Adult Spinal Deformity: A Comprehensive Review of Current Advances and Future Directions
Article information
Abstract
Owing to rapidly changing global demographics, adult spinal deformity (ASD) now accounts for a significant proportion of the Global Burden of Disease. Sagittal imbalance caused by age-related degenerative changes leads to back pain, neurological deficits, and deformity, which negatively affect the health-related quality of life (HRQoL) of patients. Along with the recognized regional, global, and sagittal spinopelvic parameters, poor paraspinal muscle quality has recently been acknowledged as a key determinant of the clinical outcomes of ASD. Although the Scoliosis Research Society-Schwab ASD classification system incorporates the radiological factors related to HRQoL, it cannot accurately predict the mechanical complications. With the rapid advances in surgical techniques, many surgical options for ASD have been developed, ranging from minimally invasive surgery to osteotomies. Therefore, structured patient-specific management is important in surgical decision-making, selecting the proper surgical technique, and to prevent serious complications in patients with ASD. Moreover, utilizing the latest technologies such as robotic-assisted surgery and machine learning, should help in minimizing the surgical risks and complications in the future.
Introduction
Owing to the progressive population aging, adult spinal deformity (ASD) now accounts for a considerable proportion of the Global Burden of Disease [1-3]. ASD is a complex spectrum of disorders primarily affecting the lumbar and thoracolumbar spine that cause deformities in the coronal and sagittal planes [1,4]. The prevalence rate of ASD is higher in individuals aged >60 years. Multiple agerelated factors like reduced bone mineral density (BMD), spinal degeneration, restricted mobility, and imbalanced gait posture contribute to the progression of ASD [1].
ASD such as lumbar degenerative scoliosis, dynamic sagittal imbalance (DSI), and iatrogenic flat back can debilitate the patients by disrupting the structural support of the spinal column [5]. Structural imbalance caused by age-related pathological and asymmetrical load-bearing and degeneration or collapse of the motion segments leading to back pain or neurological deficit and deformation were shown to adversely affect the health-related quality of life (HRQoL) [1]. Furthermore, ASD can exacerbate the disability in patients with comorbid conditions such as hypertension, diabetes, or mental disorders [1,6].
Owing to the complexity of ASD, the surgical procedure for its treatment should be decided while considering various factors such as perioperative mobility of the patient and cost of the surgery [6]. Previously, conservative treatment was the mainstay for treating ASD due to the challenges involved in surgical intervention; however, the new concept of spinopelvic alignment, focus on improving the HRQoL, and recent technical advances have made surgery the predominant treatment method for ASD [7]. Nonetheless, clinical obstacles such as postoperative mechanical complications, neurovascular injuries, and pseudoarthroses still persist. Many state-of-the-art techniques, from minimally invasive surgeries to artificial intelligence (AI) methods for predicting complications, have been developed for the treatment of ASD. Herein, we comprehensively discuss the current advances and future directions for ASD treatment.
Prevalence and Etiopathogenesis of Adult Spinal Deformity
The term ASD encompasses a broad spectrum of conditions that may cause abnormal spinal alignment, pain, disability, neurological deficit, and/or functional loss [1]. Spinal malalignment is defined as the abnormal spinal curvature in any of the axial, coronal, and/or sagittal planes [4,8]. These abnormal curvatures include scoliosis in the coronal plane, kyphosis or lordosis in the sagittal plane, and kyphoscoliosis in both the coronal and sagittal planes [8-10].
The reported prevalence of ASD ranges between 2% and 32%, and ASD affects up to 68% of the elderly population [11]. ASD has a negative impact on the HRQoL of elderly patients and its prevalence is increasing due to demographic shift, increased life expectancy, and effective diagnosis of the disorder [12,13]. The physical burden of ASD is higher in the general population than the other chronic conditions, like arthritis, diabetes mellitus, congestive heart failure, and chronic lung disease [14].
Degeneration of the spine begins in the load-bearing structures, including intervertebral disks and facet joints [15]. Age-related progression of degenerative changes in bone and soft tissue leads to radiculopathy or instability caused by rotatory subluxation or spondylolisthesis [16]. The initial process of degeneration entails microstructural and macrostructural changes in the intervertebral disk, including loss of water and proteoglycan content, increased enzyme degradation, and loss of disk height. Loss of the shock-absorbing capacity of intervertebral disks leads to pathological changes in the facet joints (facet joint arthritis) and posterior elements. This can increase the load on the anterior part of the vertebral body that contributes to abnormal bone remodeling and instability [15,16]. Abnormal bone remodeling cycles affect the quality of paraspinal muscles, which aggravates the spinal deformity (Fig. 1) [17].
