The Prevalence of Coexisting Lumbar Spondylosis and Knee Osteoarthritis: A Systematic Review and Meta-Analysis
Article information
Abstract
Study Design
A systematic review.
Purpose
This study aimed to evaluate the prevalence of coexisting lumbar spondylosis (LS) and knee osteoarthritis (KOA), which has clinical implications on the screening, diagnosis, and management of orthopedic patients.
Overview of Literature
Due to current global health trends, the number of affected patients is expected to increase substantially. However, no prior systematic reviews have discussed this topic.
Methods
A systematic literature search was conducted in June 2021 in the PubMed, Embase, Scopus, CINAHL, and Cochrane CENTRAL databases. Clinical and epidemiological studies that reported quantitative data on the prevalence of coexisting LS and KOA were included. Studies which reported data on only LS or KOA alone were excluded. Odds ratios (ORs) and 95% confidence intervals (CI) for LS or KOA were retrieved or calculated for meta-analysis. Fixed-effects and random-effects models were used, and statistical significance was considered when p<0.05. Heterogeneity was evaluated using Cochran’s Q test and the I2 statistic. Risk of bias was assessed using the MINORs (methodological index for nonrandomized studies) criteria.
Results
This review included nine studies (5,758 patients). Four studies (4,164 patients) defined KOA and LS by a Kellgren-Lawrence (KL) grade of ≥2 and were included in the meta-analysis. Two other studies defined KOA and LS by a joint space narrowing grade of ≥2. The remaining three studies reported other outcomes. The combined ORs of having KOA of KL grade ≥2 due to LS was 1.75 (95% CI, 1.22–2.50; p=0.002), while the combined OR of having LS of KL grade ≥2 due to KOA was 1.84 (95% CI, 1.23–2.77; p=0.003).
Conclusions
In patients with either KOA or LS, the odds of having a concurrent knee-spine presentation are significantly increased. This may have implications for clinical decision-making and treatment strategies. Further high-level studies with larger patient populations are required to confirm these results in specific populations.
Introduction
Osteoarthritis is a multifactorial disease, with the knee and lumbar spine among the most commonly affected sites [1]. Spondylosis is a nonspecific term that encompasses degenerative conditions of the intervertebral discs, vertebral bodies, and associated joints of the spine, including spinal osteoarthritis [2]. Both knee osteoarthritis (KOA) and lumbar spondylosis (LS) are complex joint disorders with multiple risk factors.
The prevalence of LS and KOA been reported in separate populations has. In 2018, Ravindra et al. [3] estimated that 266 million individuals worldwide suffered from lumbar degenerative diseases, whereas in 2020, Cui et al. [4] estimated that 654.1 million persons suffered from KOA. However, recent studies suggest that LS and KOA are risk factors for one another [5,6], because of the compensatory adjustments in weightbearing and posture in reaction to the disruption of the body’s mechanical alignment. In fact, both conditions can coexist in a single patient [7], with worse clinical and surgical outcomes in LS patients who undergo knee surgery [8,9].
The prevalence of coexisting LS and KOA has clinical implications on the screening, diagnosis, and management of orthopedic patients. To our knowledge, no systematic reviews or meta-analyses have been published on this topic, and there is limited epidemiological data on the concurrent existence of both diseases in a single population. Moreover, the trend of ageing populations and increasing prevalence of obesity suggest that the number of affected patients is likely to increase substantially [10]. Thus, there is a need to elucidate the true association between LS and KOA, as well as quantify the extent of their association. Therefore, the purpose of this study is to qualitatively and quantitatively assess the prevalence of coexisting LS and KOA.
Materials and Methods
1. Information sources and study selection
An electronic search was performed by two independent authors (blinded for peer-review) in the PubMed, Embase, Scopus, CINAHL, and Cochrane CENTRAL databases to identify all relevant studies published from inception to 12 June 2021. The search string was as follows: “(((“Osteoarthritis, Spine” OR “Spinal Diseases” OR “Spine osteoarthr*” OR “lumbar osteoarthr*” OR “lumbar spondylosis*” OR “lumbosacral spondylosis*” OR “spinal stenosis*” OR “spinal degenerative joint disease*” OR radiculopathy* OR myelopathy*) AND (“osteoarthritis, Knee” OR “Knee osteoarthr*” OR “tibiofemoral osteoarthr*” OR “patellofemoral osteoarthr*” OR “tricompartmental osteoarthr*”)) OR “knee-spine syndrome”) AND (prevalence or prevalence* OR epidemiology OR epidemiologic studies).” Database searches were supplemented by a manual search of the citations in identified studies. The search workflow adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [11], and is showcased in Fig. 1.
