The Transaxillary Approach as a Direct Route in the Management of Upper Thoracic Spine Pathology: A Technical Note with Case Series
Article information
Abstract
This retrospective case series of prospective data aims to describe the transaxillary approach for the treatment of upper thoracic spine pathology. Various surgical techniques and approaches have been reported across the literature to address upper thoracic spine pathology, including the cervicothoracic approach, anterior transsternal approach, posterolateral approach, supraclavicular approach, and lateral parascapular approaches. These techniques are invasive. A minimally invasive, less morbid, and direct access approach to the pathology of the upper thoracic spine has not been reported in the literature. Patients with pathology affecting the first thoracic vertebra up to the sixth thoracic vertebra were classified into the upper thoracic spine group. Patients with pathology below the sixth thoracic vertebra were excluded. Patients not having a minimum follow-up of 12 months were also excluded. The study analyzed 18 patients. The mean preoperative modified Japanese Orthopedic Association score was 7.2±1.44, which improved to 10.16±1.2 (p<0.05). The majority (14/18) of the patients had an excellent outcome. Three patients had good outcomes, and one patient had a fair outcome. Five cases of intraoperative dural leak were recorded, and one patient had postoperative neurological deficit. The transaxillary approach is a safe, viable, muscle-sparing, and minimally invasive approach for ventral pathologies of the upper thoracic spine.
Introduction
Upper thoracic spine disorders present a challenge to surgeons because of their unique anatomy [1]. The upper thoracic spine is near vital neurovascular structures, mediastinal viscera, and a narrow thoracic outlet. Surgical access to treat disorders involving the vertebral body or ventral dura of the upper thoracic spine remains debatable [1]. The posterior spinal approach, which is the workhorse of any spine surgeon, is not feasible in the upper thoracic region because of various anatomical reasons in addition to the presence of a scapula and strong shoulder muscles [2]. Pathologies commonly affecting the upper thoracic spine include fractures, tubercular osteomyelitis, ossification of the posterior longitudinal ligament (OPLL), and disk herniations [3–6]. While tubercular osteomyelitis is very commonly seen in the southern Asian subcontinent, the incidence of OPLL is high in East Asian populations [7–9]. Conversely, thoracic disk herniations are rare when compared with their cervical and lumbar counterparts, with an approximate incidence of 0.25%–0.5% [10]. If these lesions are present in the upper thoracic region, they pose a significant challenge to the treating surgeon.
Various surgical techniques and approaches have been reported across the literature to address upper thoracic spine pathology, including the cervicothoracic, anterior transsternal, anterolateral thoracotomy, posterior, posterolateral, supraclavicular, and lateral parascapular approaches [1,11]. Although these approaches can address the upper thoracic spine pathology, they are invasive and lead to significant postoperative morbidity and muscle weakness [11]. This calls for an approach that is less invasive and less morbid and gives direct access to the pathological site. Herein, we describe our experience with the transaxillary approach for treating upper thoracic spine pathology.
Technical Notes
1. Study design and participants
After approval from the institutional review board (IRB) of Wooridul Spine Hospital (2023-01-WSH-002), a retrospective review of the prospectively collected database was performed. As the study is retrospective, IRB is exempted from obtaining specific consent from each patient for the study. However, general consent was obtained from all the patients regarding the usage of data for academic purposes. The database was searched for patients with thoracic spine lesions using the codes M-4884. Patients with pathology affecting the first thoracic vertebra up to the sixth thoracic vertebra were classified into the upper thoracic spine group [1]. Consecutive patients who underwent a transaxillary approach to treat such lesions were included in the study. Patients with pathology below the sixth thoracic vertebra were excluded. Patients not having a minimum follow-up of 12 months and those with incomplete clinical details were also excluded.
Demographic and baseline variables, such as age, sex, level of vertebral involvement, presenting symptoms, type of vertebral pathology, and medical comorbidities, were recorded. Clinical data such as Visual Analog Scale (VAS), Oswestry Disability Index (ODI), neurology (modified Japanese Orthopedic Association [JOA] score), operative duration, length of hospital stay, and complications were recorded. The improvement rate (IR) in neurological status was evaluated by the modified JOA score using the following formula [12,13].
The maximum score according to the JOA classification for thoracic myelopathy is 11. The IR was used to define the surgical outcome as follows: excellent (IR ≥75%), good (75%> IR ≥50%), fair (50%> IR ≥25%), and poor (IR <25%) [12,13].
