1. Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am 1993 75:1298–307.


2. Buttermann GR. Anterior Cervical discectomy and fusion outcomes over 10 years: a prospective study. Spine (Phila Pa 1976) 2018 43:207–14.

4. Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am 1958 40-A:607–24.


5. Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2003 28:134–9.


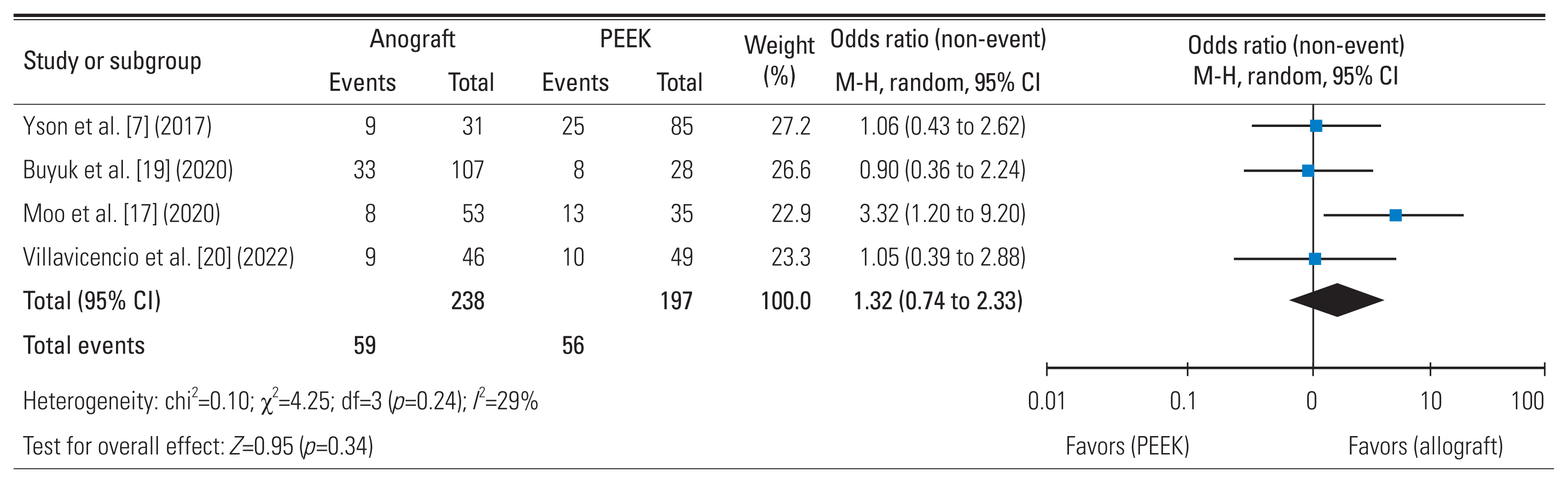
7. Yson SC, Sembrano JN, Santos ER. Comparison of allograft and polyetheretherketone (PEEK) cage subsidence rates in anterior cervical discectomy and fusion (ACDF). J Clin Neurosci 2017 38:118–21.


10. Fatima N, Massaad E, Shankar GM, Shin JH. Structural allograft versus polyetheretherketone implants in patients undergoing spinal fusion surgery: a systematic review and meta-analysis. World Neurosurg 2020 136:101–9.


