 |
 |
- Search
| Asian Spine J > Volume 17(3); 2023 > Article |
|
Abstract
Acknowledgments
Notes
Author Contributions
Conceptualization: DKP; data curation: AKD, AG; formal analysis: AKD, RK; funding acquisition: AKD, AG; methodology: DKP; project administration: not applicable; visualization: DKP, RK; writing–original draft: AKD; Writing–review & editing: AKD, DKP, AG, RK; and final approval of the manuscript: all authors.
Fig. 2
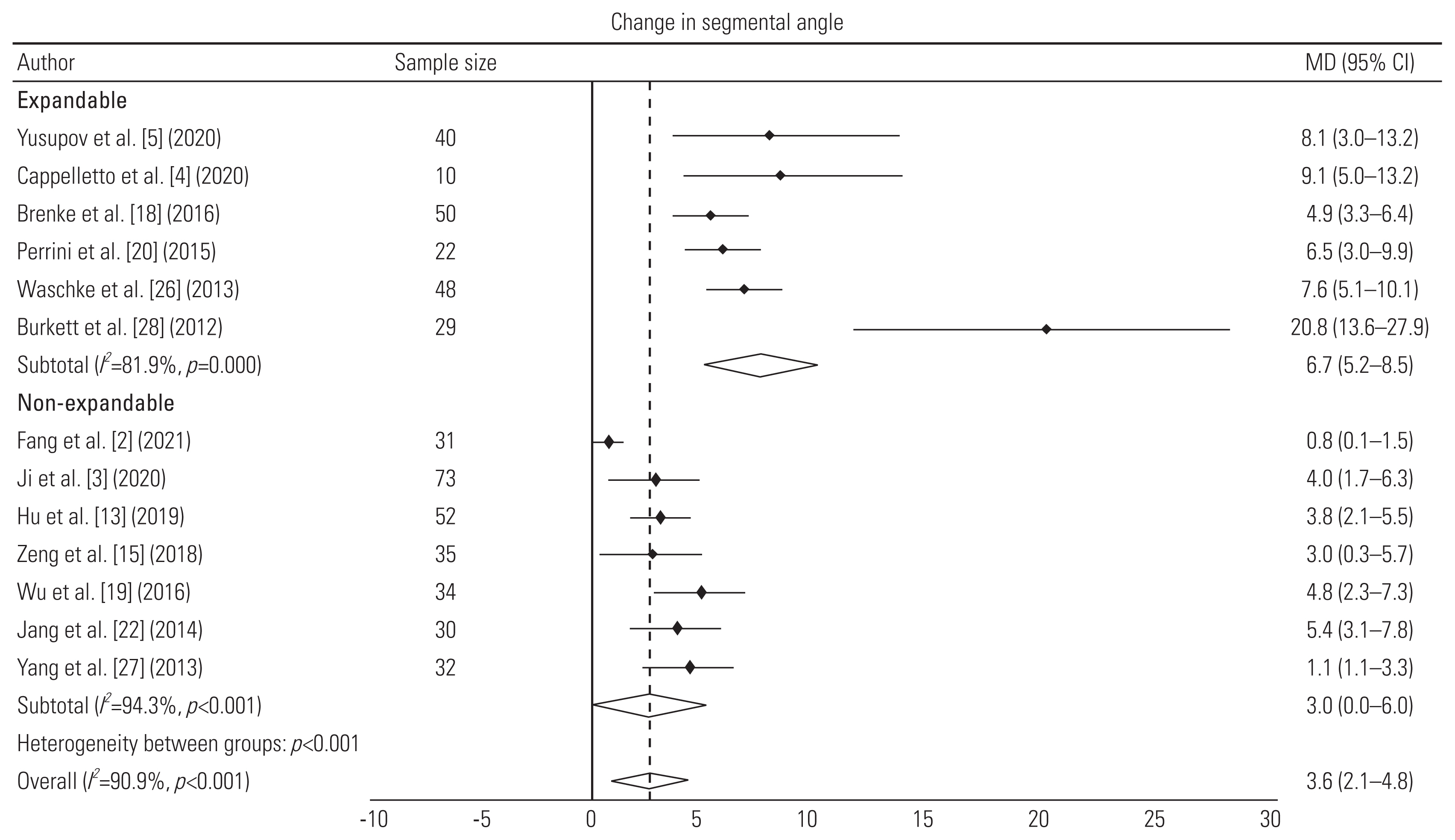
Fig. 3
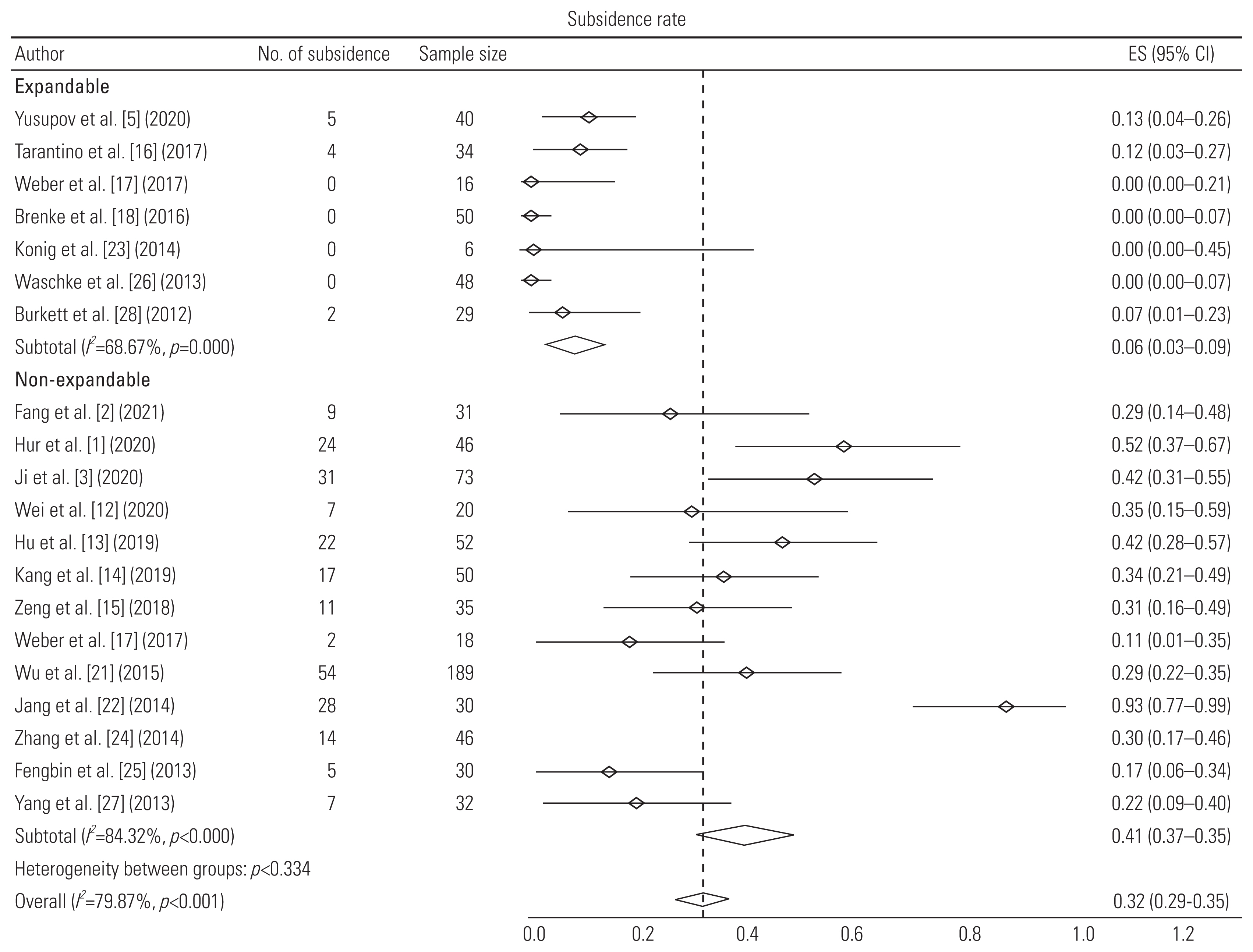
Fig. 4

Fig. 5
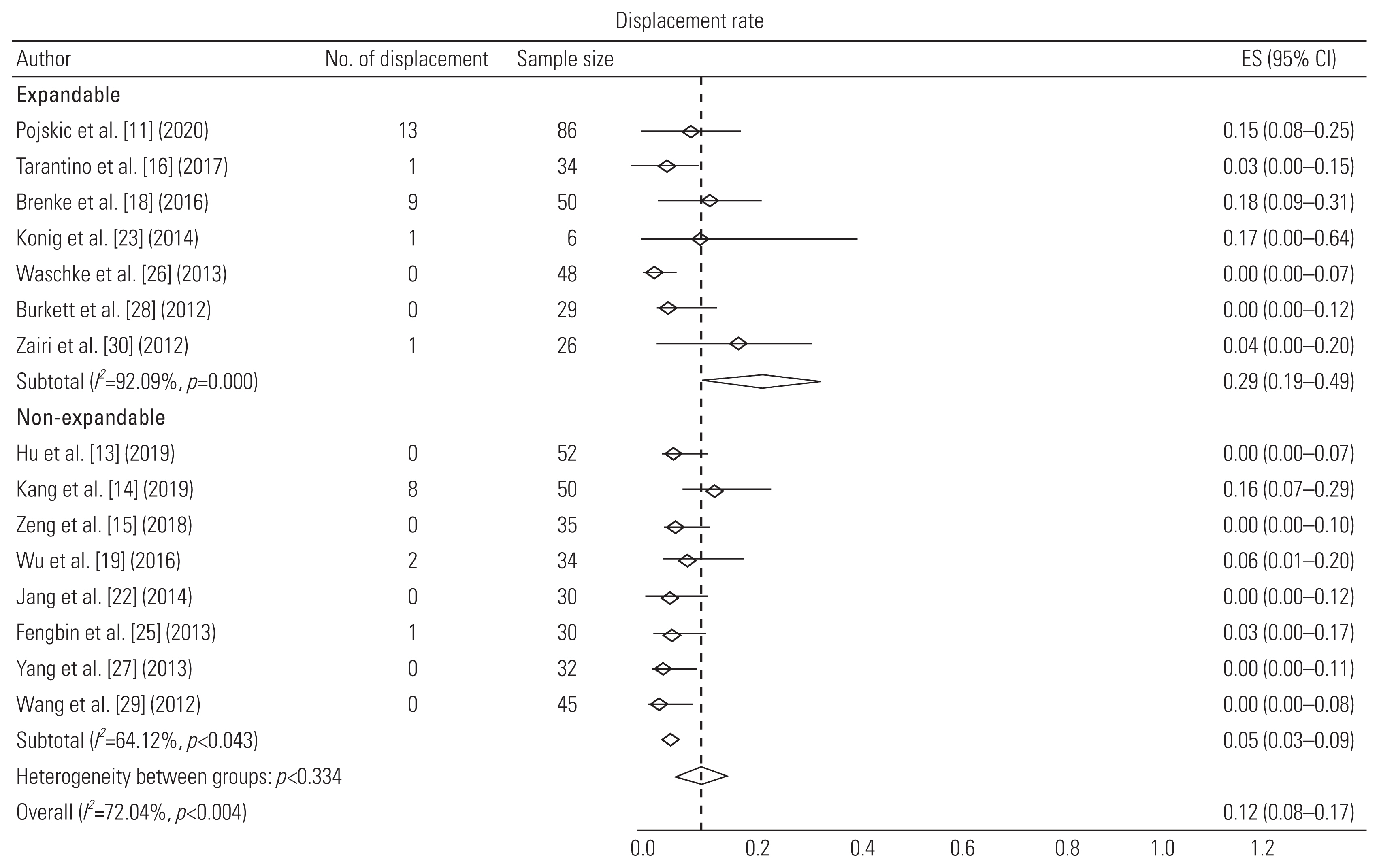
Fig. 6
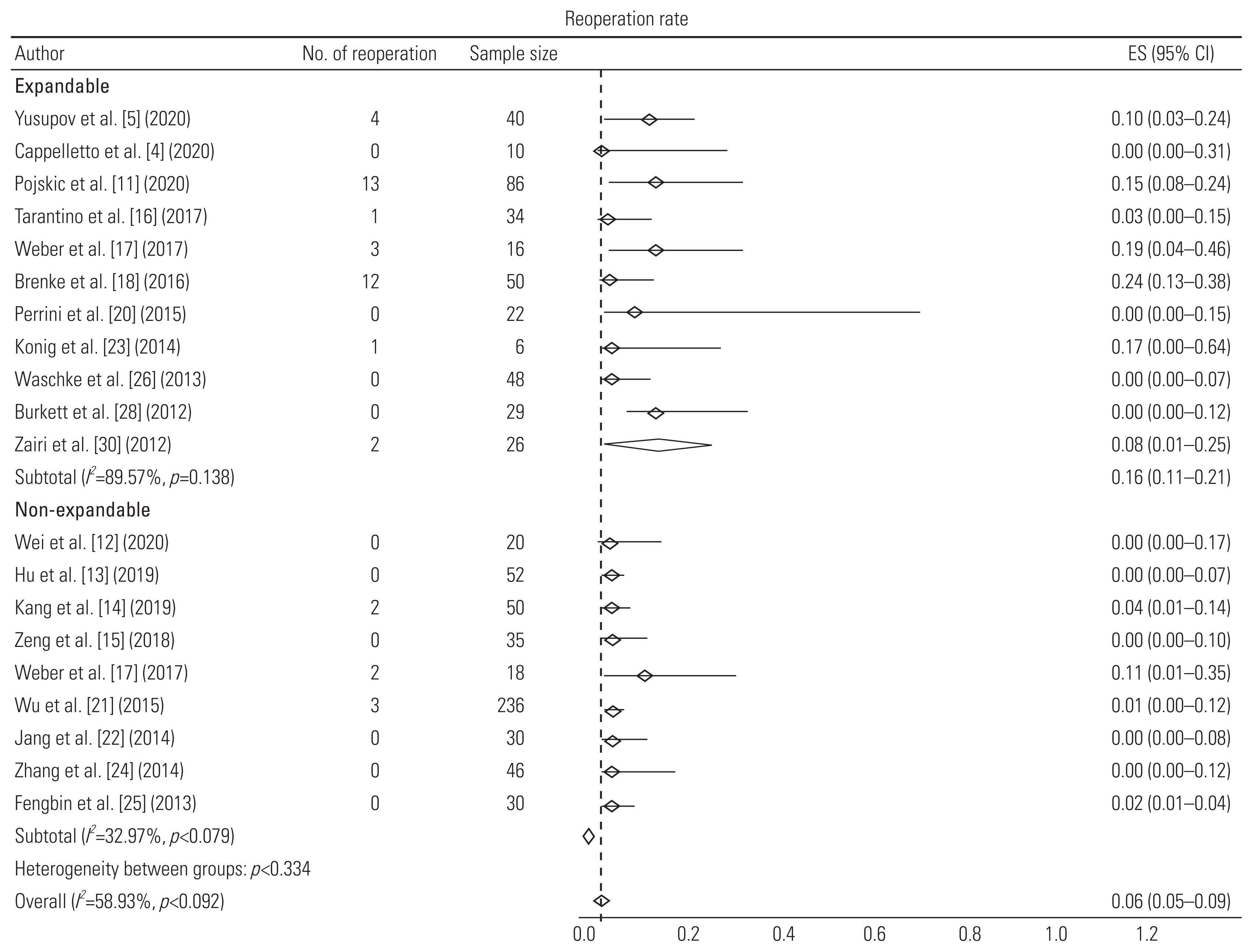
Table 1
Table 2
| Author (year) | Criteria | Quality rating | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | ||
| Fang et al. [2] (2021) | Y | Y | N | Y | N | Y | Y | NR | Y | Y | Y | NA | Fair |
| Yusupov et al. [5] (2020) | Y | Y | Y | Y | N | Y | Y | NR | N | Y | Y | NA | Fair |