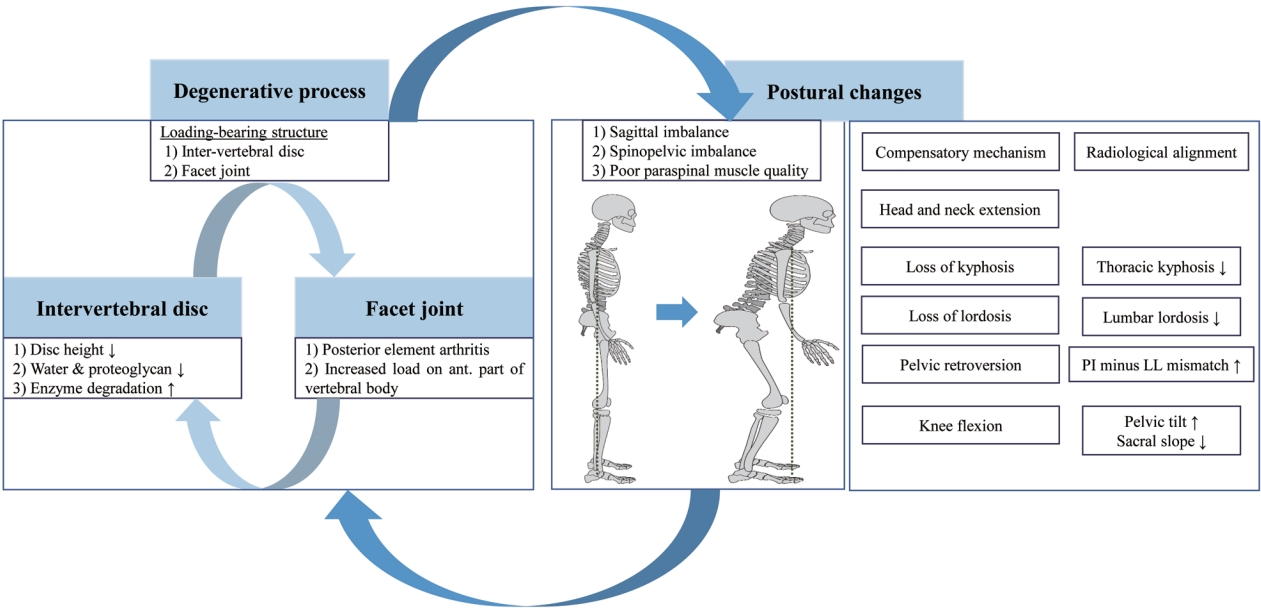
Etiopathogenesis and postural change in adult spinal deformity. The degenerative process is initiated by load-bearing structures, including intervertebral discs and facet joints, and leads to postural changes. Abnormal bone remodeling cycles then affect paraspinal muscle quality, which aggravates spinal deformity. To maintain an upright posture, compensatory mechanisms are driven by loss of lordosis (decreased lumbar lordosis) and lead sequentially to low thoracic kyphosis, pelvic retroversion (increased pelvic tilt), knee flexion, and head and neck extension. These changes present as sagittal and spinopelvic imbalance (increased pelvic incidence minus lumbar lordosis mismatch and increased sagittal vertical axis) and contribute to poor health-related quality of life.
Compensatory mechanisms, which are loss of lordosis and pelvic retroversion, are needed to counteract the pathological changes in the spine for maintaining an erect position [15,16]. Loss of lumbar lordosis (LL) is the main driver of compensatory mechanisms and is caused by age-related degenerative changes, progressive idiopathic scoliosis, and iatrogenic changes [5]. It causes the trunk to tilt forward and creates sagittal imbalance that is depicted in radiographs as an increase in pelvic incidence (PI) minus LL (PI–LL mismatch) and the sagittal vertical axis (SVA) [18,19]. Loss of LL is followed by sagittal imbalance that involves thoracic spine straightening (loss of thoracic kyphosis [TK]), pelvic retroversion, knee flexion, and a backward tilt of the head and neck to maintain a level gaze (Fig. 1). Sagittal imbalance is associated with pain, disability, and poor HRQoL [13]. Coronal imbalance may also be correlated with back pain and generally results in an undesirable appearance [4]. Although, the surgical treatment for ASD currently focuses on correcting the sagittal imbalance, an ideal surgical intervention should consider both sagittal and coronal parameters.