Radiological outcomes, such as the amount of decompression, were evaluated by immediate postoperative magnetic resonance imaging (MRI). After the data were collected and tabulated, descriptive statistics were used for continuous variables. The t-test was used to determine the significance of changes in VAS and ODI scores before and after surgery. A p-value of <0.05 was considered significant.
2. Surgical technique
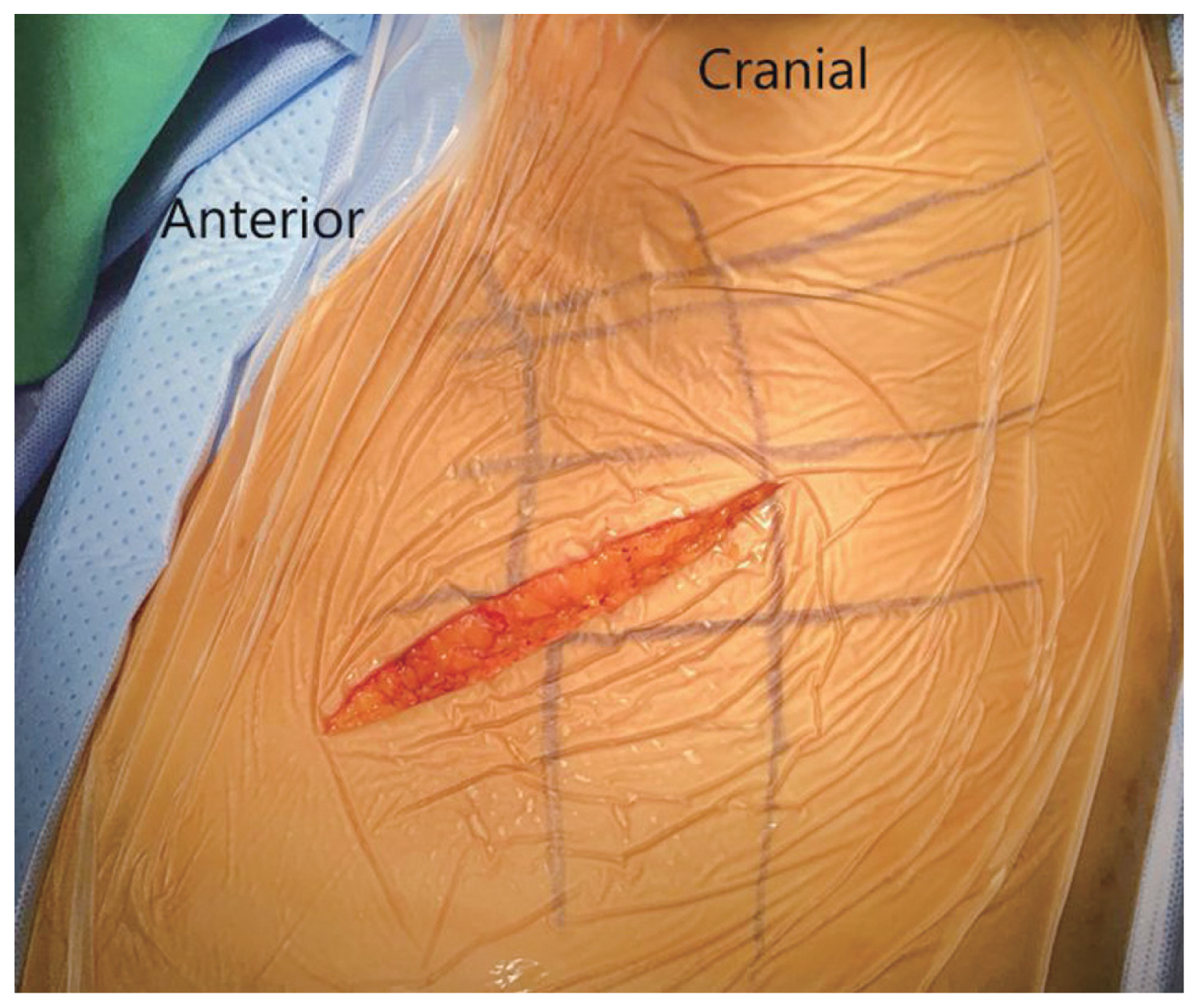
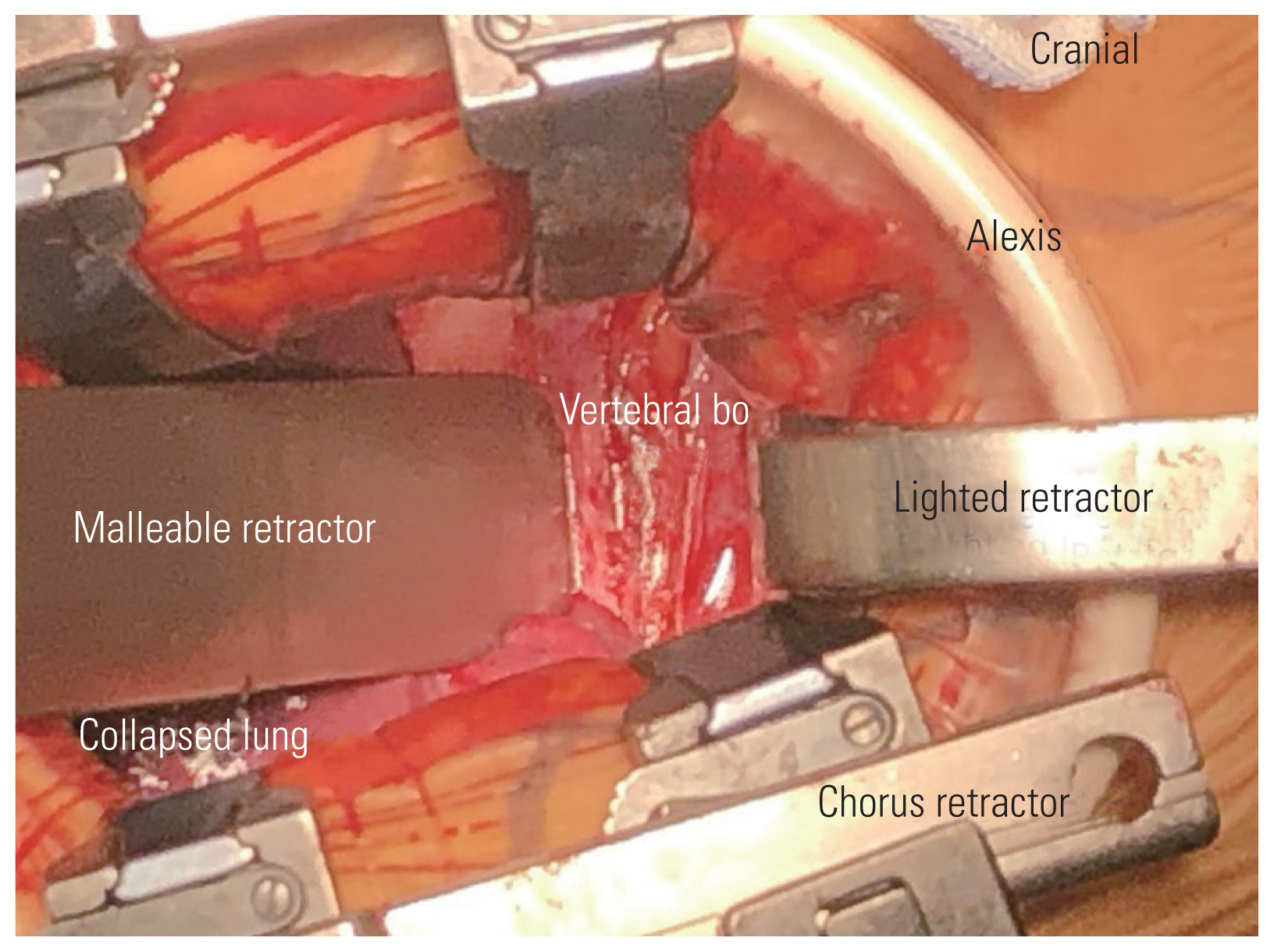
After adequate general anesthesia and a double-lumen endotracheal tube, the patient was placed in the right lateral position with the left side up and the left arm over the head supported by a forearm stand. The left-sided approach is usually chosen because of the ease of handling the aorta from the left side. The procedure was performed under continuous neuromonitoring. An incision was made for the transaxillary approach (Fig. 1). After incision and subcutaneous dissection, a plane was made between the latissimus dorsi and pectoralis muscles, and the pleural cavity was reached through the intercostal muscles (Fig. 2A, B). The rib was not resected, and a space was created by rib retraction. Once the pleural cavity has been reached, the anesthetist is requested to deflate the left lung. A double-ring retractor was used to simplify the decompression. The target area was confirmed using a pin marker under the image intensifier. The cord was decompressed according to the standard techniques described in the literature [14,15]. Once the target area was confirmed, the bone was drilled using a bone drill to separate the OPLL from the posterior vertebral body (Fig. 3). After the separation of the OPLL from the dura, the OPLL was removed from the vertebral body. Hemostasis was achieved, a chest tube was placed through a separate incision, and the wound was closed in layers.
