Management of Osteoporotic Vertebral Fracture: Review Update 2022
Article information
Abstract
A vertebral fracture is the most common type of osteoporotic fracture. Osteoporotic vertebral fractures (OVFs) cause a variety of morbidities and deaths. There are currently few “gold standard treatments” outlined for the management of OVFs in terms of quantity and quality. Conservative treatment is the primary treatment option for OVFs. The treatment of pain includes short-term bed rest, analgesic medication, anti-osteoporotic medications, exercise, and a brace. Numerous reports have been made on studies for vertebral augmentation (VA), including vertebroplasty and kyphoplasty. There is still debate and controversy about the effectiveness of VA in comparison with conservative treatment. Until more robust data are available, current evidence does not support the routine use of VA for OVF. Despite the fact that the majority of OVFs heal without surgery, 15%–35% of patients with an unstable fracture, persistent intractable back pain, or severely collapsed vertebra that causes a neurologic deficit, kyphosis, or chronic pseudarthrosis frequently require surgery. Because no single approach can guarantee the best surgical outcomes, customized surgical techniques are required. Surgeons must stay current on developments in the osteoporotic spine field and be open to new treatment options. Osteoporosis management and prevention are critical to lowering the risk of future OVFs. Clinical studies on bisphosphonate’s effects on fracture healing are lacking. Teriparatide was intermittently administered, which dramatically improved spinal fusion and fracture healing while lowering mortality risk. According to the available literature, there are no standard management methods for OVFs. More multimodal approaches, including conservative and surgical treatment, VA, and medications that treat osteoporosis and promote fracture healing, are required to improve the quality of the majority of guidelines.
Introduction
The incidence of osteoporotic vertebral fractures (OVFs) has risen along with the elderly population’s longer life expectancy. The most typical osteoporotic fracture is a vertebral fracture. OVFs are commonly treated conservatively; nonetheless, it can be difficult to manage complicated cases that require surgery. OVFs can lead to poor activities of daily living (ADL), subsequent fractures (which are four times more common), pulmonary problems (which are three times more common), and increased mortality (15% increase) [1-4]. The presence of an OVF is a major predictor of morbidities, such as back pain, spine deformities, and a decline in quality of life (QOL) [5]. The clinical practice guidelines (11 recommendations) were developed by the American Academy of Orthopedic Surgeons; there was only one strong and one moderate evidences; the remaining nine recommendations were weak or inconclusive [6]. A 2017 review of clinical recommendations for OVF found that diagnostic and treatment advice was frequently contradictory. Because there are now few studies with level I evidence available for review and few of the best OVF management guidelines, more work is needed to raise the standard of the majority of guidelines [7-11]. The purpose of this review was to provide an up-to-date summary of the available evidence on OVF management. Because they are the most common, thoracic and lumbar OVFs were the focus of this review. Cervical OVFs were excluded from this review.
Conservative Treatment
The acute pain of a new OVF is usually relieved after 6–12 weeks [12]. The most common course of treatment for a patient with an acute OVF is a conservative manner. It focuses on pain management through short-term bed rest, analgesic medicine, anti-osteoporotic medication, exercise (physiotherapy), and a brace (spinal orthosis). Because conservative treatment is highly beneficial and should be actively practiced in general, it has remained a successful primary therapeutic approach even if there are no conclusive results.
1. Pain management
Analgesic medicine is the first line of treatment. Nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, and anti-osteoporotic drugs are among the medications on the list [13]. NSAID use has linked to slowed bone healing, which increases the likelihood of nonunion, according to a number of meta-analyses and cohort studies [14]. Nonetheless, no definitive conclusions have been reached. Opioids such as oxycodone can be combined with paracetamol for patients who do not respond well to first-line pain relievers. Opioids not only have a significant impact on the management of acute pain but also have significant adverse effects (AEs), including addiction, decreased gastrointestinal motility and respiratory function, cognitive impairments with a corresponding increase in falls, and depression [15]. Anti-osteoporotic medications may be used to treat pain in OVF patients. It contains anabolic substances and conventional antiresorptive drugs, such as intravenous teriparatide (TPD) and bisphosphonates (BPs) [16]. TPD, an injectable form of parathyroid hormone (PTH), has been shown in meta-analyses to significantly reduce back pain, improve bone mineral density (BMD), and lower the risk of subsequent fracture [17]. Although it is recommended, there is little evidence that calcitonin is effective for treating persistent back pain in recent OVF patients [18].