Radiological Parameters of Adult Spinal Deformity
The role of proper alignment of the spine is to maintain an upright posture, protect neural elements, and maintain axial skeletal stability. Radiological assessment plays an important role in clinical decision-making while identifying the cause of pain and disability, predicting prognosis, and planning surgical treatment. Therefore, it is important to capture radiographic images with the patient in the correct posture: (1) whole-spine anterior-posterior (AP) and lateral views, a 30–90-cm vertical film at a distance of 72 inches (182.88 cm) from the subject; (2) a view showing the natural position of the knee and hip joints; and (3) an image while the patient is standing with the hands in the clavicle position [8,15,16]. Regional, global, coronal, and sagittal spinopelvic parameters can be calculated from the whole-spine AP and lateral view radiographs that are used to assess the spinal alignment of patients with ASD (Fig. 2A, B). Measurement of radiological parameters has not been standardized yet, but one of the widely used methods for analyzing the anatomical shape of the spine is summarized in Table 1.
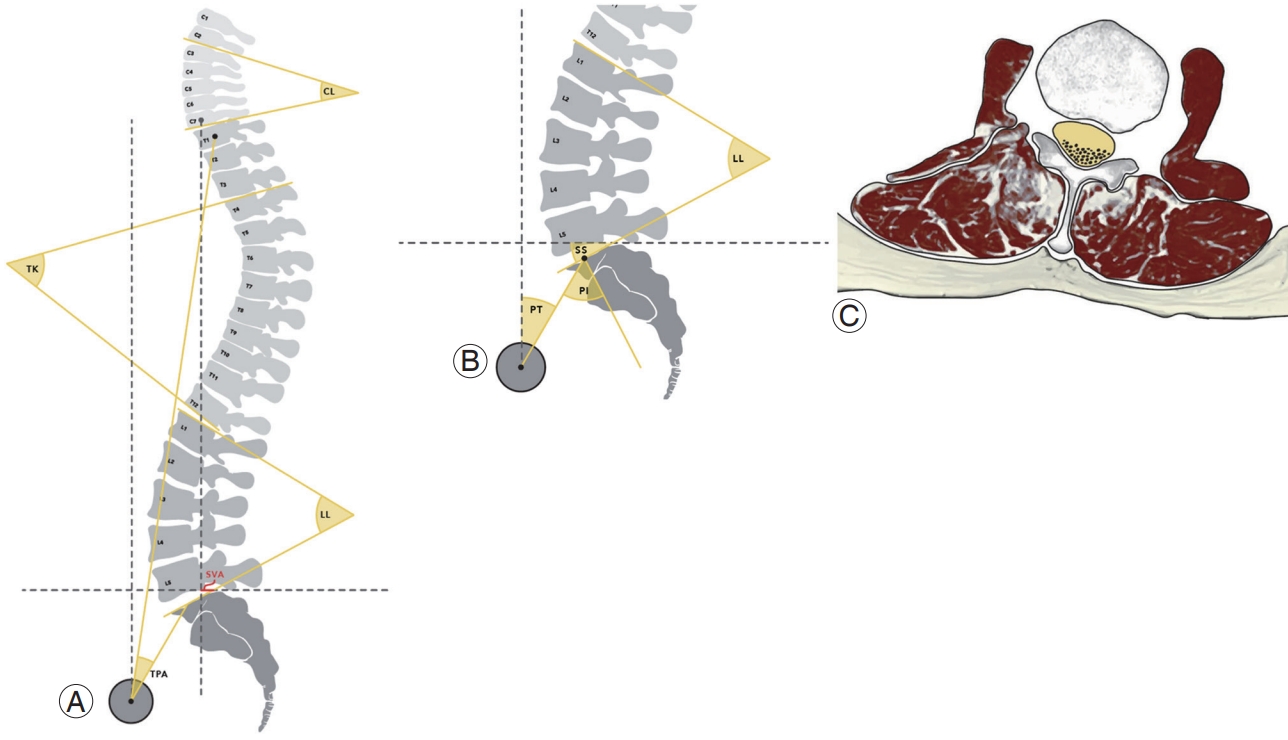
Radiological parameters of adult spinal deformity. Regional, global, and sagittal spinopelvic variables are important: (A) regional variables (cervical lordosis [CL], thoracic kyphosis [TK], and lumbar lordosis [LL]) and global variables (sagittal vertical axis [SVA] and T1 pelvic angle [TPA]); (B) sagittal spinopelvic variables (pelvic incidence [PI], pelvic tilt [PT], and sacral slope [SS]). (C) Poor paraspinal muscle quality caused by fatty infiltration is another important radiological parameter that contributes to sagittal imbalance.
