Association between Postoperative Neck Pain and Intraoperative Transcranial Motor-Evoked Potential Waveforms of the Trapezius Muscles in Patients with Cervical Myelopathy Who Underwent Cervical Laminoplasty
Article information
Abstract
Study Design
Retrospective study.
Purpose
Cervical laminoplasty is safe and effective for treating cervical myelopathy but has a higher frequency of postoperative axial pain compared to other methods. Several studies have reported on the causes of postoperative axial pain, but none have fully elucidated them. This study aimed to investigate the association between postoperative neck pain and intraoperative transcranial motor-evoked potential (MEP) waveforms of the trapezius muscles using transcranial MEPs.
Overview of Literature
Few studies have investigated the association between postoperative neck pain and intraoperative transcranial MEP waveforms of the trapezius muscles in patients with cervical laminoplasty.
Methods
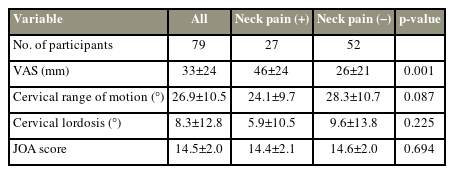
A total of 79 patients with cervical myelopathy who underwent cervical laminoplasty at our facility between June 2010 and March 2013 were included in this study. Intraoperative control and final waveform were evaluated based on the trapezius muscle MEPs by measuring the latency and amplitude. A neck pain group comprised patients with higher neck pain Visual Analog Scale scores from preoperative value to 1 year postoperatively. The cross-sectional areas of the trapezius muscles and the MEP latencies and amplitudes were compared between patients with and without neck pain.
Results
The latency and amplitude of the control waveforms were not significantly different between groups. The neck pain group had a significantly shorter final waveform latency (neck pain: 23.6±2.5, no neck pain: 25.8±4.5; p=0.019) and significantly larger amplitude (neck pain: 2,125±1,077, no neck pain: 1,630±966; p=0.041) than the no neck pain group.
Conclusions
Postoperative neck pain was associated with the final waveform latency and amplitude of the trapezius muscle MEPs during cervical laminoplasty. Intraoperative electrophysiological trapezius muscle abnormalities could cause postoperative neck pain.
Introduction
Degenerative cervical myelopathy (DCM) is the most common cause of spinal cord dysfunction in the elderly [1] and degenerative cervical spine conditions, such as spondylolisthesis, herniated discs, and posterior longitudinal ligament ossification, which cause chronic spinal cord compression. DCM is characterized by a slow progression, in stages, and worsening symptoms, such as gait abnormalities, muscle weakness, and sensory changes [2], which indicates surgical decompression to slow further neurological deterioration [3.4]. Cervical laminoplasty is a widely used surgical technique for treating DCM owing to its safe and effective posterior decompression of multistage lesions while preserving the posterior elements of the cervical spine.
Postoperative complications of laminoplasty include C5 palsy, axial pain, motor deficits, and loss of lordosis. Recent meta-analyses showed that multilevel cervical myelopathy treatment results in a significantly lower incidence of surgery-related complications following laminectomy with fusion, rather than after anterior decompression and fusion [5,6]. However, postoperative axial pain is more frequent after laminoplasty than other surgeries [6,7]. Hosono et al. [8] reported that patients who underwent laminoplasty have a significantly higher incidence of neck pain than those who underwent anterior fusion (60% versus 19%). Postoperative axial neck pain occurred in 26% of the patients who underwent laminoplasty, persisted for an average of 5.5 months, and reduced within 1–1.5 years postoperatively. Although axial pain is not a major DCM symptom, it can negatively impact the quality of life of patients; hence, preventing it may improve surgical outcomes [9].
Many studies have investigated the causes of axial pain following laminoplasty [8,10]. The muscles attached to C2 or C7, such as the posterior deep extensor and trapezius muscles, are prone to postoperative axial pain [10–12], and the motor-evoked potential (MEP) latency of the trapezius muscle is shortened in patients with cervical myelopathy experiencing neck pain [13]. However, the intraoperative relationship between the MEP waveform and the trapezius muscle, which may be a factor in postoperative neck pain, has not yet been examined.
This study aimed to elucidate the relationship between the intraoperative MEP waveforms of the trapezius muscle and postoperative neck pain. Results would provide useful information regarding surgical technique selection, preoperative planning, and postoperative rehabilitation.