2. Brace (spinal orthosis)
Patients with OVF should wear traditional three-point contact braces, hyperextension orthoses, a Jewett brace, or thoracolumbar sacral orthoses (TLSO) [19,20]. There are numerous advantages to using a brace, including being less invasive, relatively safe, and inexpensive. The goals of the braces are to promote fracture healing by stabilizing, to allow for faster mobilization, to reduce pain and fatigue, and to prevent postural forward flexion (Fig. 1) [19,21]. A TLSO was found to have significant effects on trunk muscle strength, posture, QOL, ADL, and pain in one prospective randomized study [22]. According to a recent systematic review, spinal orthoses significantly improved functional outcomes in neurologically intact patients 60 years of age and older, reducing kyphotic deformity, improving postural stability, and increasing muscular strength [23]. However, studies in patients with non-OVFs provide evidence for the effectiveness of a spinal orthosis [24]. Inadequate immobilization, sores, decreased pulmonary function and compliance, and core muscle weakness are drawbacks of spinal orthoses [25]. Clinicians lack sufficient information regarding the particular type of brace, indications, and time to remove [21]. Additionally, a number of papers claimed that there is poor compliance and wide variation in the use of spinal orthoses [26]. The strength of recommending spinal orthoses to patients with OVF remained uncertain as a result of the paucity of high-quality evidence [6,19].
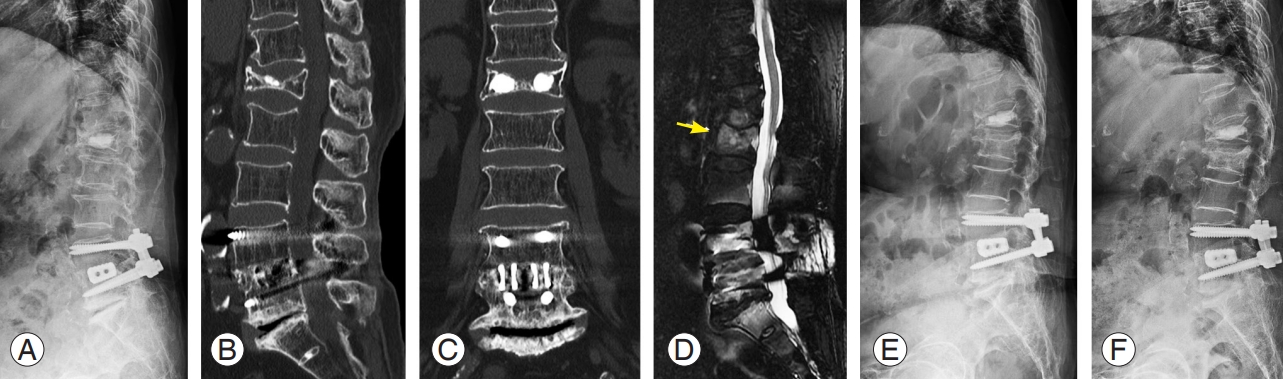
An 84-year-old female patient visited the emergency room complaining of back pain after lifting and carrying light objects indoors. (A–C) She went home after pain control because no obvious fractures were observed on the plain radiograph and computed tomography images except for old healed fractures and implants due to spinal surgery. (D) She revisited the hospital as an outpatient clinic and underwent a magnetic resonance image for persistent back pain. The sagittal image (fat-suppressed T2-weighted) shows bone marrow edema (yellow arrow) in the L2 vertebral body. (E) Further collapse was observed on the plain radiograph 1 month after injury. (F) Conservative treatment using a hard brace and teriparatide (daily injection) was performed, and no additional body height loss was observed on the plain radiograph at 2 months after injury.
3. Exercise (physiotherapy)
After the acute pain subsides, core muscle strengthening exercises are frequently recommended to reduce chronic pain, improve posture and gait, improve QOL, and strengthen the back extensors [27]. Additionally, it might reduce edema, the need for painkillers, and the danger of further falls and fractures. Continuous physical activity was linked to a lower risk of osteoporotic fractures in a nationwide population-based cohort study [28].