Several methods to evaluate sagittal imbalance have been studied. A study by Shiba et al. [20] showed the importance of the three-dimensional gait analysis in evaluating degenerative kyphotic change and identifying DSI in patients with ASD. Bae et al. [21] recommended that radiographs for DSI evaluation should be performed after the patient has walked for 10 minutes in order to obtain static images capturing the effects of patient compensation. In recent years, low-dose radiation and weightbearing full-body X-ray imaging (EOS imaging) is used to overcome the limitations of whole-spine AP and lateral view radiographs [22]. When the anterior and lateral images are captured in an EOS system, three-dimensional reconstructions of the vertebral body are made to eliminate the distortions caused by pelvic rotation during image acquisition [23].
Many multicenter prospective studies have suggested that the spinopelvic parameters, including pelvic tilt (PT), sacral slope (SS), and PI, act directly on the lumbar curve and orientation because the LL and spinopelvic parameters are correlated [19,24,25]. Therefore, it is crucial to understand the relationship among the spinal parameters for evaluating the pathological conditions of the areas between the spine and pelvis. Sagittal spinopelvic parameters are routinely evaluated in patients with ASD because they lead to to pain, disability, and poor HRQoL of the patients. Schwab et al. described the thresholds for these parameters (PT >22° and PI–LL mismatch >11°) based on clinical outcomes [24]. Similarly, surgical restoration of the PI was found to affect the pelvis and lumbar curvatures and orientations; therefore, these parameters must be evaluated during preoperative and postoperative radiological examinations [13].
The role of the paraspinal muscles in ASD has received special attention because of the recent findings on sagittal alignment [26]. Poor paraspinal muscle quality can lead to sagittal imbalance with a greater influence than sarcopenia, and it negatively affects the HRQoL [17]. The main role of the paraspinal muscles, especially the multifidus and psoas muscles, is to stabilize the spinal column and maintain skeletal balance [21]. Degenerative changes due to fat infiltration disrupt the muscle, causing skeletal imbalance and leading to poor sagittal spinopelvic alignment (Fig. 2C). Jun et al. [27] suggested a correlation among fatty degeneration (FD) and various sagittal parameters. They found that increased FD was closely correlated with decreased LL (Pearson’s coefficient=−0.505), which was further correlated with decreased TK (Pearson’s coefficient=0.471), which in turn was correlated with increased SVA (Pearson’s coefficient=−0.283). Moreover, Miura et al. [28] found that DSI profoundly affects FD of the paraspinal muscle during gait in patients with ASD. Therefore, spine surgeons must pay careful attention to the quality of the paraspinal muscle, as well as BMD, age, pain, disability, and spinal alignment during treatment of ASD patients.
Development of an Adult Spinal Deformity Classification System
Ideally, classification systems should be able to reflect the etiopathophysiology of a condition, predict prognosis and clinical outcome, and provide the information required for clinical decision-making and patient management [1]. In 2005, Aebi [29] first proposed a classification system for ASD based on etiology that is useful for understanding the etiopathophysiology of ASD. However, an important study by Bess et al. [30] on “high-impact, clinically significant radiographic parameters” [18,19] highlighted the need for modification of this classification system. In 2012, the Scoliosis Research Society (SRS) Adult Deformity Committee reflected on the pelvic parameters that are substantially correlated with the HRQoL [13]. The resulting SRS-Schwab ASD classification is the currently used standardized and recognized system with acceptable reliability, prognostic and decision-making results [25].
The SRS-Schwab system includes a coronal curve type and sagittal modifiers (PI–LL mismatch, SVA, and PT) [1]. The coronal curve type is based on the spine location and Cobb’s angle (>30°) of the scoliotic curves, as follows: (1) curve type T: thoracic only; thoracic major curve of >30° at the apical level of T9 or higher; (2) curve type L: thoracolumbar/lumbar only; isolated thoracolumbar or lumbar curve >30° at the apical level of T10 or lower; (3) curve type D: double major curve with thoracic and thoracolumbar/lumbar curve of >30°; and (4) curve type N: normal; no definite coronal deformity (all coronal curves <30°).
Sagittal modifiers involving a PI–LL mismatch, SVA, and PT are described as follows: (1) PI minus LL mismatch: 0 (<10°), + (between 10°–20°), and ++ (>20°); (2) SVA: 0 (<40 mm), + (40–95 mm), and ++ (>95 mm); and (3) PT: 0 (<20°), + (20°–30°), and ++ (>30°).
