||||||
| Perrini et al. [14] (2017) | 146.34±46.10 | 154.55±40.59 | NA | 154.55±40.59 (12 mo) | −15.64±8.06 (12 mo) | NA | 2 | 2 |
|
|
||||||||
| Shin et al. [21] (2014) | NA | NA | NA | 11.32±6.91 | 14.73±9.22 | NA | 1 (5%) | 6 (30%) |
|
|
||||||||
| Zhang et al. [33] (2018) | 126.0±13.2 | 143.4±17.9 | 70.0±15.8 | 18.7±8.6 | 17.7±8.3 | 75.4±23.0 | 0 | 4 |
|
|
||||||||
| Zhou et al. [10] (2018) | 1 level: 69.3±9.6; 2 levels: 117.2±12.3; 3 levels: 138.5±7.9 | 1 level: 83.7±7.7; 2 levels: 138.5±14.1; 3 levels: 152.6±12.4 | 1 level: 24.6±2.2; 2 levels: 39.6±1.4; 3 levels: 56.6±1.9 | No significant difference | - | 1 level: 34.2±2.3; 2 levels: 53.5±1.4; 3 levels: 82.5±3.4 | 0 | 1 (2.13%) |
|
|
||||||||
| Zhu et al. [11] (2019) | 100.8±24.22 | 130±18.13 | 29.6±9.82 | 15.5±5.93 | 20.4±7.32 | 30.5±11.63 | 2 (6.7%) | 10 (31.2%) |
Table 5
Outcomes discussed in included studies (2)
| Reference | Segmental angle (°) | Disc height (mm) | Subsidence rate | Odom’s criteria | Robinson’s criteria | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| SAC | SAC | ACP | ACP | SAC | ACP | SAC | ACP | SAC | ACP | |
| Chen et al. [20] (2016) | 10.8±6.5 (24-mo postop) | 6.5±0.8 (24-mo postop) | 6.7±1.0 (24-mo postop) | 11.4±7.5 (24-mo postop) | 14/84 (16.67%) (24-mo postop) | 5/78 (6.41%) (24-mo postop) | Satisfactory: 28 (100%); not satisfactory: 0 | Satisfactory: 26 (100%); not satisfactory: 0 | NA | NA |
|
|
||||||||||
| Ji et al. [28] (2015) | NA | 5.83±0.71 (1 level lower fusion segment, 24-mo postop) | 5.65±0.61 (1 level lower fusion segment, 24-mo postop) | NA | NA | NA | NA | NA | Satisfactory: 19 (86%); not satisfactory: 3 (14%) | Satisfactory: 19 (95%); not satisfactory: 1 (5%) |
|
|
||||||||||
| Joo et al. [27] (2010) | −4.36 (−7 to −1) | NA | NA | −4.20 (−6 to −1) | 7 (31.81%) | 6 (30%) | NA | NA | Satisfactory: 19 (86.36%); not satisfactory: 3 (13.64%) | Satisfactory: 17 (85%); not satisfactory: 3 (15%) |
|
|
||||||||||
| Kim et al. [25] (2013) | 0.21±3.13 (12 mo) | NA | NA | 0.8±2.83 (12 mo) | Anterior disc space=10 (34%); posterior disc space=7 (24%) | Anterior disc space=7 (30%); posterior disc space=6 (26%) | NA | NA | NA | NA |
|
|
||||||||||
| Kim et al. [29] (2017) | 1 level: 1.04±2.37; 2 levels: 1.37±2.56 | Compared to lower disc: 1 level: 6.45±1.43; 2 levels: 7.18±0.63; compared to upper disc: 1 level: 6.41±0.85; 2 levels: 6.65±0.9 | Compared to lower disc: 1 level: 6.83±1.17; 2 levels: 6.84±0.90; compared to upper disc: 1 level: 6.58±1.06; 2 levels: 6.66±1.17 | 1 level: 1.78±2.48; 2 levels: 3.38±1.94 | 1 level: 11 (45.8%); 2 levels: 8 (66.6%) | 1 level: 9 (32.1%); 2 levels: 6 (30%) | NA | NA | NA | NA |
|
|
||||||||||
| Lee et al. [24] (2013) | −3.10 (12 mo) | NA | NA | −5.71 (12 mo) | 51 (58.62%) | 15 (38.46%) | NA | NA | NA | NA |
|
|
||||||||||
| Li et al. [13] (2017) | NA | 6.63±0.79 | 7.09±1.02 | NA | 12 (17.65%) | 9 (12.86%) | NA | NA | NA | NA |
|
|
||||||||||
| Lu et al. [5] (2018) | NA | 6.4±1.0 (24 mo) | 6.9±0.9 (24 mo) | NA | 2 (4.5%) | 0 (0%) | NA | NA | NA | NA |
|
|
||||||||||
| Oh et al. [22] (2013) | 0.09±8.26 | 49.81±4.20 | 52.95±3.45 | 1.50±6.66 | 10 (35.71%) | 3 (11.54%) | NA | NA | Satisfactory: 23 (82.14%); not satisfactory: 5 (17.86%) | Satisfactory: 25 (96.15%); not satisfactory: 1 (3.85%) |
|
|
||||||||||
| Perrini et al. [14] (2017) | −0.02±8.44 (12 mo) | 36.75±3.90 (12 mo) | 39.51±3.50 (12 mo) | −7.68±4.82 (12 mo) | 40 (71.42%) | 2 (13.67%) | NA | NA | Satisfactory: 49 (87.5%); not satisfactory: 7 (12.5%) | Satisfactory: 18 (81.82%); not satisfactory: 4 (18.18%) |
|
|
||||||||||
| Shin et al. [21] (2014) | −2.69±5.26 | 32.40±3.47 | 34.71±3.88 | 3.85±4.87 | 14 (70%) | 9 (45%) | Satisfactory: 20 (100%); not satisfactory: 0 | Satisfactory: 20 (100%); not satisfactory: 0 | NA | NA |
|
|
||||||||||
| Zhang et al. [33] (2018) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
|
|
||||||||||
| Zhou et al. [10] (2018) | NA | NA | NA | NA | 4 (4.21%) | 3 (3.52%) | Satisfactory: 41 (80.39%); not satisfactory: 10 (19.61%) | Satisfactory: 38 (80.85%); not satisfactory: 9 (19.15%) | NA | NA |
|
|
||||||||||
| Zhu et al. [11] (2019) | 9.5±4.33 | 78.4±6.17 | 81.1±8.15 | 10.1±3.71 | 17/90 (18.8%) | 8/96 (8.3%) | Satisfactory: 27 (90%); not satisfactory: 3 (10%) | Satisfactory: 29 (90.6%); not satisfactory: 3 (9.4%) | NA | NA |
References
1. Cloward RB. The anterior approach for removal of ruptured cervical disks. J Neurosurg 1958;15:602–17.


2. Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am 1958;40-A:607–24.


3. Yin M, Ma J, Huang Q, et al. The new Zero-P implant can effectively reduce the risk of postoperative dysphagia and complications compared with the traditional anterior cage and plate: a systematic review and meta-analysis. BMC Musculoskelet Disord 2016;17:430.



4. El-Tantawy A. Is it possible to eliminate the plate-related problems and still achieve satisfactory outcome after multilevel anterior cervical discectomy? Eur J Orthop Surg Traumatol 2015;25(Suppl 1): S135–45.


5. Lu Y, Bao W, Wang Z, et al. Comparison of the clinical effects of zero-profile anchored spacer (ROI-C) and conventional cage-plate construct for the treatment of noncontiguous bilevel of cervical degenerative disc disease (CDDD): a minimum 2-year follow-up. Medicine (Baltimore) 2018;97:e9808.



6. Sun Z, Liu Z, Hu W, Yang Y, Xiao X, Wang X. Zero-profile versus cage and plate in anterior cervical discectomy and fusion with a minimum 2 years of follow-up: a meta-analysis. World Neurosurg 2018;120:e551–61.


7. Yang Z, Zhao Y, Luo J. Incidence of dysphagia of zero-profile spacer versus cage-plate after anterior cervical discectomy and fusion: a meta-analysis. Medicine (Baltimore) 2019;98:e15767.



8. Zhao Y, Yang S, Huo Y, Li Z, Yang D, Ding W. Locking stand-alone cage versus anterior plate construct in anterior cervical discectomy and fusion: a systematic review and meta-analysis based on randomized controlled trials. Eur Spine J 2020;29:2734–44.


9. Nambiar M, Phan K, Cunningham JE, Yang Y, Turner PL, Mobbs R. Locking stand-alone cages versus anterior plate constructs in single-level fusion for degenerative cervical disease: a systematic review and meta-analysis. Eur Spine J 2017;26:2258–66.


10. Zhou J, Li J, Lin H, Li X, Zhou X, Dong J. A comparison of a self-locking stand-alone cage and anterior cervical plate for ACDF: minimum 3-year assessment of radiographic and clinical outcomes. Clin Neurol Neurosurg 2018;170:73–8.


11. Zhu D, Zhang D, Liu B, Li C, Zhu J. Can self-locking cages offer the same clinical outcomes as anterior cage-with-plate fixation for 3-level anterior cervical discectomy and fusion (ACDF) in mid-term follow-up? Med Sci Monit 2019;25:547–57.



12. Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310–7.