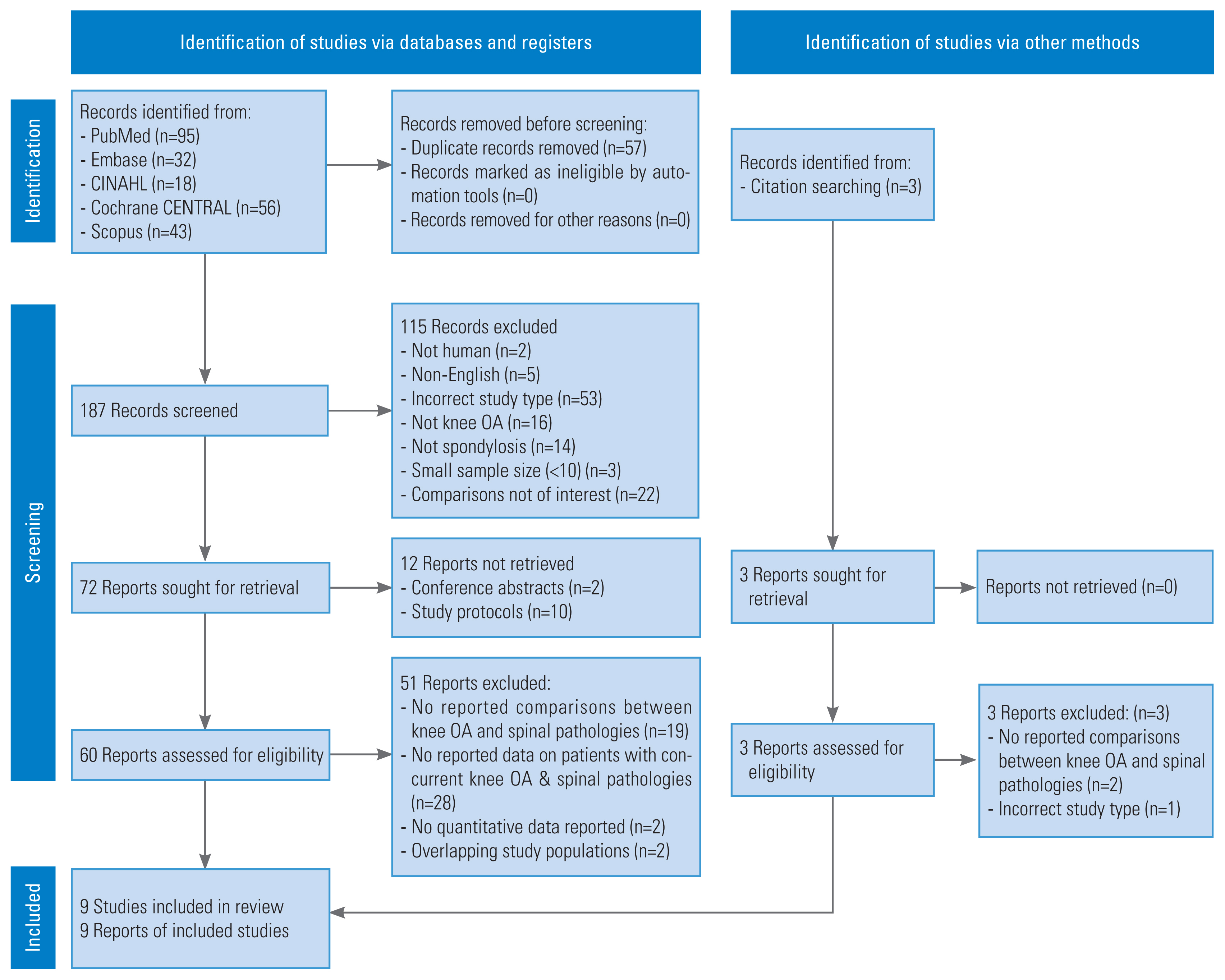
The search workflow was performed in accordance to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). OA, osteoarthritis.
Two reviewers (blinded for peer-review) independently assessed each study to determine their eligibility for inclusion in the analysis. Duplicate studies were removed using the EndNote X9 software (Clarivate, Philadelphia, PA, USA). Afterwards, the studies were transferred to Microsoft Excel (Microsoft Corp., Redmond, WA, USA) for title and abstract screening, followed by full text screening. The eligibility criteria used are listed below. After completing the independent screening process, disagreements were resolved via consensus discussion between the authors. The list of excluded articles was uploaded to Figshare [12].