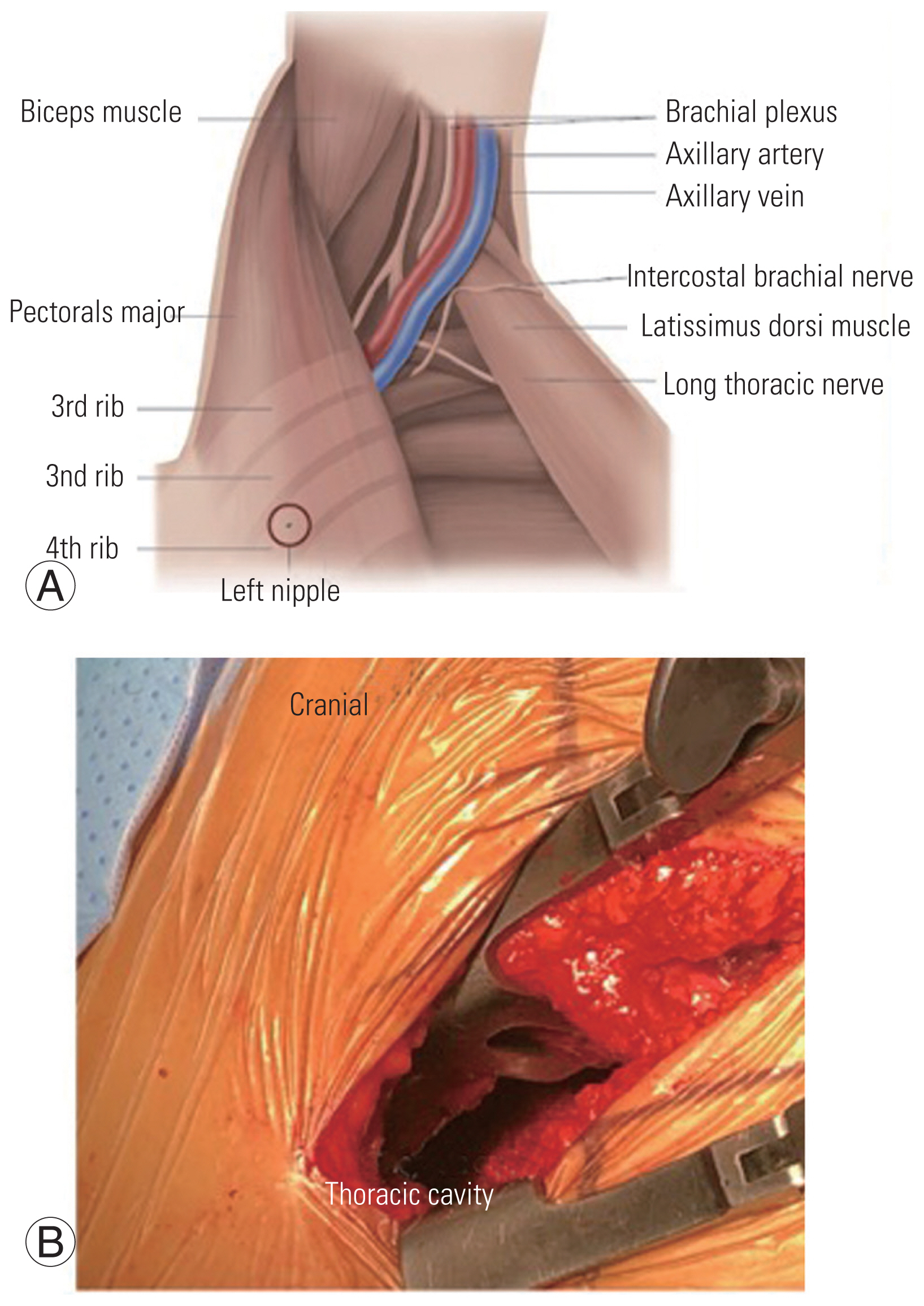
(A) An illustration showing the plane between latissimus dorsi and pectoralis major with major vessels in the field. (B) Figure showing the access to thoracic cavity and vertebral body.