13. Li Z, Zhao Y, Tang J, et al. A comparison of a new zero-profile, stand-alone Fidji cervical cage and anterior cervical plate for single and multilevel ACDF: a minimum 2-year follow-up study. Eur Spine J 2017;26:1129–39.


14. Perrini P, Cagnazzo F, Benedetto N, Morganti R, Gambacciani C. Cage with anterior plating is advantageous over the stand-alone cage for segmental lordosis in the treatment of two-level cervical degenerative spondylopathy: a retrospective study. Clin Neurol Neurosurg 2017;163:27–32.


15. Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine (Phila Pa 1976) 2002;27:2453–8.

16. Kalb S, Reis MT, Cowperthwaite MC, et al. Dysphagia after anterior cervical spine surgery: incidence and risk factors. World Neurosurg 2012;77:183–7.


17. Lee MJ, Bazaz R, Furey CG, Yoo J. Influence of anterior cervical plate design on dysphagia: a 2-year prospective longitudinal follow-up study. J Spinal Disord Tech 2005;18:406–9.

18. Grasso G, Leone L, Torregrossa F. Dysphagia prevention in anterior cervical discectomy surgery: results from a prospective clinical study. World Neurosurg 2019;125:e1176–82.


19. Patel NP, Wolcott WP, Johnson JP, et al. Esophageal injury associated with anterior cervical spine surgery. Surg Neurol 2008;69:20–4.


20. Chen Y, Lu G, Wang B, Li L, Kuang L. A comparison of anterior cervical discectomy and fusion (ACDF) using self-locking stand-alone polyetheretherketone (PEEK) cage with ACDF using cage and plate in the treatment of three-level cervical degenerative spondylopathy: a retrospective study with 2-year follow-up. Eur Spine J 2016;25:2255–62.


21. Shin JS, Oh SH, Cho PG. Surgical outcome of a zero-profile device comparing with stand-alone cage and anterior cervical plate with iliac bone graft in the anterior cervical discectomy and fusion. Korean J Spine 2014;11:169–77.



22. Oh JK, Kim TY, Lee HS, et al. Stand-alone cervical cages versus anterior cervical plate in 2-level cervical anterior interbody fusion patients: clinical outcomes and radiologic changes. J Spinal Disord Tech 2013;26:415–20.

23. Nemoto O, Kitada A, Naitou S, Tachibana A, Ito Y, Fujikawa A. Stand-alone anchored cage versus cage with plating for single-level anterior cervical discectomy and fusion: a prospective, randomized, controlled study with a 2-year follow-up. Eur J Orthop Surg Traumatol 2015;25(Suppl 1): S127–34.


24. Lee CH, Hyun SJ, Kim MJ, et al. Comparative analysis of 3 different construct systems for single-level anterior cervical discectomy and fusion: stand-alone cage, iliac graft plus plate augmentation, and cage plus plating. J Spinal Disord Tech 2013;26:112–8.

25. Kim CH, Chung CK, Hahn S. Autologous iliac bone graft with anterior plating is advantageous over the stand-alone cage for segmental lordosis in single-level cervical disc disease. Neurosurgery 2013;72:257–66.


26. Kim YS, Park JY, Moon BJ, Kim SD, Lee JK. Is stand alone PEEK cage the gold standard in multilevel anterior cervical discectomy and fusion (ACDF)?: results of a minimum 1-year follow up. J Clin Neurosci 2018;47:341–6.


27. Joo YH, Lee JW, Kwon KY, Rhee JJ, Lee HK. Comparison of fusion with cage alone and plate instrumentation in two-level cervical degenerative disease. J Korean Neurosurg Soc 2010;48:342–6.



28. Ji GY, Oh CH, Shin DA, et al. Stand-alone cervical cages versus anterior cervical plates in 2-level cervical anterior interbody fusion patients: analysis of adjacent segment degeneration. J Spinal Disord Tech 2015;28:E433–8.

29. Kim SY, Yoon SH, Kim D, Oh CH, Oh S. A prospective study with cage-only or cage-with-plate fixation in anterior cervical discectomy and interbody fusion of one and two levels. J Korean Neurosurg Soc 2017;60:691–700.



30. Song KJ, Taghavi CE, Lee KB, Song JH, Eun JP. The efficacy of plate construct augmentation versus cage alone in anterior cervical fusion. Spine (Phila Pa 1976) 2009;34:2886–92.


31. Riley LH Jr, Robinson RA, Johnson KA, Walker AE. The results of anterior interbody fusion of the cervical spine: review of ninety-three consecutive cases. J Neurosurg 1969;30:127–33.

32. Broekema AE, Molenberg R, Kuijlen JM, Groen RJ, Reneman MF, Soer R. The Odom criteria: validated at last: a clinimetric evaluation in cervical spine surgery. J Bone Joint Surg Am 2019;101:1301–8.