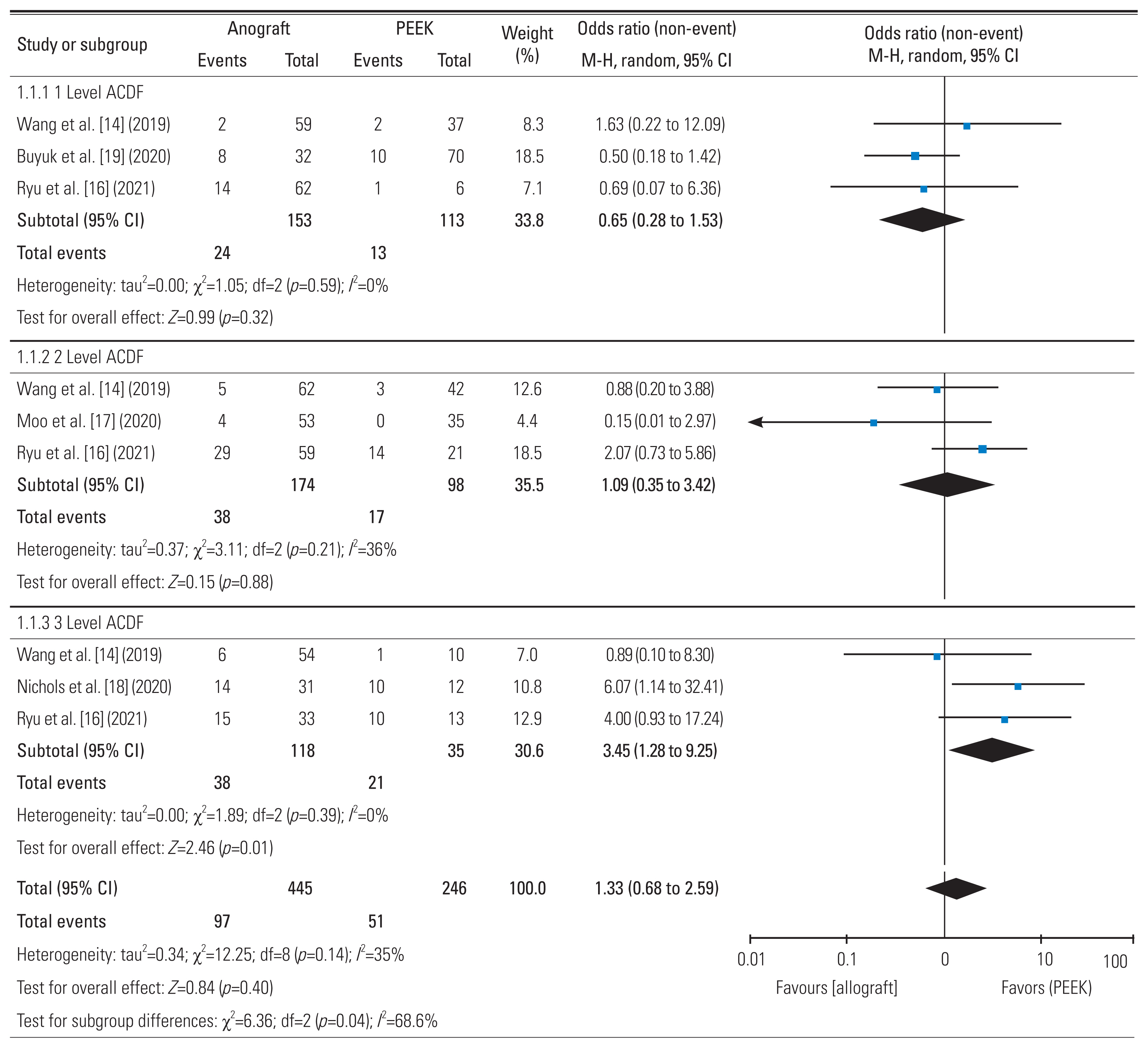
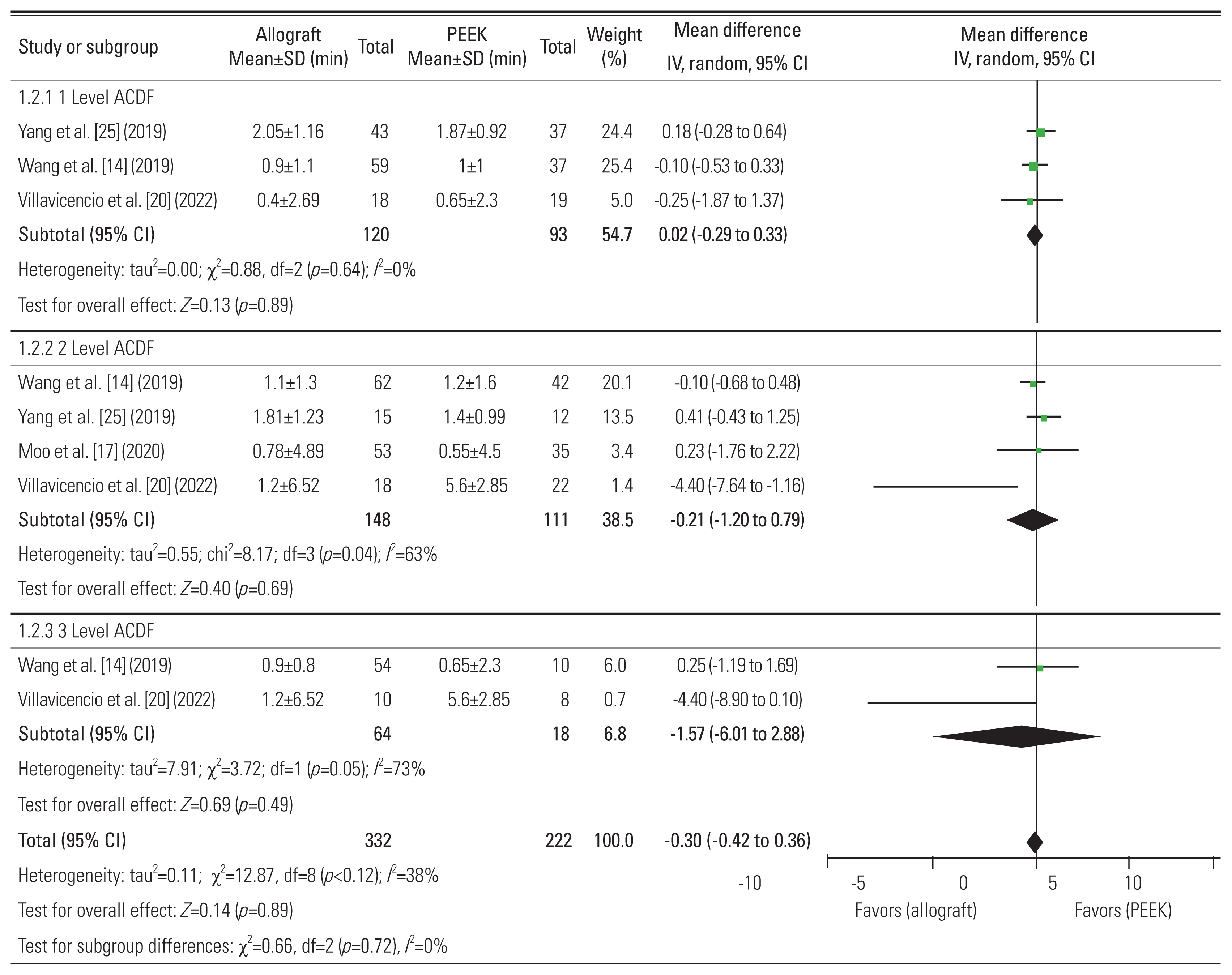
14. Wang M, Chou D, Chang CC, et al. Anterior cervical discectomy and fusion performed using structural allograft or polyetheretherketone: pseudarthrosis and revision surgery rates with minimum 2-year follow-up. J Neurosurg Spine 2019 32:562–9.

