Biomechanical Study on Three Screw-Based Atlantoaxial Fixation Techniques: A Finite Element Study
Article information
Abstract
Study Design
This is a finite element study.
Purpose
This study is aimed to compare the biomechanical behaviors of three screw-based atlantoaxial fixation techniques.
Overview of Literature
Screw-based constructs that are widely used to stabilize the atlantoaxial joint come with their own challenges in surgery. Clinical and in vitro studies have compared the effectiveness of screw-based constructs in joint fixation. Nevertheless, there is limited information regarding the biomechanical behavior of these constructs, such as the stresses and strains they experience.
Methods
A finite element model of the upper cervical spine was developed. A type II dens fracture was induced in the intact model to produce the injured model. The following three constructs were simulated on the intact and injured models: transarticular screw (C1–C2TA), lateral mass screw in C1 and pedicle screw in C2 (C1LM1–C2PD), and lateral mass screw in C1 and translaminar screw in C2 (C1LM1–C2TL).
Results
In the intact model, flexion–extension range of motion (ROM) was reduced by up to 99% with C11–C2TA and 98% with C1LM1–C2PD and C1LM1–C2TL. The lateral bending ROM in the intact model was reduced by 100%, 95%, and 75% with C11–C2TA, C1LM1–C2PD, and C1LM1–C2TL, respectively. The axial rotation ROM in the intact model was reduced by 99%, 98%, and 99% with C11–C2TA, C1LM1–C2PD, and C1LM1–C2TL, respectively. The largest maximum von Mises stress was predicted for C1LM1–C2TL (332 MPa) followed by C1LM1–C2PD (307 MPa) and C11–C2TA (133 MPa). Maximum stress was predicted to be at the lateral mass screw head of the C1LM1–C2TL construct.
Conclusions
Our model indicates that the biomechanical stability of the atlantoaxial joint in lateral bending with translaminar screws is not as reliable as that with transarticular and pedicle screws. Translaminar screws experience large stresses that may lead to failure of the construct before the required bony fusion occurs.
Introduction
The atlantoaxial region is composed of the atlas, axis, and ligaments and allows a variety of complex motions [1]. Atlantoaxial instability (AAI) is extremely dangerous and disrupts the daily life of patients with AAI. Major causes of AAI are trauma, congenital abnormalities, inflammation, Down syndrome, and tumors [2]. If AAI is not treated appropriately, it may cause permanent neurological disorders or deformities [3].
Posterior atlantoaxial fixation can effectively treat AAI. The first posterior atlantoaxial fixation technique, reported by Gallie [4] in 1939, involved the use of sublaminar wires. Another wiring technique introduced by Brooks–Jenkins provided similar stability to Gallie’s technique during flexion and extension but more rotational stability [5]. Because of the risk of spinal cord injury when using sublaminar wires, other techniques involving the use of braided cables were introduced [6]. The limitations of wiring techniques have led to the development of more reliable methods.
Screw-based fixation techniques are currently widely accepted as treatments for AAI. The transarticular screw technique proposed by Magerl and Seemann [7] is used for posterior atlantoaxial fixation and has fusion rates of up to 100% [8]. Two transarticular screws are inserted in the axis and directed toward the anterior arch of the atlas. Despite its many advantages, this technique has several drawbacks, the main one being that it requires preliminary reduction of the joint [8]. Screw malpositioning, breakage, and neural and vascular injury may also occur [9]. Harms and Melcher [8] proposed a pedicle screw technique based on the rod cantilever concept that minimizes the risk of injury to the vertebral artery [10]. Here, independent screws are inserted into C1 and C2 and connected using rods. This technique is surgically less demanding than the transarticular screw technique and can be used on most patients [11]. Nevertheless, the risk of injury to the vertebral artery still exists. Wright [12] proposed a translaminar screw technique using two screws bilaterally crossing in C2. This technique poses no risk to the vertebral artery and is not affected by variations in individual anatomy [13]. C2 instrumentation is commonly done using pedicle or translaminar screws with reasonable results [2]. A retrospective clinical study by Parker et al. [14] showed that pedicle screws breached the pedicle in 7% of patients, whereas the translaminar screws breached the lamina in only 1.3% of patients. One year postoperatively, 0% and 6% of patients treated with pedicle and translaminar screws, respectively, required revision surgery [14]. However, the results of their study cannot be generalized as direct comparisons cannot be made between the pedicle and translaminar screws because of anatomical differences among their patients. Furthermore, when comparing the outcomes of surgery, they did not account for biomechanical variations between translaminar and pedicle screws caused by differences in bone density.
Biomechanical evaluations of these atlantoaxial fixation techniques have been conducted in many in vitro studies. However, in vitro studies provide limited information regarding the biomechanical behavior of atlantoaxial joints with implants. Finite element (FE) models can provide insights on load-sharing, stress, and strain on the joints and implants. In this study, a novel FE model of the upper cervical spine was developed and validated using information from in vitro studies. Simulations were conducted in three main loading directions. Transarticular, C2 pedicle, and C2 translaminar screw constructs were used, and their influences on the range of motion (ROM) of the joint and the stresses on the implants were evaluated.