3. Case presentation
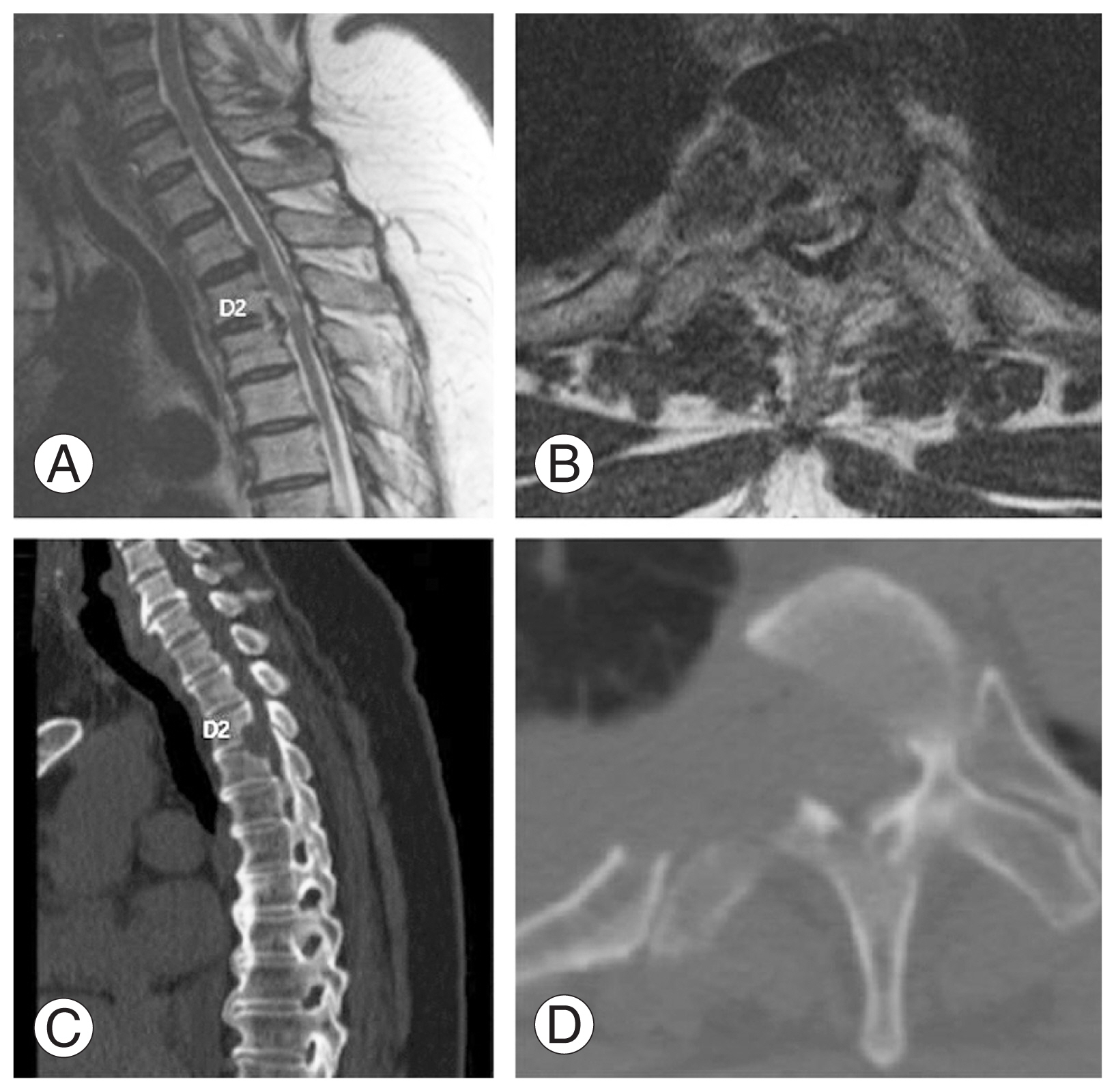
A 56-year-old woman visited the spine clinic with bilateral lower limb weakness and upper back pain for 6 months. The weakness gradually progressed, and the patient was unable to perform her daily activities. On clinical examination, no visible deformity or tenderness over the spine was noted. Tone was increased in bilateral lower limbs with decreased motor power. The results of the neurological examination are presented in Table 1. Her ODI was 74/100, and her VAS score was 9/10. Her modified JOA score was 8/11. Radiography, MRI, and computed tomography (CT) were performed, which showed OPLL at the D2–D3 vertebral level. Significant thecal sac compression was noted at the D2–D3 level (Fig. 4A–D). Further blood investigations were performed, and the patient was counseled regarding the need for decompression. The transaxillary approach was chosen to decompress the thecal sac at the involved level. Postoperatively, the patient was mobilized, and her neurologic status recovered to the ambulatory level. Postoperative MRI and CT showed good decompression of the D2–D3 level (Fig. 5A–D). The patient’s postoperative ODI, VAS, and modified JOA scores were 48/100, 2/10, and 10/11, respectively. The patient had a follow-up of 135 months, and the OPLL had not recurred at the operated site.