15. Bergin SM, Wang TY, Park C, et al. Pseudarthrosis rate following anterior cervical discectomy with fusion using an allograft cellular bone matrix: a multi-institutional analysis. Neurosurg Focus 2021 50:E6.

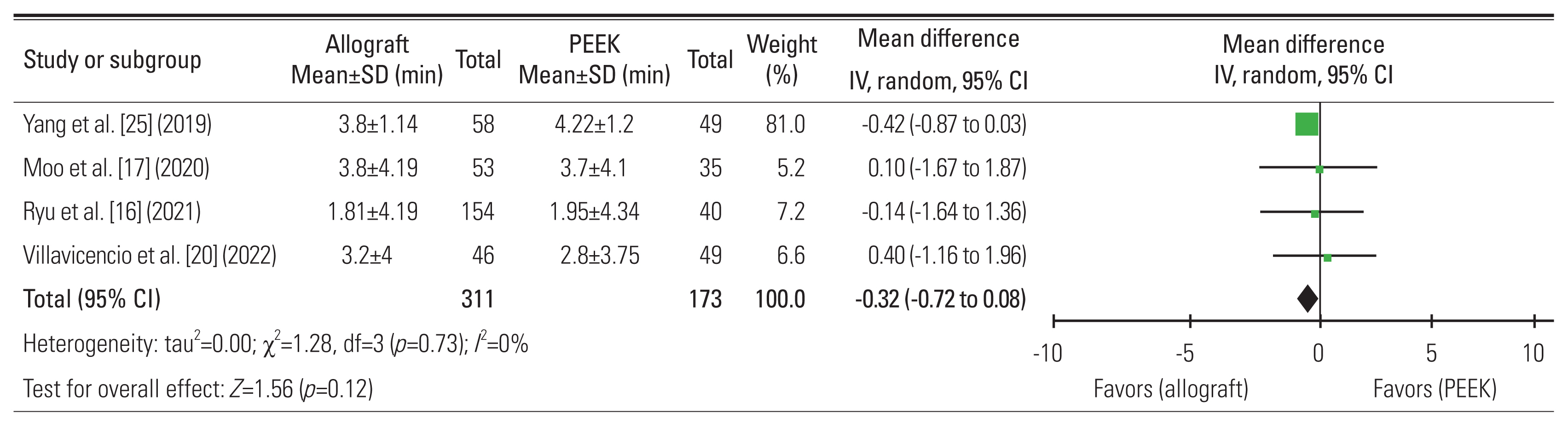
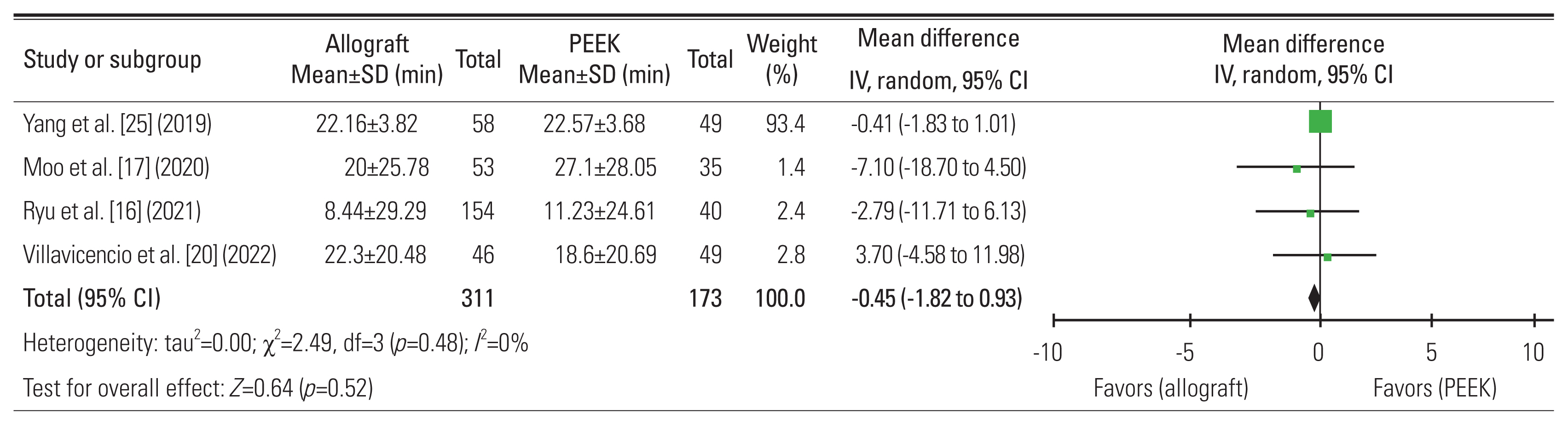
16. Ryu WHA, Richards D, Kerolus MG, et al. Nonunion rates after anterior cervical discectomy and fusion: comparison of polyetheretherketone vs structural allograft implants. Neurosurgery 2021 89:94–101.

18. Nichols NM, Jamieson A, Wang M, Chou D, Mummaneni PV, Tan LA. Characterizing the fusion order and level-specific rates of arthrodesis in 3-level anterior cervical discectomy and fusion: a radiographic study. J Clin Neurosci 2020 81:328–33.


20. Villavicencio AT, Nelson EL, Rajpal S, Beasley K, Burneikiene S. Prospective, randomized, blinded clinical trial comparing PEEK and allograft spacers in patients undergoing anterior cervical discectomy and fusion surgeries. Spine (Phila Pa 1976) 2022 47:1043–54.