Materials and Methods
1. Ethical statement
This study was conducted in compliance with the tenants of the Declaration of Helsinki and was approved by the Research Ethics and Conflicts Committee of the National Center for Geriatrics and Gerontology (approval no., 433). All the patients provided informed consent for participation in the study.
2. Patients and demographics
Patients who underwent double-door laminoplasty using hydroxyapatite spacers for cervical myelopathy at our institute between June 2010 and March 2013 were consecutively enrolled. Myelopathy diagnosis was confirmed by a thorough neurological examination and imaging that indicated spinal cord compression, which was generally associated with an intramedullary high-intensity area on T2-weighted magnetic resonance imaging (MRI). Spine surgeons in our institute determined the surgical indications based on the symptoms and the medical images of patients with cervical myelopathy.
3. Surgical procedure and apparatus
The surgical procedure for conventional double-door laminoplasty has been described in detail in a previous study [14]. Briefly, midline spinous processes were split using a high-speed burr under microscopy. Hydroxyapatite spacers (Apacerum; Asahi Optical Co. Ltd., Tokyo, Japan) were placed between the split laminae and fixed with nonabsorbable sutures to maintain an enlarged spinal canal. When performing a C7 laminoplasty, C7 lamina was resected in a dome shape and preserves the C7 spinous process. Patients wore a cervical orthosis for 2 weeks following the surgery [15].
Surgery was performed at the location of stenosis, C3–C7 level. In this study, patients who underwent C3–C6, C3–C7, C4–C6, and C4–C7 laminoplasty were included. Those who underwent prior cervical spine surgery and had missing data on MEP wave, preoperative or 1-year postoperative Visual Analog Scale (VAS) scores, or MRI were excluded.
4. Assessment and analysis
Neck pain was evaluated using the VAS scores obtained preoperatively and 1 year postoperatively. Patients with postoperative VAS scores higher than the preoperative scores were defined as having prolonged postoperative neck pain.
Trapezius muscle mass was assessed using a 1.5 T MRI scanner. Axial T2-weighted slices at the C3/C4, C4/C5, and C5/C6 intervertebral levels were obtained to measure the cross-sectional area (CSA) of the multifidus, the semispinalis cervicis, the semispinalis capitis, the splenius capitis, and the trapezius muscles using an area calculation software (SYNAPSE; Fujifilm Medical, Tokyo, Japan) (Fig. 1). One investigator (S.I.) performed the CSA measurements, who