| Cappelletto et al. [4] (2020) | Y | N | Y | CD | N | Y | Y | NR | Y | Y | Y | NA | Poor |
| Ji et al. [3] (2020) | Y | Y | N | Y | CD | Y | Y | NR | Y | Y | Y | NA | Fair |
| Hur et al. [1] (2020) | Y | Y | N | Y | CD | Y | Y | Y | CD | Y | Y | NA | Fair |
| Pojskic et al. [11] (2020) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NA | Good |
| Wei et al. [12] (2020) | Y | Y | N | Y | N | Y | Y | NR | Y | Y | Y | NA | Fair |
| Hu et al. [13] (2019) | Y | Y | N | Y | CD | CD | Y | Y | Y | Y | Y | NA | Fair |
| Kang et al. [14] (2019) | Y | Y | N | Y | CD | Y | Y | NR | Y | Y | Y | NA | Fair |
| Doria et al. [7] (2018) | Y | Y | N | Y | CD | Y | Y | Y | Y | Y | Y | NA | Good |
| Zeng et al. [15] (2018) | Y | Y | N | Y | CD | Y | Y | Y | Y | Y | Y | NA | Good |
| Tarantino et al. [16] (2017) | N | Y | N | Y | CD | Y | Y | Y | Y | Y | Y | NA | Fair |