For the sagittal modifiers, an increased PI–LL mismatch is correlated with a decreased SRS score and 12-item Short Form Survey score and increased Oswestry Disability Index (ODI) score that indicate poor clinical outcome and high disability. SVA also showed a significant positive correlation with pain and disability [30,31]. PT is a dynamic parameter that reflects compensatory pelvic retroversion to maintain an erect posture when PT >20° which correlates to disability and pain [5]. Thus, sagittal modifiers within the normal range are an important element in surgical planning for ASD. From multicenter prospective studies, Schwab et al. [13,24] suggested a predictive threshold for sagittal modifiers and clinical outcomes. They reported that a predictive ODI score of ≥40 was significantly correlated with clinical outcomes when the PI minus LL, SVA, and PT were ≥11°, ≥47 mm, and ≥22°, respectively [13]. Other studies, including those by Terran et al. [25] and Smith et al. [12], showed similar results of a strong correlation between patient-reported surgical outcomes and radiological parameters.
The SRS-Schwab system has merits in predicting patientreported clinical outcomes but has some limitations [19]. First, it cannot help in selecting the surgical technique or determining the fusion level in detail. Second, age and agerelated factors are not considered in the SRS-Schwab classification system. Third, the preventive radiological threshold for mechanical complications that require revision surgery, including proximal junctional kyphosis (PJK) and proximal junctional failure (PJF), are not considered in this system [32]. A study by Nakashima et al. [33] proposed a new global spinal balance classification that considers the differences in the individuals’ height and physique [33]. They also pointed out that a stooping posture caused by paraspinal muscle weakness and pelvic compensation by hip extensor muscles are a part of the pathophysiology of ASD [33]. Kwon et al. [34] recently suggested a complement SRS-Schwab ASD classification that considers pelvic compensation (success: high PT/PI and SVA; failure: low PT/PI and SVA). They observed that patients with ASD have pelvic compensation failure in spite of better preserved paraspinal muscles and identified pelvic compensation as an independent factor [34]. Based on these studies, as previously mentioned, the SRS-Schwab classification system needs to be further developed to improve treatment decision-making for ASD [35].
Decision-Making for Management of Adult Spinal Deformity: Conservative Treatment versus Surgical Treatment
To date, there are no definitive guidelines for treatment decision-making for patients with ASD [3]. In the past years, conservative treatment of ASD was preferred [29] because surgical intervention warranted prolonged recovery time, involved difficult perioperative management, and was associated with a high complication rate and costs [36-38]. Conservative treatment includes physical therapy, consuming oral or parenteral analgesics, and brace, but this approach does not improve disability or the quality of life of patients with ASD [39]. Multicenter prospective studies have shown that surgical treatments can considerably improve patient-reported clinical outcomes such as ODI, SRS, and HRQoL scores [1,16]. A meta-analysis conducted in 2022 showed that surgical intervention improved the patient-reported clinical outcomes, including pain and disability (measured using SRS-22 score, ODI, Visual Analog Scale, and Numeric Rating Scale) and correction of scoliotic curves [39]. Common complications of surgical treatment include mechanical and neurological deficits, cardiopulmonary and gastrointestinal problems, and infections. However, conservative treatment has a complication rate of 32.6%, owing to the use of oral analgesics such as non-steroidal anti-inflammatory drugs. Glassman et al. [40] performed a cost-effectiveness analysis for adult lumbar scoliosis surgery that revealed a high economic burden of surgical treatments ($111,451 for the surgical group versus $29,124 for the non-surgical group). To summarize, surgical treatment is now the mainstay for managing ASD, but surgical decision-making should entail a careful consideration of the condition of the patients.
After deciding on surgical treatment for ASD, meticulous preoperative planning and adopting a systemic approach for each patient are important. First, setting patient-specific targets for radiological parameters based on the SRS-Schwab classification is essential for preventing under- or over-correction [41]. Structural surgical planning has three main steps: identification of deformity drivers, setting alignment target, and determining flexibility [5]. Generally, the main cause of ASD is the loss of LL that is quantified by a PI–LL mismatch in the SRS-Schwab classification. After loss of LL, the compensatory mechanism in the pelvis for maintaining a standing posture is quantified by PT and SVA. In setting the alignment target, the Lafage formula can be applied for preoperative and intraoperative planning [38,42]. Age-specific alignment standards have been established for PT, PI–LL mismatch, and SVA [43]. This formula correlates optimal SVA to reflect the PT and compensatory mechanisms. Moreover, higher correction of SVA for age-specific alignment targets than the other targets may achieve better clinical and radiological outcomes and prevent complications [44]. To assess flexibility, Bridwell [45] categorized captured lateral supine radiographs into three groups: (1) totally flexible deformity that corrects in the supine position, (2) deformity that partially corrects, and (3) totally inflexible deformity. Of the three categories, more rigid deformities require more aggressive surgical osteotomy interventions to achieve proper correction. Scheer et al. [41] revealed that undercorrection of a sagittal deformity was associated with poor HRQoL, but overcorrection increased the risk of mechanical complications, particularly PJK. These results indicate that moderate alignment goals are associated with more satisfactory HRQoL which is contrary to the existing practice of preferring over-correction in order to mitigate subsequent loss of correction.