The primary outcome of this study was to assess the prevalence of coexisting LS and KOA. The secondary outcomes were to identify other outcomes associated with either LS or KOA from the literature and provide explanations on their potential associations.
2. Eligibility criteria
This review was registered on the PROSPERO (International Prospective Register of Systematic Reviews) database (registration no., CRD42021268542). We included clinical or epidemiological studies of any level of evidence which reported quantitative data on the prevalence of coexisting LS and KOA.
Case reports, review articles, published abstracts, studies involving less than ten patients, and duplicate data were excluded from this review. Studies which reported data on only LS or KOA alone were excluded. Articles not written in English and those with unavailable access to the full text were also excluded.
3. Data extraction
Data from the texts, figures, and tables of included studies were extracted to Microsoft Excel (Microsoft Corp.) for analysis and review. The information recorded included: (1) study details, including study design, country of origin, and level of evidence, (2) study objectives, (3) study population details, including number of patients, the size of the control group (if any), mean age, mean body mass index (BMI), gender split, and country of study, (4) outcomes studied and definition for the presence of LS and KOA, (5) unadjusted and adjusted odds ratios (ORs) for the presence of KOA in LS patients and the presence of LS in KOA patients, and (6) covariates adjusted for (if any). Data was extracted by two independent authors, and the Microsoft Excel sheets (Microsoft Corp.) were cross-checked between authors before any qualitative or quantitative analyses were performed.
4. Statistical analysis
All eligible studies were included in a narrative qualitative synthesis. We initially considered performing a meta-analysis for each eligible outcome. However, due to different diagnostic criteria for LS and KOA, and the small number of studies, a meta-analysis was only appropriate for two outcomes: the presence of KOA in LS patients, and the presence of LS in KOA patients defined by a Kellgren-Lawrence (KL) [13] grade ≥2. Fixed-effects and random-effects models were calculated using the Review Manager ver. 5.4 software (Cochrane, London, UK) using the Mantel-Haenszel method of weighting [14]. The measurement effects are presented on forest plots using ORs with 95% confidence intervals (CIs), and statistical significance was set at p<0.05. Heterogeneity was examined using Cochran’s Q test and the I2 statistic. Analyses with high heterogeneity were re-checked to ensure that the extracted data was correct, then random-effects models were used. Subgroup analyses were attempted, but due to the small number of studies reporting the necessary data, the analyses were not undertaken.
5. Quality assessment of studies
All included studies were assessed for quality and rigor against the methodological index for nonrandomized studies (MINORS), and a global score was assigned to each in Table 1. The MINORS score is a summation of individual item scores (0 to 2 for each item), with maximum scores of 24 and 16 for comparative and noncomparative studies, respectively [15]. Two authors, who were blinded for peer-review, independently reviewed each reference. Disagreements were resolved via consensus discussion between the authors.
Results
1. Study characteristics
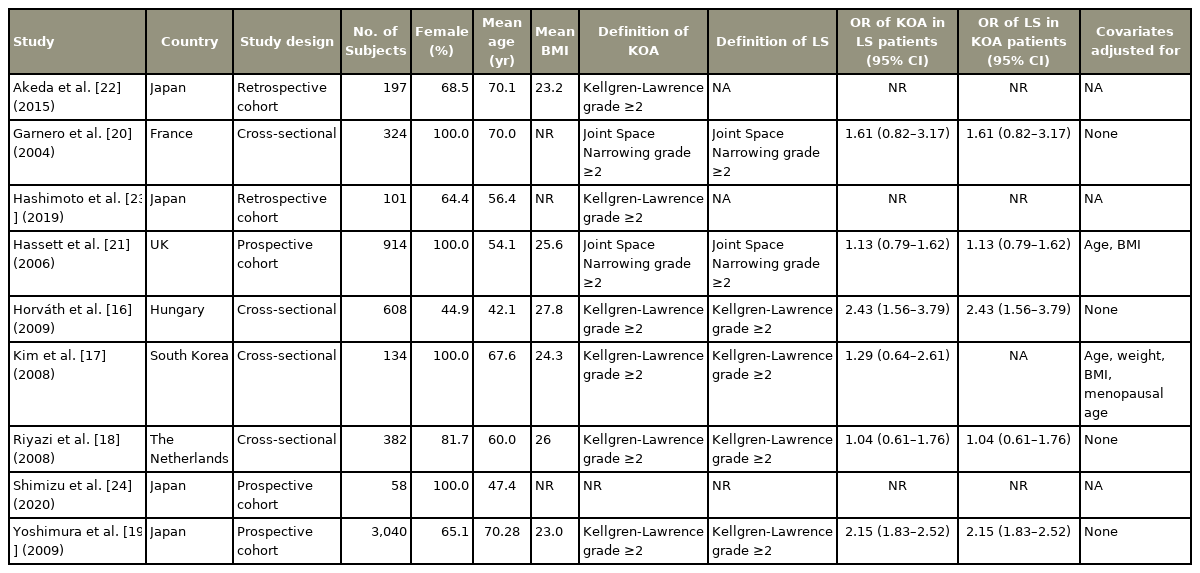