manually traced the defined region of interest within the fascial border of each muscle bilaterally on the T2-weighted images. The measurements were repeated twice, and the results were averaged. The CSAs of the right and left sides were combined for each patient. To assess the intra- and interobserver errors, two investigators (S.I. and Y.S.) independently measured five randomly chosen MR images [16]. The CSA atrophy rate of each muscle was calculated as follows: (preoperative CSA−postoperative CSA)/preoperative CSA×100.
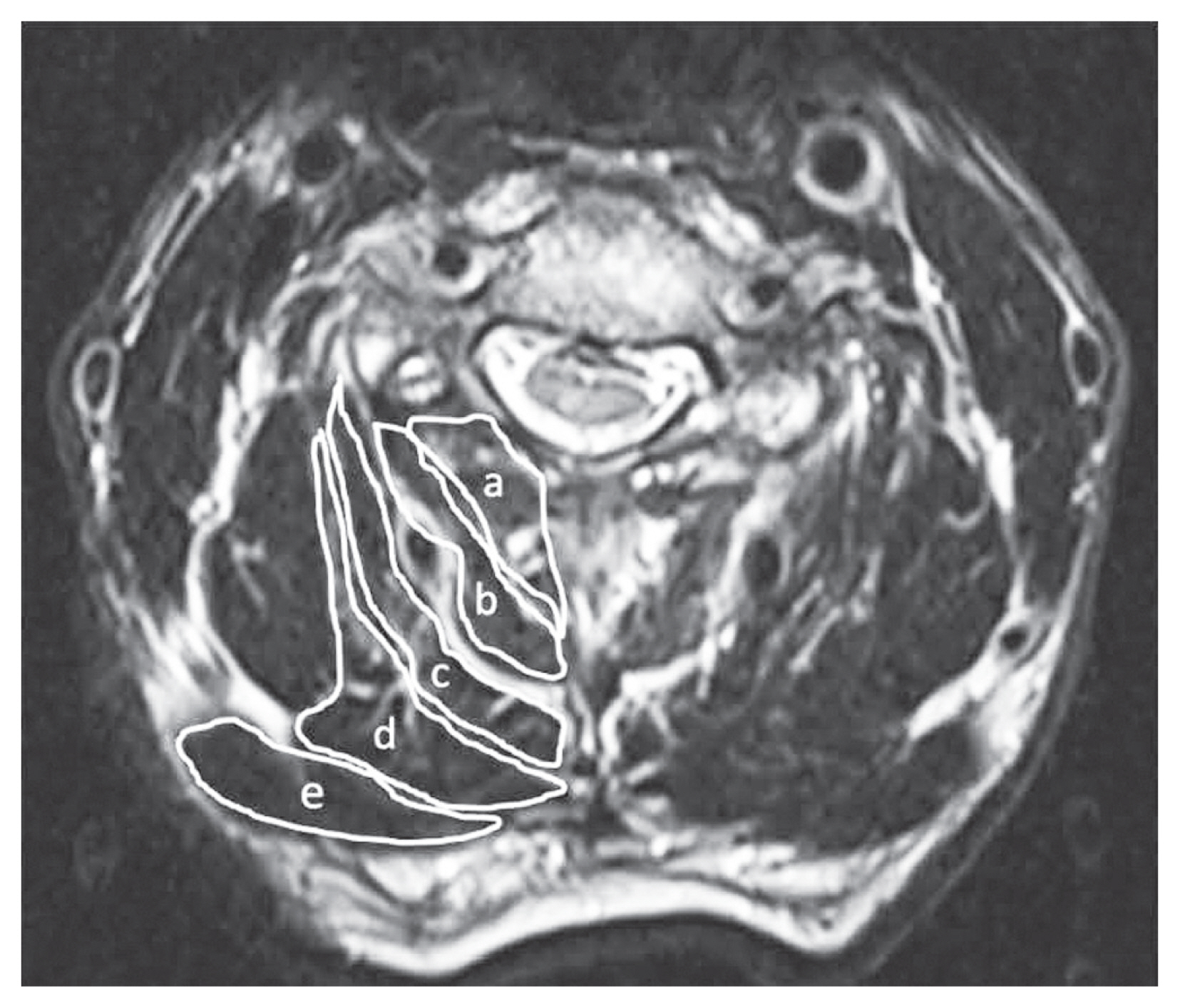
Cross-sectional preoperative magnetic resonance imaging (T2-weighted axial) of the trapezius muscles at the C3–4, C4–5, and C5–6 intervertebral levels. a, multifidus; b, semispinalis cervicis; c, semispinalis capitis; d, splenius capitis; e, trapezius.
The Japanese Orthopedic Association (JOA) score, cervical lordosis, and range of motion were assessed on the radiographs preoperatively and 1 year postoperatively. Postoperative 1-year evaluations were conducted on the same day and were defined as evaluations made within 10–14 months postoperatively (363.2±15.4 days, 312–400 days). Transcranial stimulation was performed using a D185 MultiPulse stimulator (Digitimer Ltd., Tokyo, Japan) to measure the trapezius muscle MEPs. The stimulation parameters were as follows: five consecutive stimuli with 2-ms interstimulus intervals, 500 V, a 50–1,000-Hz filter, and 100-ms epoch time with ≤20 individual recorded responses. The stimulated point was 2 cm anterior and 6 cm lateral from the Cz location (International 10–20 System) over the cerebral cortex motor area. MEPs were recorded from the bilateral trapezius muscles using the Neuromaster MEE-1,200 series (Nihon Kohden, Tokyo, Japan). To derive the compound action potential of the trapezius muscles, needle electrodes placed at the midpoint between the seventh cervical spinous process and the acromion were used [13]. The latency and amplitude were measured using the control waveform at the start of surgery and the final waveform at the end of surgery.
The drugs initially administered were propofol (3–4 μg/mL), fentanyl (2 μg/kg), and vecuronium (0.12–0.16 mg/kg). Anesthesia was maintained using propofol (3 μg/mL), fentanyl (1–2.5 μg/kg/hr), and vecuronium (0.01–0.04 mg/kg/hr). Benzodiazepines were administered as a pre-anesthetic medication but were limited or not used at all due to their suppression of the latency and amplitude. The patients were maintained under normothermia, and the anesthetist attempted to maintain end-tidal CO2 in the normal range throughout the surgery [15].