Current Techniques for Surgical Treatment of Adult Spinal Deformity
Radical advances in the past 2 decades have led to the invention of many surgical techniques, implants, and neurophysiological monitoring options [46,47]. The main surgical treatments for ASD are fusion, decompression, and osteotomy. There is no clear consensus on deciding the optimal surgical technique, but Silva and Lenke [48] introduced a general six-tiered hierarchy for surgical treatment of adult degenerative scoliosis: (1) decompression alone; (2) decompression with limited instrumented posterior spinal fusion; (3) decompression with lumbar curve instrumented fusion; (4) decompression with anterior and posterior spinal instrumented fusion; (5) thoracic instrumentation and extended fusion; and (6) inclusion of osteotomies for specific deformities.
For the subset of patients with minor spinal deformities and predominant issue of radiculopathy from isolated spinal stenosis, minimally invasive surgery (MIS) may be a good option for reducing morbidity owing to smaller incision, less soft tissue dissection, and faster postoperative recovery than the other surgical techniques [49]. Minimally invasive laminectomy with or without a foraminotomy is advantageous for older patients with degenerative scoliosis because this surgical procedure relieves radicular symptoms and decreases the risks associated with open procedures [50]. However, MIS must be carefully chosen because it may not effectively reduce back pain or sagittal imbalance [39]. Furthermore, these procedures are associated with a risk of complications such as recurrent radiculopathy and mechanical instability that may require posterior spinal instrumented fusion in the future.
Short-level fusion (limited fusion) is a potentially appropriate option for patients with mild or moderate ASD. Single- or two-level degenerative diseases include spinal stenosis with spondylolisthesis and/or degenerative disk disease that present as back pain and radiculopathy [4,51]. In such cases, decompression with short-level fusion can relieve the symptoms and restore the spinal alignment. Furthermore, short-level fusion can be combined with MIS techniques [49].
Recently, circumferential MIS has been introduced for 360° correction with anterior spinal column support and percutaneous posterior instrumentation [52]. Lateral lumber interbody fusion (LLIF) followed by percutaneous pedicle screw fixation by splitting of the psoas muscle is a relatively new circumferential MIS technique that permits the placement of anterior interbody support and performing posterior spinal instrumentation [53]. Access to the anterior column of the spine by surgical intervention has a few advantages compared with the conventional approaches. First, it prevents injury to the posterior tension band that protects against adjacent segment disease. Second, it offers better access to the intervertebral disk than posterior approaches, allowing the insertion of a large cage and improvement of the fusion rate. Third, placement of a large cage increases disk height, offering indirect nerve decompression and is more advantageous than the traditional anterior approach, which is associated with the risk of visceral organ damage and lumbar plexus injury [54]. Currently, the oblique lumbar interbody fusion (OLIF) technique is widely employed in patients with ASD. This technique was developed by complementing the LLIF technique [55]. In OLIF surgery, correction is directly performed on the anterior column of the spine, while preserving the psoas, anterior, and posterior paraspinal muscles. In ASD with severe sagittal deformity, a hybrid surgical approach (combination of OLIF and open posterior approach) is used to correct both coronal and sagittal deformities and reduce perioperative morbidity [39,50]. Circumferential MIS techniques for ASD have shown satisfactory clinical and radiological outcomes [56]. A meta-analysis found similarity between the complication rates of circumferential MIS and open approaches [57]. As elderly patients with degenerative scoliosis have poor bone quality and comorbidities, circumferential MIS can correct the sagittal imbalance and deformity and can result in faster recovery with few complications [50].