A tailored rehabilitation program based on balance and muscle strength tests has recently been proposed as an effective treatment option for basic motor function improvement and disability reduction [29,30]. A retrospective observational study found that compliance with a home exercise program was 62.86%, with several causes of non-compliance, including the absence of supervision by health personnel and a lack of motivation [31]. The use of exercise is still controversial, and more research is needed to maximize its clinical relevance [20]. According to a 2018 Task Force Report from the American Society for Bone and Mineral Research (ASBMR), exercise may increase mobility, lessen discomfort, and reduce fear of falling. However, they also mentioned that it is unclear whether exercise lowers falls, strengthens back extensors, and improves balance [32].
Vertebral Augmentation
There have been numerous studies published on vertebral augmentation (VA), which includes kyphoplasty (KP) and vertebroplasty (VP). Fourteen randomized controlled trials investigating the role of VA have been published and over 4,000 articles on VP alone. Despite this, there is still disagreement regarding the effectiveness of VA. Although reaching a firm conclusion is challenging, it is widely accepted that the VA group demonstrated a pain-reduction effect when compared with the control group during the acute phase, but there was no discernible difference in the long-term follow-up results. VA has several advantages, including local anesthesia, mechanical stabilization with cement injection, and analgesic effect from the thermal reaction of polymethyl methacrylate (PMMA) cement [33]. According to several studies, treating local kyphosis and relieving pain with VA may have significantly improved sagittal imbalance [34]. The position statement was presented by American Society spinal intervention groups, which also emphasized the multiple AEs of bed rest, including muscle weakness, pressure sores, and deep vein thrombosis [35]. Patients with osteoporotic burst fractures (OBF) are the subject of several studies. VA reported one case of a patient with OBF who had a poor general condition but no neurologic deficit and received a satisfactory outcome [36]. Short segment fixation combined VA (hybrid procedure) has been introduced as a different treatment approach for OBF [37]. However, because these studies have limited surgical indications, then VA should be performed through a comprehensive evaluation for vertebral instability to avoid serious complications. Several papers proposed VA as an alternative treatment option for intravertebral cleft or vertebral osteonecrosis, but a large number of other studies reported a high failure rate (Fig. 2) [38]. An intravertebral cleft or vertebral instability may be the primary cause of a delayed neurologic deficit and a pain determinant. These results suggest that stabilization therapy has important implications [39]. VA recently showed positive outcomes in mid and long-term periods, and it has been demonstrated to be a cost-effective alternative to conservative treatment in studies conducted in a variety of healthcare settings [40,41]. A meta-analysis suggested facet joint block may be considered complementary to VA in the management of residual back pain, but it may not be effectively used as an alternative therapy [42]. Despite numerous studies pointing to the benefits of VA, there is high heterogeneity across each of these trials, which is the main reason why the evidence is not completely accepted [43]. As a result, clinicians found it challenging to apply the findings to standard clinical practice.
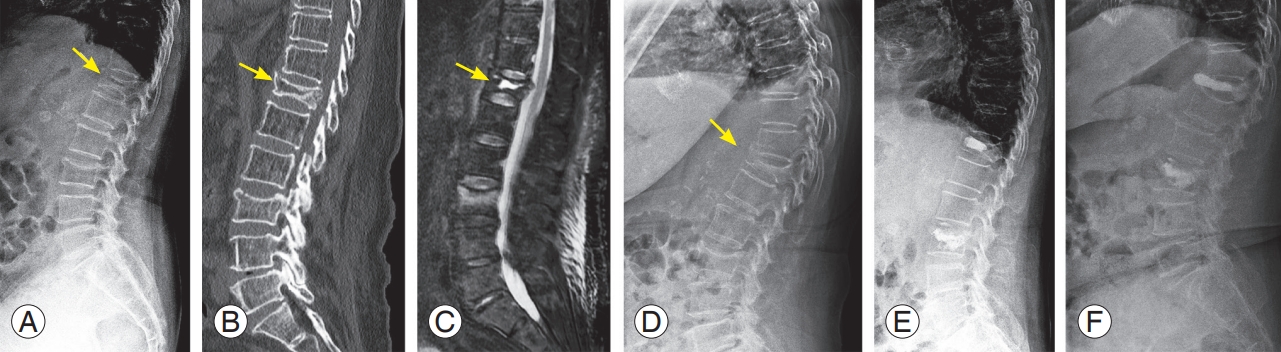
A 79-year-old female with multiple osteoporotic vertebral fractures at T12 and L3. (A) Plain radiograph in supine position shows a decreased body height at L3 and intravertebral cleft at T12 (yellow arrow). (B) Sagittal computed tomography image displays vertebral body bone defect at T12 (yellow arrow). (C) Sagittal magnetic resonance image (fat-suppressed T2-weighted) show intraosseous fluid collection (yellow arrow). (D) Plain radiograph in a standing position shows a significantly collapsed body at T12 (yellow arrow). (E) The patient was treated by kyphoplasty using a balloon at T12 and vertebroplasty at L3. (F) After falling again two months after surgery, the patient suffered a re-fracture at T12, cement dislocation and severe body re-collapse were observed.