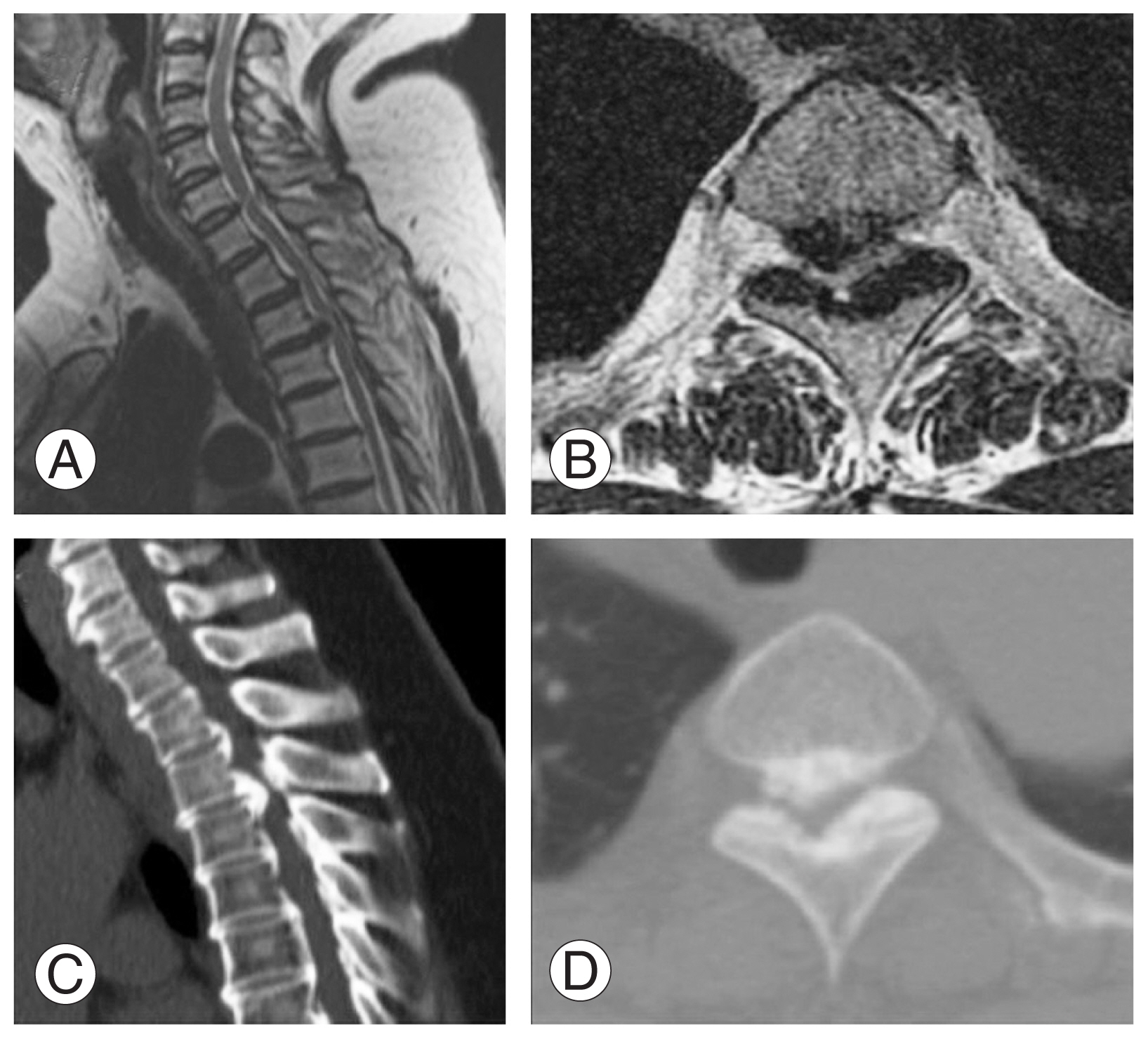
(A, B) Preoperative sagittal and axial magnetic resonance imaging showing ossification of the posterior longitudinal ligament (OPLL) at D2–D3 with significant thecal sac compression. (C, D) Preoperative sagittal and axial computed tomography scan showing OPLL at D2–D3.