| Weber et al. [17] (2017) | Y | Y | N | Y | CD | Y | Y | NR | Y | Y | Y | NA | Fair |
| Brenke et al. [18] (2016) | Y | Y | Y | Y | CD | Y | Y | Y | Y | Y | N | NA | Good |
| Wu et al. [19] (2016) | Y | Y | N | Y | CD | Y | Y | NR | Y | Y | Y | NA | Fair |
| Perrini et al. [20] (2015) | Y | Y | N | Y | N | Y | Y | CD | Y | Y | Y | NA | Fair |
| Wu et al. [21] (2015) | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | NA | Good |
| Jang et al. [22] (2014) | Y | Y | N | Y | CD | Y | Y | Y | Y | Y | Y | NA | Good |
| Konig et al. [23] (2014) | N | N | CD | CD | CD | Y | Y | NR | CD | NA | N | NA | Poor |
| Zhang et al. [24] (2014) | Y | Y | N | Y | CD | Y | Y | NR | Y | Y | Y | NA | Fair |
| Fengbin et al. [25] (2013) | Y | Y | N | Y | CD | Y | Y | Y | Y | Y | Y | NA | Good |
| Waschke et al. [26] (2013) | N | Y | Y | Y | CD | Y | Y | Y | Y | Y | Y | NA | Good |