Although VA has low reported complication rates in general, it is important to discuss the serious nature of these problems (2% in KP and 3.9% in VP) [44]. The likelihood of major AEs, including infection, neural tissue damage, thecal sac compression, pulmonary embolism, and respiratory failure, was identified in five trials (821 VP cases). Cement extravasation is a common severe complication. In 473 VA cases, 87.5% for VP and 49.2% for KP were found [45]. In comparison with 1.6% to 3.0% for VP, two meta-analyses determined that the symptomatic leakage rate for KP is between 0% and 0.3% [46]. Precautions have been suggested to reduce the risk of cement leakage, including (1) careful preoperative evaluation, (2) a total cement injection volume less than or equal to the void left by the balloon, (3) a small volume of cement (0.2–0.5 mL) each time, (4) regular evaluation by fluoroscopic imaging, (5) use of high-viscosity cement in a doughy state, and (6) injection time of 3–4 minutes after cement mixing [36].
Re-fractures (VA index level) or subsequent fractures (adjacent level) have also been mentioned as a cause for concern [47]. Risk factors for index level re-fracture include intravertebral cleft and severe kyphosis, increased psoas muscle fatty infiltration, thoracolumbar level, solid lump cement distribution pattern, and higher restoration of body height [48,49]. A common complication is adjacent-segment fracture (ASF), which has a risk of 2% to 23% in KP and up to 52% in VP. The majority of ASFs were observed within two months of VA [50]. The following are hypotheses regarding the potential causes of the rising ASF rate. By increasing the stiffness of the cemented vertebra, it is possible to produce 35 times harder and 12 times stiffer than those in the control group. Unusual loading distribution can result in a 13%–18% increase in adjacent-level pressure [51]. Until recently, the impact of VA on later ASF was not well understood. According to some authors, restoring sagittal balance and physiologic loading by VA may reduce ASF, which was primarily caused by underlying osteoporosis and altered mechanical load caused by spinal deformity [52]. Although this is still debatable, it is possible to conclude that VA has no effect on the likelihood of future fractures. To overcome ASF, prophylactic VP for the adjacent level was attempted. It was suggested that adjacent levels be given a mechanical property gradient by injecting cement based on the findings of various investigations [53,54]. However, its preventive effects have not been completely confirmed.
After randomized studies were published in 2009 demonstrating that the VA was not superior to a sham treatment [8,9], numerous studies have been published that contradict the positive effects of VA. There have also been debate and worldwide concern about study design and results [55]. Because of the controversy surrounding its use in OVFs, the 2018 ASBMR Task Force Report stated that existing research does not support the routine use of VA. Patients should be made aware of the evidence when it is presented [32]. Based on the findings to date, the impact of VA cannot be determined definitively. Only in a small percentage of non-responders to conservative treatment is VA performed properly to anticipate early pain management while accounting for major complications. Because the potential advantages of VA are unknown until more robust data are available, they should not be routinely provided to OVF patients.
Surgical Treatments
Approximately 15%–35% of patients will experience persistent pain, decreased pulmonary function, spinal deformity, and neurological deficits that will necessitate surgical intervention [56,57].
1. Surgical indication
Surgery is recommended for individuals who have significant vertebral instability (unstable fractures), clinical symptoms (persistent intractable back pain or neurological deficit), and radiological deformity (kyphosis or pseudarthrosis). The incomplete or delayed neurological deficit is believed to be the result of progressive kyphosis or dynamic instability, which repeatedly causes microtrauma [58,59]. Dynamic MRI can be a useful tool in making an accurate diagnosis for these patients [60].