21. Gillis CC, Kaszuba MC, Traynelis VC. Cervical radiographic parameters in 1- and 2-level anterior cervical discectomy and fusion. J Neurosurg Spine 2016 25:421–9.


23. Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther 1991 14:409–15.

25. Yang S, Yu Y, Liu X, et al. Clinical and radiological results comparison of allograft and polyetheretherketone cage for one to two-level anterior cervical discectomy and fusion: a CONSORT-compliant article. Medicine (Baltimore) 2019 98:e17935.


26. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials 2006 27:324–34.


27. Kersten RF, van Gaalen SM, de Gast A, Oner FC. Polyetheretherketone (PEEK) cages in cervical applications: a systematic review. Spine J 2015 15:1446–60.


30. Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: what they can offer and what they cannot. J Bone Joint Surg Br 2007 89:574–9.

31. Lee DH, Cho JH, Hwang CJ, et al. What is the fate of pseudarthrosis detected 1 year after anterior cervical discectomy and fusion? Spine (Phila Pa 1976) 2018 43:E23–8.


33. Veeravagu A, Cole T, Jiang B, Ratliff JK. Revision rates and complication incidence in single- and multilevel anterior cervical discectomy and fusion procedures: an administrative database study. Spine J 2014 14:1125–31.


35. Bolesta MJ, Rechtine GR, Chrin AM. Three- and four-level anterior cervical discectomy and fusion with plate fixation: a prospective study. Spine (Phila Pa 1976) 2000 25:2040–6.

36. Steinmetz MP, Warbel A, Whitfield M, Bingaman W. Preliminary experience with the DOC dynamic cervical implant for the treatment of multilevel cervical spondylosis. J Neurosurg 2002 97:330–6.


37. Tye GW, Graham RS, Broaddus WC, Young HF. Graft subsidence after instrument-assisted anterior cervical fusion. J Neurosurg 2002 97:186–92.


38. Yue WM, Brodner W, Highland TR. Long-term results after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year radiologic and clinical follow-up study. Spine (Phila Pa 1976) 2005 30:2138–44.

39. van Jonbergen HP, Spruit M, Anderson PG, Pavlov PW. Anterior cervical interbody fusion with a titanium box cage: early radiological assessment of fusion and subsidence. Spine J 2005 5:645–9.


41. Schmieder K, Wolzik-Grossmann M, Pechlivanis I, Engelhardt M, Scholz M, Harders A. Subsidence of the wing titanium cage after anterior cervical interbody fusion: 2-year follow-up study. J Neurosurg Spine 2006 4:447–53.


42. Hakalo J, Pezowicz C, Wronski J, Bedzinski R, Kasprowicz M. The process of subsidence after cervical stabilizations by cage alone, cage with plate and plate-cage. A biomechanical comparative study. Neurol Neurochir Pol 2007 41:411–6.

44. Niu CC, Liao JC, Chen WJ, Chen LH. Outcomes of interbody fusion cages used in 1 and 2-levels anterior cervical discectomy and fusion: titanium cages versus polyetheretherketone (PEEK) cages. J Spinal Disord Tech 2010 23:310–6.

47. Karikari IO, Jain D, Owens TR, et al. Impact of subsidence on clinical outcomes and radiographic fusion rates in anterior cervical discectomy and fusion: a systematic review. J Spinal Disord Tech 2014 27:1–10.

48. Ramakrishna S, Mayer J, Wintermantel E, Leong KW. Biomedical applications of polymer-composite materials: a review. Compos Sci Technol 2001 61:1189–224.

51. Carter N, Gianulis EC, Moore MA,Allograft structural interbody spacers compared to PEEK cages in cervical fusion: benchtop and clinical evidence. Barbeck M, Rosenberg N, Rider P, Peric Kacarevic Z, Jung O, editors. Clinical implementation of bone regeneration and maintenance. London: IntechOpen; 2021.Chapter 4:
https://doi.org/10.5772/intechopen.88091

52. Black J, Hastings G. Handbook of biomaterial properties. New York (NY): Springer; 2014.
53. Pennington Z, Mehta VA, Lubelski D, et al. Quality of life and cost implications of pseudarthrosis after anterior cervical discectomy and fusion and its subsequent revision surgery. World Neurosurg 2020 133:e592–9.