A chi-square test or Student t-test was performed to compare patient demographics, latency and amplitude of the trapezius muscle MEPs, the muscle mass of the multifidus, the semispinalis cervicis, the semispinalis capitis, the splenius capitis, and the trapezius muscles, JOA scores, cervical lordosis, and range of motion measurements on the radiographs between patients with and without neck pain. Pearson’s correlation coefficient was used to examine the relationship between the trapezius muscle mass and the latency and amplitude of the trapezius muscle MEPs. IBM SPSS Statistics ver. 22.0 software (IBM Corp., Armonk, NY, USA) was used for data aggregation and analyses, with a p-value <0.05 indicating statistical significance.
Results
A total of 79 patients who underwent surgery for cervical myelopathy between June 2010 and March 2013 were included in this study. The average age at the time of surgery was 72.6±8.6 years. A total of 23, 33, six, and 17 patients underwent C3–C6, C3–C7, C4–C6, and C4–C7 laminoplasty, respectively. Of the 79 patients, 27 and 52 were assigned to the neck pain and no neck pain groups, respectively. Sex, age, height, body weight, preoperative and postoperative range of motion, cervical lordosis, JOA score (Tables 1, 2), and the preoperative and postoperative trapezius muscle CSAs were not significantly different between groups, except for the CSA atrophy rate (%) of the trapezius muscle (neck pain group: 13.3±13.2, no neck pain group: 5.3±15.5; p=0.025) (Table 3). In addition, preoperative and postoperative muscle CSAs and the CSA atrophy rate of the multifidus, the semispinalis cervicis, the semispinalis capitis, and the splenius capitis were not significantly different (Table 4).
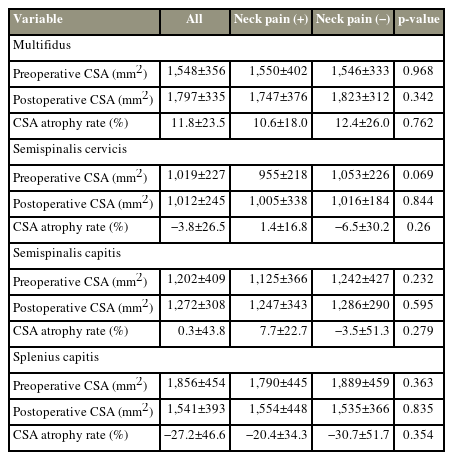
The CSA of the multifidus, the semispinalis cervicis, the semispinalis capitis, and the splenius capitis
The latency and amplitude of the control waveforms were also not significantly different between groups. However, the neck pain group had a significantly shorter final waveform latency (neck pain group: 23.6±2.5 ma, no neck pain: 25.8±4.5 ma; p=0.019) and larger final waveform amplitude (neck pain: 2,125±1,077 μV, no neck pain: 1,630±966 μV; p=0.041) than the no neck pain group (Table 5).
Furthermore, given the significant differences in the atrophy rate of trapezius muscle CSA and the final waveform latencies and amplitudes, we examined the relationship between trapezius muscle mass and the MEPs and found that the trapezius muscle mass is positively correlated to latency (Fig. 2) but negatively correlated to amplitude (Fig. 3). Finally, the estimated intra-class correlation was 0.840 and the interobserver error was 0.830, indicating that the independent findings showed good agreement.

Association between the final waveform latency and the atrophy rate of trapezius muscles (r=−0.239, p=0.034).
Discussion
In this study, patients with postoperative neck pain had shortened latency and increased amplitude in the final waveform of the intraoperative trapezius MEP. In addition, patients with postoperative neck pain experienced trapezius atrophy, which was negatively correlated with the latency and positively correlated with the amplitude of the final trapezius MEP waveform. Despite the several reports on neck pain following cervical laminoplasty [8,10], to the best of our knowledge, this is the first report on the relationship between intraoperative MEP waveform and neck pain.