2. Surgical methods
Because perioperative complications and implant failures were observed in 18.1% and 41.2% of cases, respectively, specific surgical approaches for OVF are required [61]. The following surgical fusion methods are commonly used: anterior spinal fusion, posterior spinal fusion, combined anterior and posterior spinal fusion, posterior three-column osteotomy with shortening osteotomy or vertebral column excision, and VP with posterior spinal fusion (Fig. 3). All five methods produced comparable neurological recovery, functional improvements, and complication rates [62]. Because the load-sharing concept can cause an implant failure in a flexion moment during a standing or sitting position, longer instrumented fusion constructs are required in the posterior alone instrumentation, along with pedicle screw fixation (PSF) and more anchors [62]. Recent reports from several authors describe so-called hybrid stabilization, a minimally invasive fixation for OBF that combines KP [63,64].
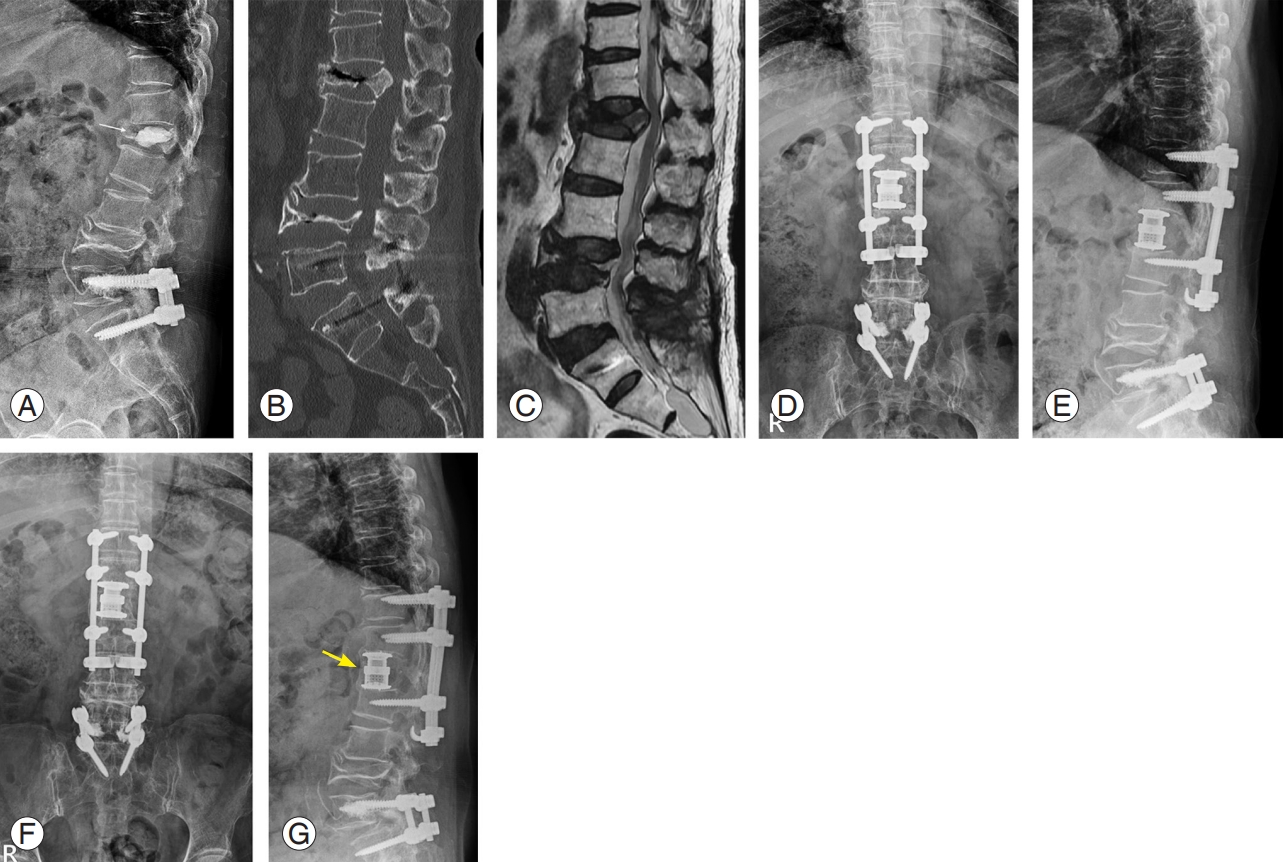
A 64-year-old female with an osteoporotic burst fracture at L1. She was treated by vertebral augmentation at another hospital; however, she complained of persistent back pain and gait disturbance. (A) Plain radiograph shows a decreased body height and cement leakage into intervertebral disc space at L1 (yellow arrow). (B) Sagittal computed tomography image display a nonunion and fragment retropulsion into the spinal canal. (C) Sagittal magnetic resonance image (T2-weighted) of lumbar spine displays a compressed spinal cord at L1 (yellow arrow). (D, E) We performed combined anterior and posterior spinal fusion including anterior corpectomy, expandable cage insertion, and multilevel pedicle screw fixations with sublaminar hooks. (F, G) Plain radiographs show the stable status of instruments and complete anterior bony bridge (yellow arrow).