Osteotomies are powerful surgical techniques for reshaping and reconstructing stiff or severe spinal deformities [58]. However, in-depth knowledge of the spinal anatomy and biomechanics is an essential prerequisite for performing osteotomies because of the associated risk of serious complications [58]. Therefore, they have a steep learning curve of at least 100 cases according to Raad et al. [59]. The decision to perform spinal osteotomy should be made while considering the patient’s symptoms, bone and muscle quality, etiopathophysiology, curve type, and operative goal. Schwab et al. [60] proposed an anatomybased osteotomy classification system with six grades corresponding to increased posterior-based destabilization (Fig. 3): (1) grade 1: resection of the inferior facets; (2) grade 2: resection of both the inferior and superior facets, along with other posterior ligamentous and osseous structures; (3) grade 3: removal of the posterior elements and pedicles and a part of the vertebral body; (4) grade 4: wider resections that also include at least a portion of an adjacent disk; (5) grade 5: removal of one vertebra and adjacent disks; and (6) grade 6: any resection that exceeds grade 5.
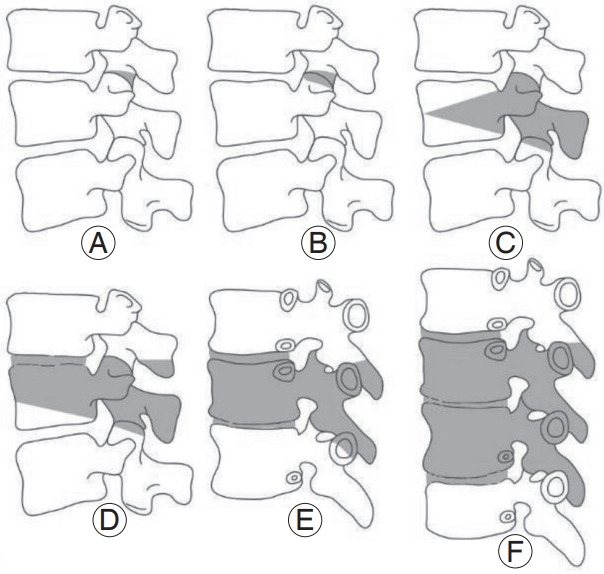
Anatomical osteotomy classification. Schwab et al. [60] developed a comprehensive anatomy based spinal osteotomy grading system with six grades that correspond to an increasing potential for destabilization. (A) Grade 1: resection of the inferior facets; (B) grade 2: resection of both the inferior and superior facets, along with other posterior ligamentous and osseous structures; (C) grade 3: removal of the posterior elements and pedicles and part of the vertebral body; (D) grade 4: wider resections that include at least a portion of an adjacent disc; (E) grade 5: removal of one vertebra and adjacent discs; and (F) grade 6; any resection that exceeds a grade 5. Reproduced from Kim HJ, et al. Asian Spine J 2020;14:886-97 [15].
The two main osteotomy techniques are posterior column osteotomy (Smith-Peterson osteotomy [SPO], chevron osteotomy, and Ponte osteotomy) and threecolumn osteotomy (pedicle subtraction osteotomy [PSO] and vertebral column resection [VCR]) [58]. Many spine surgeons, including Bridwell [45], have suggested that the osteotomy technique should be selected on the basis of the angularity, flexibility, and location of deformity. SPO is an opening wedge osteotomy with a hinge at the posterior portion of the intervertebral disk space [61]. After resection of the posterior elements, including the bilateral facet joints, lamina, and posterior ligaments, deformity correction is performed by closing the wedge posteriorly, and an expected correction of 10°–15° per level of osteotomy [62]. Ponte osteotomy is a modified version of SPO with additional removal of both the superior and posterior facets [63]. Three-column osteotomy is a universal concept that entails resection of the spine from the posterior column and the middle column to the anterior column [58]. PSO is a closing-wedge osteotomy accomplished by resection of the posterior elements and vertebral body [64]. After a V-shaped wedged vertebral lesion is resected, the middle column is posteriorly shortened that achieves sagittal plane correction of 30°–35° in lumbar lesions and 20°–25° in thoracic lesions. It is mainly used for correcting the lumbar lesions of the iatrogenic flat-back deformity [64]. VCR is complete removal of ≥1 vertebral bodies along with the adjacent disks, pedicles, and every posterior segments [65]. It is a powerful approach for correcting severe/rigid deformities and enabling sagittal deformity correction of up to 45° [58,65]. Ever since their inception by Suk et al. [66-68], posterior-only VCRs have been widely performed for severe and rigid thoracic deformities [69] despite of the technical challenges and involved risks of serious complications [70]. In terms of Schwab’s anatomybased osteotomy classification system, grades 2, 3, and 4 are associated with posterior column osteotomy (mainly SPO), PSO, and extended PSO or bone-disk-bone osteotomy, respectively, while grades 5 and 6 are associated with VCR [58].