Studies involving monkeys and cats have reported that cortical stimulation produces several descending volleys in the pyramidal tract at 1–2-ms intervals, and the same is expected to occur in humans. During the response latency of the cortex, at rest, the membrane potential of most anterior horn cells is low, generally 5–6 mV below threshold. Therefore, anterior horn cells are not excited unless multiple descending volleys are evoked. Firing is not possible with the first descending volley but occurs with the second and third descending volleys. In studies comparing resting and voluntary muscle contractions, the latency is shortened during voluntary contractions because during voluntary contraction, the membrane potential of the anterior horn cells is almost at threshold and can fire at the first descending volley [17]. Therefore, shortening of muscle latency may be related to an increase in the membrane potential of the anterior horn cells, which may result in stronger muscle tone than at rest. In this study, the latency was shorter in the neck pain group than in the no neck pain group. Thus, patients with neck pain may have higher MEPs of anterior horn cells and increased muscle tone. Furthermore, elevated muscle tone may be related to abnormalities in the descending control of the upper central nervous system. If so, elevated membrane potentials could lead to increased muscle tone, which in turn could also cause abnormalities in the descending control of sensation, leading to pain [18–20].
Trapezius muscle surface electromyography indicated that electrophysiological activity increases in patients with neck pain [21]. In addition, a previous study using intraoperative MEP of the trapezius muscles in patients with cervical myelopathy reported that the latency was shortened and the amplitude tended to increase in patients with neck pain [13]. In this study, the latency was shortened and the amplitude was increased in patients with postoperative neck pain, indicating that neck pain and trapezius muscle tone may have increased immediately after surgery. This may have resulted in disuse, leading to muscle atrophy. Alternatively, the shortening of the latency and increase in amplitude represent an increase in muscle activity, and since muscle activity increases with muscle injury, the degree of trapezius muscle injury at the time of surgery may have been greater in the patients with residual postoperative neck pain [22].
An imbalance between the upper and lower trapezius muscles also causes neck pain [23–26]. In this study, the upper trapezius muscle was damaged and atrophied after cervical laminoplasty, thereby increasing the imbalance leading to a greater degree of atrophy resulting in neck pain. In the MEP study, the corticospinal tract was excited and the electrophysiological activity increased when muscle damage occurred, suggesting that the electrophysiological activity increased with increasing muscle damage [27]. The results of this study showed that shortened latency and increased amplitude correlated with postoperative muscle atrophy, and the increased electrophysiological activity could represent more muscle damage.
The importance of preserving the C7 spinous process has been reported as a factor in the prevention of postoperative neck pain [12]. In this study, as we included only cases in which the C7 spinous processes were preserved, our findings suggest that preserving the trapezius muscle could be desirable in preventing neck pain following cervical laminoplasty. To preserve the paraspinal muscle, cervical laminoplasty may be performed in the same way as a lumbar spinous process-splitting laminectomy for lumbar canal stenosis [28]. Future consideration and prospective investigation of this approach are needed to reduce postoperative neck pain.
This study has certain limitations, including the anesthetic influence on the MEP wavelengths, as the anesthetic could not be completely withheld. However, an intraoperative total propofol dose >1,550 mg influenced waveforms [29], suggesting that the anesthetic influence on the waveforms in our study was minimal. The innervation of the trapezius muscle is C3 or C4, and the MEP of the trapezius muscle may be affected by stenosis at C2/3 and C3/4; however, the effect of stenosis in that area was not investigated in this study. We believe that our axial pain assessment was inadequate based on several factors, including cervical pathology and psychological issues. A 1-year postoperative follow-up period was considered short. Despite the variations in the number of cervical laminae that required surgical intervention, the surgical procedure was uniform. Although the effect of cervical alignment may cause differences in the muscle CSA in each individual, the atrophy rate was calculated considering the individuals’ pre- and postoperative comparisons, which were corrected for cervical alignment.
Conclusions
Postoperative neck pain following cervical laminoplasty was associated with electrophysiological abnormalities in the final waveform of the trapezius MEP and trapezius atrophy. The final waveform latency and amplitude of the trapezius muscle MEP were also correlated with postoperative trapezius atrophy. Thus, intraoperative electrophysiological abnormalities of the trapezius muscles could induce postoperative neck pain and trapezius muscle atrophy.
Acknowledgments
The authors would like to thank the medical engineering division in national center for geriatrics and gerontology for technical support.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
SI (first author) conceived and designed the study. SI (first author), Y.S., and A.H. performed the research. All authors analyzed the data. Y.S. contributed new methods and models. SI (first author) wrote the paper. All authors approve of the final version of the manuscript.