4. Main outcomes
The study analyzed 18 patients, which included two male and 16 female patients. The mean age was 55±12 years. Of the 18 patients, 14 had OPLL and four had thoracic disk herniations. Nine patients had pathology at more than one level. All patients had a minimum follow-up of 12 months. The maximum follow-up period was 138 months. The mean duration of symptoms before surgery was 9±4 months. The mean operative duration was 261.38±94.8 minutes. The mean blood loss was 812±1191 mL. The mean hospital stay was 26.53±16.18 days. The mean preoperative modified JOA score was 7.2±1.44 which improved to 10.16±1.2 (p<0.05). The mean preoperative VAS score was 7.83±1.04. The VAS score improved to 2±0.59 at the final follow-up (p<0.05). The mean preoperative ODI score was 73.3±11.3, which improved to 36.78±7.7 at the final follow-up (p<0.05). The majority (14/18) of the patients had an excellent outcome according to the IR. Three patients had good outcomes, and one patient had a fair outcome. There were five cases of intraoperative dural leak. All patients were treated with dural graft and TachoSil [16]. One patient developed a postoperative neurological deficit because of the mass effect of the TachoSil and a decompression had to be performed in the immediate postoperative period. In our case series, serious complications occur in any patient. Demographic details of the patients are enumerated in Table 2. The clinical details are described in Table 3.
Discussion
The choice of surgical approach by a surgeon is guided by the location of the pathology in the spine. While lumbar and cervical spine pathologies can be treated by defined and straightforward approaches, approaches for the thoracic spine, particularly the upper thoracic spine, are debated. Many approaches have been described in the literature, including both anterior and posterior routes [17]. The posterior transpedicular approach or laminectomy has limited access and does not allow direct visualization of ventral lesions [18]. Approaches such as costotransversectomy or the lateral extracavitary approach are difficult above the T4 vertebra because of the scapula [18]. The anterior cervical approach rarely provides access below C7 and has a wide individual variation depending on the morphology of the neck [18]. The sternum-splitting approach with the anterior cervical approach gives access up to the T5 vertebra but requires mobilization of the aerodigestive tracts, great vessels, recurrent laryngeal nerve, and thoracic duct. Data retrieved mainly from cardiothoracic surgeries indicate significant morbidity and mortality following sternotomy, with an incidence as high as 25% [19,20]. When an anterolateral approach is used, impairment of the latissimus dorsi muscle along with the parascapular muscles leads to restricted shoulder movements in the postoperative period [1]. Although all the above approaches were successful in treating the pathology, they were invasive and involved the resection of normal structures such as the ribs, muscles, and sternum, which can result in significant morbidity [21].
In view of these disadvantages, alternate anterior approaches to the upper thoracic spine are needed, and various anterior techniques have been reported in the literature. The anterior approach to the upper thoracic spine remains a challenge for spine surgeons because of anatomical considerations. During the evolution of anterior approaches for the spine, Tangco et al. [22] first described thoracotomy for tuberculosis of the spine in the thoracic region. Urschel [23] introduced the transaxillary approach not for spinal pathology but for rib resection in thoracic outlet syndrome. This was then popularized by vascular surgeons to address vascular lesions and by neurosurgeons to perform sympathectomy [24].