A total of nine studies with 5,758 patients were included in this systematic review [16–24]: four cross-sectional studies, three prospective cohort studies, and two retrospective cohort studies. The study characteristics are summarized in Table 2. Four studies totaling 4,164 patients defined LS and KOA by a KL grade of ≥2 and were included in the meta-analysis [16–19]. Two other studies defined LS and KOA by a joint space narrowing (JSN) grade of ≥2 [20,21,25]. The remaining three studies reported other outcomes [22–24]. The first study reported the change in disc height index (DHI) in patients with KOA [22]. The second study reported the proportion of patients with KOA having a concurrent mechanical diagnosis and therapy classification of spinal derangement [23]. The third study reported the correlation coefficients between changes in KL grade of the knee joint and lumbar spine [24].
2. Meta-analysis
1) ORs of KOA exposure in LS patients (KL grade ≥2)
The ORs of KOA exposure in LS patients were derived from four studies [16–19]. Based on the random-effects model, the combined OR of having KOA of KL grade ≥2 due to LS was 1.75 (95% CI, 1.22–2.50; p=0.002), as shown in Fig. 2. Based on the fixed-effects model, the combined OR of having KOA of KL grade ≥2 due to LS was 2.02 (95% CI, 1.75–2.32; p=0.003), as shown in Fig. 2.
2) ORs of LS exposure in KOA patients (KL grade ≥2)
The ORs of LS exposure in KOA patients were derived from three studies [16,18,19]. Based on the random-effects model, the combined OR of having LS of KL grade ≥2 due to KOA was 1.84 (95% CI, 1.23–2.77; p=0.003), as shown in Fig. 3. Based on the fixed-effects model, the combined OR of having LS of KL grade ≥2 due to KOA was 2.06 (95% CI, 1.78–2.37; p=0.003), as shown in Fig. 3.
3. Narrative synthesis
1) ORs of KOA exposure in LS patients (female, JSN grade ≥2)
We reviewed two studies with female-only patient populations that defined KOA and LS by a JSN grade of ≥2 [20,21]. Garnero et al. [20] reported an OR of 1.61 (95% CI, 0.82–3.17), while Hassett et al. [21] reported an OR of 1.13 (95% CI, 0.79–1.62). A forest plot was not generated due to the small number of studies and statistically insignificant ORs (p=0.20 and p>0.05, respectively).
2) Other reported outcomes
Akeda et al. [22] reported a significant association between radiographic KOA and an increased risk of lumbar intervertebral disc height narrowing (p=0.0197). Shimizu et al. [24] reported significant positive correlation coefficients between KOA and changes in LS at both L3/4 (r=0.383, p=0.004) and L4/5 (r=0.333, p=0.012). On the other hand, Hashimoto et al. [23] observed that the percentage of patients with spinal derangement was greater among those without radiographic findings of KOA (p<0.001). Spinal derangement was defined as a state where mechanical loading in specific direction results in symptomatic and functional improvements which rapidly occur and last after loading. All three of these studies defined KOA and LS by a KL grade of ≥2.
Discussion
The prevalence of coexisting LS and KOA has clinical implications on the screening, diagnosis, and management of orthopedic patients. Due to the upwards trends of obesity and ageing populations, the number of affected patients is likely to increase substantially, but a literature search revealed no prior systematic review on the topic. In line with this, we aimed to qualitatively and quantitatively assess the prevalence of coexisting LS and KOA. We found significantly higher odds of KOA exposure in LS patients, as well as of LS exposure in KOA patients. Although there was discordance between papers in terms of diagnostic criteria (i.e., KL grade versus JSN grade), studies reporting other outcomes supported the correlation between LS and KOA. However, the lack of large-scale epidemiological studies and sufficient data for subgroup analyses makes it difficult to ascertain the true prevalence of coexisting LS and KOA in specific demographic groups.