| Yang et al. [27] (2013) | Y | Y | N | Y | CD | Y | Y | Y | Y | Y | Y | NA | Good |
| Burkett et al. [28] (2012) | N | Y | Y | Y | CD | Y | Y | NR | Y | Y | Y | NA | Fair |
| Wang et al. [29] (2012) | N | Y | N | Y | CD | Y | Y | NR | N | Y | Y | NA | Poor |
| Zairi et al. [30] (2012) | Y | Y | N | Y | CD | Y | Y | NR | Y | Y | Y | NA | Fair |
Q1: Was the study question or objective clearly stated?; Q2: Were eligibility criteria for the study population prespecified and clearly described?; Q3: Were the participants in the study representative of those who would be eligible for the intervention in the general or clinical population of interest?; Q4: Were all eligible participants that met the prespecified entry criteria enrolled?; Q5: Was the sample size sufficiently large to provide confidence in the findings?; Q6: Was the intervention clearly described and delivered consistently across the study population?; Q7: Were the outcome measures prespecified, clearly defined, valid, reliable, and assessed consistently across all study participants?; Q8: Were the people assessing the outcomes blinded to the participants’ interventions?; Q9: Was the loss to follow-up after baseline 20% or less? Were those lost to follow-up accounted for in the analysis?; Q10: Did the statistical methods examine changes in outcome measures from before to after the intervention? Were statistical tests done that provided p-values for the pre-to-post changes?; Q11: Were outcome measures of interest taken multiple times before the intervention and multiple times after the intervention?; Q12: If the intervention was conducted at a group level, did the statistical analysis take into account the use of individual-level data to determine effects at the group level?