In this paper, the transaxillary approach is classically a muscle-sparing approach where the latissimus dorsi is spared, and the plane is developed by lifting the latissimus dorsi (Fig. 2A) [10]. The traditional anterior transthoracic approaches use the fourth or fifth intercostal space to enter the thoracic cavity, and many surgeons resect a rib to gain more working space. The transaxillary approach in our study was proximal, and we did not resect any rib, thereby preserving as much anatomy as possible [10]. When the pathology was at the lung apex or high thoracic level, a transpleural approach was preferred because of the structural complexity of performing an extrapleural dissection. In addition, this approach resulted in minimal morbidity caused by the chest drain; however, for surgeries at the midthoracic level, our preference was the extrapleural approach. Advances in anesthesia and retractor systems have further helped in the effective implementation of the transaxillary approach. A double-lumen endotracheal tube is used to deflate the ipsilateral lung while working near the vertebral bodies. A double retractor system provides a safe corridor for the working space and prevents injuries to the viscera and great vessels (Fig. 3). In our series, complications included dural leakage in five patients. Cho et al. [16] compared the outcomes of the management of dural leak during the anterior decompression of OPLL in the thoracic spine with and without a subarachnoid drain. The study group concluded that dural leaks during anterior decompressions can be managed by dural grafts alone without the need for a drain. The study group utilized the concept of volume-controlled pseudomeningocele described by Cho et al. [16]. We used the same technique in our study, and none of the patients required revision surgery for dural leaks. However, if the cerebrospinal fluid (CSF) leak is associated with persistent postural headache, poor wound healing, and appreciable collection of CSF in the subcutaneous region of the back, a subarachnoid drain may be necessary to control symptoms. In the study conducted by Kanematsu et al. [25], eight patients were treated by anterior transthoracic techniques, and the OPLL existed from the T3 to T9 vertebral levels. The mean blood loss was 346 mL, and the mean operative duration was 450 minutes. Four cases of intraoperative CSF leakage were encountered. The mean JOA score improved significantly during the follow-up period [25].
On comparing the transaxillary approaches with other approaches such as transsternal approaches, Falavigna et al. [26] treated 19 consecutive patients with disk herniations and spondylodiskitis of the upper thoracic spine with the transsternal approach. Patients who underwent sternotomy were reported to have longer hospital stays and had more severe postoperative pain than patients who did not undergo sternotomy [26]. In another study by Brogna et al. [2], 18 patients with proximal thoracic spines affected mainly by thoracic disk metastasis were treated by a mini-sternotomy approach. The average operative duration was 210 minutes, and the average blood loss was 800 mL. Because this study did not deal with any OPLL cases, no incidences of CSF leaks were recorded. Most of the patients experienced improvement in postoperative neurologic status. Few complications were noted, such as pneumonia in one patient and persistent intercostal pain in two patients that required intercostal blocks. The authors also noted the difficulty in mobilizing the viscera, such as the trachea and esophagus, along with large vessels [2]. Although the operative duration and blood loss were somewhat similar to those of the transaxillary approach, postoperative pain and lung complications such as pneumonia were more common in the transsternal approach. The transaxillary approach has many advantages such as minimal invasion, muscle sparing, avoidance of rib resection, and mobilization of great vessels. Therefore, this approach can be considered a viable option for upper thoracic lesions, particularly those involving the ventral aspect of the spinal cord. The transaxillary approach has a few limitations. Because this is a single-center study with a small sample size, no comparative group was employed to tell that this technique is the best option. The reproducibility of this technique in all centers is a challenge. It cannot be performed in patients who have compromised pulmonary reserve and who cannot tolerate single-lung ventilation. It is also difficult to employ this technique in patients with complex anatomy of the great vessels around the aortic arch.
The transaxillary approach is a safe, viable, muscle-sparing, and minimally invasive approach for the treatment of ventral pathologies of the upper thoracic spine. When this approach is used in selected indications, it provides reasonable outcomes in terms of neurological improvement with minimal or no approach-related complications. Furthermore, a large series using this approach with a long follow-up is necessary to examine the versatility of the approach and expand the indications over and above the present indications.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualisation: SHL, JB; data collection: JWS; statistical analysis: UDH; manuscript writing: SI; proof reading: HCL, SHL, JB; and final approval of the manuscript: all authors.