3. Surgical strategies and techniques
1) Screw characteristics
In the general population, a larger screw diameter can increase pullout strength [65]. However, osteoporotic bone conditions should be considered when performing PSF in OVF patients. The thin cortex of the pedicle in OVF patients can negate the enhanced fixation strength provided by larger diameter screws and increase the risk of pedicle fracture if the screw diameter is greater than 70% of the pedicle diameter [66]. Increasing screw length improves screw pullout strength, though this effect may be less pronounced in OVF patients [67].
2) Screw fixation techniques
Superior fixation strength and resistance to screw pullout may be provided by pedicle screws (PS) when they are inserted with a triangulation trajectory and engaged subchondral bone [68]. According to some authors, a minimum of three fixation points should be placed above and below the deformity’s apex [69]. The ideal fusion length, meanwhile, is still up for debate. When the screw is under-tapped (1 mm), the pedicle can help improve screw purchase [70].
3) Bone–screw interface
Because the bone–screw interface is critical for preventing screw pullout, expandable PS and VA have been the subject of extensive research [71,72]. Representative materials include PMMA bone cement and hydroxyapatite cement (HAC). PMMA bone cement has a two-fold to three-fold improvement in pullout strength [73]. Surgeons, like VA personnel, must be aware of the risks associated with PMMA cement leakage. Although there has not been enough research done on HAC augmentation in OVF patients, it has the potential to be a safe and efficient replacement for PMMA. The disadvantage of non-PMMA cement is that it takes 4–24 hours to reach maximum stiffness, whereas PMMA reaches stiffness immediately. Expandable PS, such as an expansion peg (a smaller gauge screw), is expected to be one of the novel techniques to improve the bone–screw interaction. It moves into the slotted area of the screw, expands, and spreads, causing the screw diameters to rise and the pullout strength to double [74]. More controlled trials and comparative studies are needed to reach a firmer conclusion.
4) Sublaminar wire and hooks
Combining PS and additional offset sublaminar hooks, also known as pediculolaminar fixation, can increase stiffness and pullout strength by up to 100% [75]. PSF should not be used in patients with BMD less than 0.3 g/cm2 in a biomechanical investigation [76]. The cortices of the laminae are significantly more powerful than the marrow within pedicles in OVF patients. Laminae also have a higher proportion of cortical bone than cancellous bone, making them less susceptible to osteoporosis [77]. Although spinal loop rectangle and sublaminar wiring construct are viable options for stabilizing OVF, sublaminar hooks are believed to be more resistant to posteriorly directed stresses. Hooks, however, should not be used as the sole means of fixation.
5) Supplementary interbody fusion
Lumbar interbody fusion may result in anterior column support. To avoid cage subsidence, endplate damage, delayed fusion, or pseudarthrosis, meticulous and thorough cartilaginous endplate removal is crucial. A suitable-sized interbody spacer or cage and enough amount of bone graft are also necessary for a successful fusion [78].
6) Overall guidelines
Other methods proposed by several included the use of bicortical screws, cross-links, various fixation devices, transverse connectors, and modified screw designs and trajectories (Table 1) [79,80]. In addition to the methods mentioned above, Hu [81] provided additional rules to increase the rigidity of the construct. To avoid hardware pullout from excessive corrective forces, avoid the following: (1) terminating anterior instrumentation within the kyphotic segment, (2) accepting an incomplete correction of the deformity, and (3) penetrating the contralateral vertebral body cortex when performing anterior instrumentation. A comprehensive review published in 2020 provided an overview of advancements in osteoporotic spine fixation [82].