Table 3
| Author | Study design | Sample size | Avg age (yr) | Indications | Level of corpectomy (no. of patients) | Type of cage | Bone graft | Follow-up (mo) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| E group | |||||||||
| Yusupov et al. [5] (2020) | Retrospective study | 40 (M=24, F=16) | 56±15 | Spondylosis | 1 (32), 2 (8) | Expandable titanium cage (MediExpand) with anterior fixation | Autologous bone graft | 14.8±7 | Change in SA: 7.8°; subsidence: 5; no. of reoperations: 4 |
| Cappelletto et al. [4] (2020) | Retrospective study | 10 | 55 (18–78) | Spondylosis | 1 (8), 2 (1), 3 (1) | CAPRI Expandable Cage and X-Core with anterior plating | Bone graft; β-tricalcium phosphate | 12 (6–48) | Mean increase in 9.1° lordosis; 90% fusion rate; no reoperations |
| Pojskic et al. [11] (2020) | Retrospective study | 46 (M=23, F=23) | 61.3 (11–89) | Spondylosis | 1 (14), 2 (30), 3 (2) | Expandable titanium cage (X-Core) with anterior plating | Bone morphogenetic protein | 30.7 | Fusion rate: 95%; preoperative angle=14.1° (0°–78.1°); postoperative=12.3° (3°–38.6°); subsidence in 13 patients out of 56 (not included in analysis due to different definition); displacement: 6; reoperations: 6 |
| Tarantino et al. [16] (2017) | Prospective study | 34 (M=18, F=16) | 68 (55–81) | Spondylosis | 1 (21), 2 (9), 3 (4) | Anterior distraction device (ADD Plus) with anterior plating | Not mentioned | 24 | Subsidence: 4; cage displacement: 1; reoperation due to displacement: 1 |
| Brenke et al. [18] (2016) | Retrospective study | 50 (M=28, F=22) | 61 (31–84) | Spondylosis | 1 (32), 2 (15), 3 (3) | ADD together with an anterior plate | Not mentioned | 7.3 | Gain of 4.86° lordosis; no subsidence; 9 cage displacements; 12 reoperations (implant related: 2) |
| Perrini et al. [20] (2015) | Retrospective study | 22 (M=17, F=5) | 61±9.66 | Spondylosis | 1 (15), 2 (7) | Expandable titanium cage (ADD) with anterior plating | Autologous bone graft | 39.77±16.27 | Change in SA: 6.46°; fusion rate: 100%; no reoperation |
| Konig et al. [23] (2014) | Retrospective study | 6 | 62.2 (41–79) | Spondylosis | 1, 2, or 3 | Expandable titanium cage (ADD plus) with anterior fixation | Not mentioned | 15 | No subsidence; 1 cage displacement and 1 reoperation (cage displacement) |
| Waschke et al. [26] (2013) | Retrospective study | 48 (M=20, F=28) | 61 (40–79) | Spondylosis | 1 (31), 2 (17) | Expandable titanium cages (Tecorp-C, Scient’x) with anterior plate system | Autologous bone graft | 23 (8–42) | Mean increase in 7.6° lordosis; 79% fusion rate; no subsidence; displacement and reoperation |
| Burkett et al. [28] (2012) | Retrospective study | 29 (M=20, F=9) | 48 (14–81) | Spondylosis | 1 (23), 2 (4), 3 (2) | Expandable titanium cage with anterior fixation | Bone graft; hydroxyapatite and calcium triphosphate | 9.5 (3–24) | Mean increase in 20.8° lordosis; 100% fusion rate; 2 subsidence; no displacement and no reoperation |
| Zairi et al. [30] (2012) | Retrospective study | 26 (M=14, F=12) | 60 (38–81) | Spondylosis | 1 (15), 2 (8), 3 (3) | Expandable titanium cage (Tecorp, Scient’x) with anterior plate fixation | Autologous bone graft | 30 (24–48) | 100% fusion rate; 1 cage displacement and 2 reoperations due to cage displacement and epidural hematoma |
| NE group | |||||||||
| Fang et al. [2] (2021) | Retrospective study | 31 (M=15, F=16) | 59.17±9.24 | Spondylosis | 1 (31) | TMC with anterior plating | Autologous bone graft | 12 | Change in SA: 0.76°; subsidence in 9 cages |
| Hur et al. [1] (2020) | Retrospective study | 46 (M=22, F=24) | 62.5±9.51 | Spondylosis | 1 (46) | TMC (Medtronic Sofamor Danek, Memphis) with anterior plating system | Autologous bone graft | 34.3±6.7 | 100% fusion rate at the end of follow-up and subsidence in 24 |