Considering the risks of VCR, including high complication rates, longer surgical times, and significantly higher blood loss, posterior multi-crack osteotomy techniques could be the more appropriate alternative procedures, as recently reported by Yang et al. [71]. These techniques offer few advantages: (1) preserved anterior vertebral body and longitudinal ligament complex that supports the axis during surgery and (2) the possibility of a multi-level osteotomy with minimal blood loss and nerve injury.
Mechanical Complications after Adult Spinal Deformity Surgery
According to a systematic review, the reported complication rates after ASD surgery range from 9.5% to 81.52%, and the need for revision surgery ranges from 1.7% to 40.0% [72]. Among the many complications of ASD surgery, mechanical complications including PJK, PJF, and implant-related failures that require revision surgery have an incidence rate of 20%–40% [73,74]. As mentioned above, the SRS-Schwab ASD classification has poor predictive ability for mechanical complications, and several criteria such as Roussouly theoretical apex of lordosis and global alignment of proportion (GAP) score have been proposed to prevent mechanical complications [73]. Roussouly et al. [75] distributed four lordotic arcs based on the PI. In 2018, Sebaaly et al. [76] suggested that restoring the apex of LL to its theoretical value offered sagittal balance and decreased the incidence of PJK. Similar to the concepts of the Roussouly classification, GAP scores were proposed as a new PI-based proportional method for predicting mechanical complications after ASD surgery. These scores consider relative pelvic version, relative LL, lordosis distribution index, relative spinopelvic alignment, and age of the subjects [77]. GAP scores predicted PJK relatively well [78]; however, other studies have reported conflicting results because the predictive model used for GAP scores does not consider surgical factors, BMD, and body mass index [79]. In a recent study by Teles et al. [32], neither the Roussouly theoretical apex of lordosis nor GAP scores were found to have adequate ability to predict mechanical complications after ASD surgery. Therefore, postoperative mechanical complications should be considered multifactorial and not only to be associated with alignment measures.
Future Directions
As radical technical developments continue, advanced computational technologies such as smart materials, robotic-assisted spine surgery, and AI, are likely to be used for treatment of ASD in the future [80,81]. Smart materials can be designed that can alter their properties in response to external stimuli [82]. These materials can recognize changes in their surroundings and manifest a predetermined response within a set. Shape-memory alloys, introduced in 1938, have super-elasticity, excellent fatigue behavior, and high damping capacity [82,83]. Therefore, these alloys have been used in spinal instruments and MIS systems for making patient-specific biomechanical spine models [83].
Pedicle screw instrumentation is the mainstay of surgical treatment in the field of spine. Whereas, pedicle screw malposition is a major complication during surgical procedures [84]. Robotic-assisted spine surgery has shown several advantages over conventional instrumentation systems [84,85]: (1) accuracy and safety in pedicle screw insertion; (2) precise selection of screw size; (3) proper screw positioning that can be determined by a computed tomography scan during preoperative planning; and (4) reduction in radiation exposure and operative time.
These merits render this approach suitable for cases of severe spinal deformity. However, it is still under clinical investigation, especially for its use in severe scoliosis of thoracic spine. Nevertheless, it has good prospects for application in circumferential MIS [86]. Therefore, robotic surgery is expected to confer a distinct leverage in the management of ASD, but further research is needed.
Machine learning (ML), which is an AI technique, will eventually provide predictive systems to aid in treatment decision-making in ASD [80]. ML uses computational algorithms from repeatedly trained data to develop mathematical models built using multiple variables and large quantities of data [81]. These complex algorithms can detect more subtle patterns in data than traditional statistical methods [87]. Kim et al. [88] used ML to predict surgical complications in patients who underwent elective ASD surgery and found that it improved their risk prognostication system. Furthermore, Martini et al. [89] used ML to identify several important factors associated with unplanned 30-day readmission. These studies demonstrated the capabilities of ML in the field of ASD, but further research is required to optimize its clinical application.
Conclusions
ASD has recently become a matter of concern because of its effect on the HRQoL of the patients and accounts for a significant proportion of the Global Burden of Diseases in society. Understanding the etiopathophysiology of ASD is critical in patient-specific decision making in clinical settings. Surgical treatment of ASD should aim at the restoration of age-specific alignment to improve the HRQoL, while also considering patient’s comorbidities and the risk factors associated with frequently encountered minor, occasional, and unfortunately catastrophic complications.
Notes
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: HJK, JHY, DGC, LGL; data curation: HJK; formal analysis: HJK, JHY; methodology: HJK, DGC, SIS; project administration: DGC; visualization: HJK, SWS, YJN, SCP; writing–original draft: HJK, JHY, DGC; writing–review & editing: LGL, SWS, SIS.