LS and KOA are multifactorial pathologies that are influenced by both mechanical deformities and biological abnormalities [26]. An association between spondylosis and KOA has been examined and established in current literature [6,21,27], and has even been referred to as “knee-spine syndrome” [28]. In this review, studies that used KL grading reported a 100% increased odds of exposure to both LS and KOA, which is in line with existing literature on the positive correlation between the two diseases [16–19]. The other included studies reporting correlation coefficients, changes in lumbar DHI, and spinal derangement also supported this finding. The meta-analysis also consisted of Asian (Japan and South Korea) [17,19] and European (The Netherlands and Hungary) [16,18] patients with a large age and BMI range, providing a reasonable demographic spectrum for analysis.
Discordance was observed between studies using the KL grading [16–19] and JSN grading [20,21] as diagnostic criteria. Two papers using the JSN grading reported relatively lower odds (61% and 13% increase, respectively) of KOA in female LS patients, with CIs crossing the line of no effect [20,21]. This can be attributed to the heterogeneity in study aims and patient selection, because the ORs of KOA in LS patients were not the primary outcome of the study. Furthermore, there are differences in the proportions of postmenopausal women and in the mean ages of the participants across the study populations.
Due to insufficient data, the initially planned subgroup analyses were also not possible. We were able to extract the common constitutional risk factors for LS and KOA (i.e., age, female sex, postmenopausal state, and obesity) [29,30], but the sample sizes and number of studies were limited. Future pooled results for specific patient demographics would help in the development of individualized management plans for patients with coexisting LS and KOA.
The results of this study are of clinical value and significance. By presenting preliminary quantitative evidence for the association between LS and KOA, our study can help in the evaluation of orthopedic patients with either LS or KOA for the possibility of knee-spine pathologies. This data can hopefully aid in early detection and preventive strategies, reduce public health burden, and improve the patients’ quality of life. Evidence for the coexistence of LS and KOA can also affect the surgical management of patients. Goodman et al. [7] presented a survey of 97 orthopedic surgeons regarding their preferences regarding whether to perform total knee arthroplasty or lumbar spine surgery first, given that both surgeries have been reported to affect the outcomes of each other [8,9]. It was concluded that the severity and type of knee deformity determined the sequence of treatment in both specialties. Further longitudinal cohort studies are required to investigate the effects of knee-spine pathologies on surgical outcomes. Lastly, our results also suggest that common pathophysiologic mechanisms underlie the progression of LS and KOA at different anatomical sites. This information can affect the way disease modifying drugs for osteoarthritis (DMOADs) are used in prospective intervention studies [21]. DMOADs could have a greater impact on the burden of osteoarthritis if patients with KOA are likely have further progression at other anatomical sites. Lastly, this would also influence clinician advice in patients with multisite osteoarthritis.
The findings discussed in this review should be carefully considered alongside the following limitations. Aside from the insufficient data for subgroup analyses and meta-regression, the sources of heterogeneity in the meta-analysis were also not evaluated. Unadjusted ORs were used, and the effect of confounders on results cannot be excluded. Lastly, our analysis utilized cross-sectional data, which cannot be used to describe the temporal relationship between LS and KOA. Based on our findings, there is a lack of high-level evidence evaluating coexisting LS and KOA. We hope that this review will help lead the discussion and encourage researchers to conduct more robust clinical and translational studies. This would address the factors and outcomes discussed in this review, including the effect of patient demographics, surgical outcomes, the use of DMOADs, and the temporal relationship between LS and KOA in knee-spine pathologies.
Conclusions
This is the first systematic review which evaluated the prevalence of coexisting LS and KOA. In patients with either KOA or LS, the odds of having a concurrent knee-spine presentation are significantly increased. This may have implications for clinical decision-making and treatment regimes. However, further high-level studies with larger patient populations are required to establish the concordance of results in specific populations.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
All authors contributed to the intellectual development of this paper; HRB and YH conceived and planned the study; BKB and FN performed the systematic literature search; BKB and FN wrote the manuscript; BKB, FN, YH, and HRB contributed to the interpretation of the results and provided critical feedback to the manuscript; and the final version of the paper has been seen and approved by all authors.
Acknowledgements
All work was performed remotely in view of COVID-19 pandemic restrictions.