7) Surgical outcomes and the prognosis
Although there are numerous surgical procedures, it is too difficult to obtain positive results in OVF patients. The sagittal balance that had been restored following surgery could not always be kept. Patients with Parkinson’s disease or rheumatoid arthritis frequently showed significant correction loss during follow-up (recurrence of severe local kyphosis or vertebral collapse that existed before surgery) [83]. The moderate-to-severe grade of preoperative neurological deficit, perioperative morbidity, and lack of postoperative PTH administration were strongly associated with postoperative impaired ADL [84,85]. Because no single method can guarantee the best surgical outcomes in OVF patients, customized surgical approaches are required. Surgeons must stay current on developments in the osteoporotic spine field and be open to new treatment options.
Medical Treatment
1. Anti-osteoporosis drugs
Preventing and treating osteoporosis is the most important aspect of managing OVF. Baseline treatments (calcium and vitamin D), conventional medications (BP and selective estrogen receptor modulators), and newer drugs (denosumab and TPD) can all be used to reduce the number of subsequent vertebral fractures.
2. Fracture healing
In terms of biology, BP causes a delay in maturation during endochondral repair, which results in a less developed fusion mass and a marked reduction in the union, as well as an increase in fracture callus size [86]. According to the author, BP may inhibit bone remodeling and maturation during fracture healing. However, a 1-year study of 40 osteoporosis patients found that BP therapy increased the rate of interbody fusion [87]. A meta-analysis and a systematic review reported that the clinical effects of BP on the healing of fractures and spinal fusion have not been conclusive [88].
Intermittent injection of TPD (recombinant human PTH 1–34) stimulates bone formation by stimulating osteoblast proliferation, inhibiting osteoblast apoptosis, and increasing osteoblast activity [89]. TPD greatly improved fusion and fracture healing in an animal study, and it has been observed that patients with OVF who receive conservative treatment with TPD can anticipate outcomes that are on par with those of VA treatments. The TPD group had a much greater 6-month union rate than the BP group, according to a retrospective comparison study, which raised the possibility that TPD might promote the healing of OVF fractures [90]. In reducing mechanical problems following posterior instrumented fusion for OVF, TPD outperformed BP [91].
Advanced Modality
It has been reported that sarcopenia and osteoporosis play an important role in the treatment of OVF. Several studies reported a significant correlation between vertebral instability and cross-sectional area (CSA) of erector lumbar muscles [92]. In a study on subsequent OVF, the most important issue in OVF management, CSA of the psoas muscle could be used as a standalone diagnostic tool of sarcopenia, and sarcopenia was an independent risk factor for subsequent OVF after VA [93]. These studies emphasized that muscle status, such as sarcopenia, has a superior meaning than traditional BMD [92,93]. Sarcopenia was also associated with decreased implant longevity and was identified as the primary cause of implant failures and complications in OVF patients treated with spinal fixation. The authors concluded that measuring the skeletal muscle area using an axial computed tomography of the lumbar spine might help to prevent implant-related complications via early detection and treatment of sarcopenia [94].
The role of artificial intelligence in the management of OVF is also gradually expanding. In the medical imaging field, a deep learning approach based on convolutional neural network (CNN) has gained attention, and the constructed model demonstrated high sensitivity, specificity, and accuracy with an area under the curve (AUC) of 0.949 in detecting acute OVF using magnetic resonance images. This study concluded that the performance of the CNN was comparable with that of two spine surgeons [95]. Machine learning algorithms can also be used to predict nonunion after OVF. The authors proposed extreme gradient boosting (AUC=0.845) and random forest (AUC=0.860) models as more effective predictors with good performance than conventional methods [96].
Conclusions
According to the available literature, there are still no standard methods for managing OVFs. The majority of guidelines should be improved, which will necessitate increased efforts using multimodal strategies, such as conservative and surgical treatment, VA, and medications that treat osteoporosis and promote fracture healing.
Notes
This manuscript does not provide information regarding medical devices or drugs. The first author (H.D.J.) has received research support funding from the Soonchunhyang University Research Fund. Except for that, no potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: HDJ, EHK, JCL, BJS; data curation: HDJ, EHK; formal analysis: JCL; funding acquisition: HDJ, SWC; methodology: HSK; project administration: JSC; visualization: BJS; writing–original draft: HDJ, JCL; writing–review & editing: EHK, BJS; and final approval of the manuscript: all authors.
Acknowledgements
This research was funded by the Soonchunhyang University Research Fund.