| Ji et al. [3] (2020) | Retrospective cohort study | 73 (M=37, F=36) | 58.1 (30–77) | Spondylosis | 1 (73) | TMC (DePuy Spine) with anterior plating system | Autologous bone graft | 24 | Mean increase in 4° SA; 100% fusion rate; subsidence in 31 cages; 3 reoperations due to implant failure |
| Wei et al. [12] (2020) | Prospective randomized study | 20 (M=11, F=9) | 53.8±7.8 | Spondylosis | 1 (20) | TMC with anterior plating system | Autologous bone graft | 6 | 95% fusion rate; subsidence in 7 cages; no reoperations |
| Hu et al. [13] (2019) | Retrospective study | 52 (M=28, F=24) | 54.9±9.5 | Spondylosis | 1 (52) | TMC with anterior plating system | Not mentioned | 102.4±4.6 | Mean increase in 3.8° lordosis; 95% fusion rate; subsidence in 22 cages; no cage displacement and no reoperations |
| Kang et al. [14] (2019) | Retrospective study | 50 (M=15, F=35) | 59.6±11.1 | Spondylosis | 1 (38), 2 (12) | TMC-SynMesh cage (DePuy Synthes) with anterior fixation | Autologous bone graft | 16.8±13.7 | 98% fusion rate; subsidence in 17 cages; 8 cage displacements; 2 reoperations due to C5 palsy and implant failure |
| Zeng et al. [15] (2018) | Retrospective study | 35 (M=19, F=16) | 54.4 (32–78) | Spondylosis | 1 (28), 2 (7) | TMC (DePuy Synthes) with anterior plating system | Autologous bone graft | 69.5 (60–87) | Mean increase in 3° SA; 100% fusion; subsidence 11 cages; no displacement and no reoperation |
| Wu et al. [19] (2016) | Retrospective study | 34 (M=21, F=13) | 56.7±11.06 | Spondylosis | 1 (34) | TMC with anterior plating system | Autologous bone graft | 12–42 | Mean increase in 4.78° SA; 100% fusion rate; 2 cage displacements |
| Wu et al. [21] (2015) | Retrospective study | 236 (M=128, F=108) | 51.3±6.4 | Spondylosis | 1 (236) | TMC (Mesh, Depuy) with anterior plating system | Autologous bone graft | 12 | Subsidence in 54 out of 189 patients and 3 reoperations due to implant failure |
| Jang et al. [22] (2014) | Retrospective study | 30 (M=19, F=11) | 52.4 (24–78) | Spondylosis | 1 (24), 2 (6) | TMC (Medtronic Sofamor Danek, Memphis) with anterior plating system | Autologous bone graft | 27.6 (24–49) | Mean gain of 5.4° SA; 100% fusion rate; subsidence in 28 cages; no cage displacement and reoperation |
| Zhang et al. [24] (2014) | Retrospective study | 46 (M=24, F=22) | 55.04±11.09 | Spondylosis | 1 (25), 2 (21) | TMC (Medtronic Sofamor Danek, Memphis) with anterior plating system | Autologous bone graft | 45.28±12.83 | 95.6% fusion rate; subsidence in 14 cages; no reoperation |
| Fengbin et al. [25] (2013) | Prospective randomized | 30 (M=20, F=10) | 59 (46–76) | Spondylosis | 1 (30) | TMC (DePuy) with anterior plating system | Autologous bone graft | 33±3 | 100% fusion rate; subsidence in 5 cages; 1 displacement and no reoperation |
| Yang et al. [27] (2013) | Prospective non randomized comparative | 32 (M=20, F=12) | 46.8±7.2 | Spondylosis | 1 (32) | TMC (Medtronic Sofamor Danek, Memphis) with anterior plate system | Autologous bone graft | 48 | Mean increase in 1.1° lordosis; 94% fusion rate; subsidence in 7 cages; no displacement |
| Wang et al. [29] (2012) | Prospective study | 45 (M=28, F=17) | 53.9±9.9 | Spondylosis | 1 (45) | TMC (DePuy Spine) with anterior fixation | Autologous bone graft | 14.3±6.3 | 97.8% fusion rate with no cage displacement |
| E+NE group | |||||||||
| Doria et al. [7] (2018) | Retrospective cohort study | 65 (E=26, NE=39) | 67–73 | Spondylosis | 2 or 3 | Titanium expandable cage and TMC with anterior plating | Autologous bone graft | 12 | 100% fusion rate in both expandable and non-expandable cages |
| Weber et al. [17] (2017) | Retrospective cohort study | 34 (E=16, NE=18) | 51.5±8.7(E); 61.3±11.2 (NE) | Spondylosis | E: 1 (15), 2 (1); NE: 1 (15), 2 (3) | Titanium expandable cage and TMC with anterior plating system | Autologous bone graft | E: 10.4±10.8; NE: 6.8±6.1 | No subsidence in expandable cages and 2 subsidence in mesh cage; 3 reoperations in expandable cage and 2 in mesh cage |