Materials and Methods
1. Intact finite element model
A three-dimensional (3D) FE model was developed for the upper cervical spine (C0–C1–C2). Computed tomography (CT) scans of a 35-year-old male were used to construct the geometry of the bones, after approval from the Koc University Committee on Human Research (IRB authority no., 2012.019.IRB2.009). The detailed method of creating finite models from CT scans is published previously [15,16]. CT scan data were processed using Mimics (Mimics ver. 14.1; Materialize Inc., Leuven, Belgium) to generate the 3D surfaces of the bones. The 3D geometry of the bones was meshed using IA-FEMesh (University of Iowa, Iowa City, IA, USA). Hexahedral elements were used to mesh the C1 and C2 vertebrae, and tetrahedral elements were utilized to mesh the skull. The meshed vertebrae and skull were imported into ABAQUS (ABAQUS, ver. 6.10-2; ABAQUS Inc., Providence, RI, USA) and combined with material property inputs for FE analysis (Fig. 1). Bones were simulated as isotropic linear elastic materials, and different property values were assigned to the cortical and cancellous bones. Cortical bone material properties were assigned to the skull (Table 1).
All major ligaments that stabilize the joint were modeled with different elements. The transverse ligament and tectorial membrane were modeled as a 3D structure using SolidWorks (SOLIDWORKS, ver. 2013; SolidWorks Corp., Concord, MA, USA) based on information on geometry available in literature and were defined as isotropic linear elastic materials. The ligamentum flavum, alar, apical, anterior atlantoaxial, anterior longitudinal, posterior longitudinal, capsular, and interspinous ligaments were represented by tension-only truss elements using cross-sectional areas obtained from literature [17]. Contact at the facet joints was simulated using nonlinear GAPUNI elements. Surface-to-surface finite sliding contact was permitted between the transverse ligament and odontoid process.
2. Injured and implanted FE models
Three screw-based constructs were modeled using SolidWorks: C1–C2 with a transarticular screw (C1–C2TA), C1 with a lateral mass screw and C2 with a pedicle screw (C1LM–C2PD), and C1 with a lateral mass screw and C2 with a translaminar screw (C1LM–C2TL). The C1–C2TA screws were inserted near the center of the C2 pars, angled toward the anterior arch of C1, and passed through the C1–C2 facet joint without breaching the transverse foramen. The C1 lateral mass screws were inserted just inferior to the posterior arch of C1 and directly into the lateral mass of C1. The C2 pedicle screws were inserted slightly laterally to the midpoint of the C2 pars and angled toward the C2 vertebral body passing through the C2 pedicle, almost parallel to the C2 inferior facets. The C2 translaminar screws were inserted such that the medial translaminar screw was cranial to the lateral translaminar screw. The lateral mass, pedicle, transarticular, and translaminar screws were 14 mm, 20 mm, 45 mm, and 40 mm long, respectively. All the screws were 4 mm in diameter. The connecting rod was 3.5 mm in diameter. Screw sizes were chosen on the basis of the vertebral dimensions of the models. A type II dens fracture was induced in the intact FE model to create an injured FE model. The screw constructs were added to the intact and injured FE models. Three groups of FE models were simulated under similar loading conditions: intact, implanted intact, and implanted injured.
The bone–screw and screw head–rod interfaces were constrained in all directions using the coupling option in ABAQUS. Titanium material properties were assigned to the screws and rods.
3. Loads and boundary conditions
The lower surface of the C2 vertebra was constrained in all directions. A pure moment of 1.5 nm was applied in the flexion–extension (FLEX), right–left lateral bending (LB), and right–left axial rotation (AR) planes at a flying node that was coupled to the condylar surfaces.
The intact model was used to predict ROM in the C0–C1 and C1–C2 segments, and the results were compared with values reported in literature. The implanted intact and implanted injured FE models were then used in the simulation. The maximum von Mises stresses on the implants under different loading conditions were compared.
Results
1. Model validation
The ROM predicted using the intact FE model was compared with that reported by Panjabi et al. [18–20] (Table 2). The FE model predictions for each loading condition were largely within the reported range, with minor deviations observed in C0–C1 during flexion and C1–C2 during extension and LB.
2. Implanted model
ROM reductions in the C1–C2 segments after adding the three constructs to the intact and injured models were measured and normalized using the intact ROM (Table 3). In FLEX, C1–C2TA reduced ROM by 99% in both, intact and injured models. C1LM–C2PD and C1LM–C2TL achieved similar ROM reductions (98%) in the intact model and ROM reductions of 96% and 95%, respectively, in the injured model. In LB, C1–C2TA reduced ROM by 98% and 100% in the intact and injured models, respectively. C1LM–C2PD reduced ROM by 93% in the intact model and 95% in the injured model. C1LM–C2TL reduced ROM much lesser than the other two techniques: 75% and 74% for the intact and injured models, respectively. In AR, C1–C2TA reduced ROM by 99% in both, the intact and injured models. C1LM–C2PD reduced ROM by 98%. C1LM–C2TL in the injured model showed increased stiffness from that in the intact model, achieving a 98% ROM reduction in the intact model and a 99% reduction in the injured model.
3. Stresses on the implants
Fig. 2 shows a comparison of the predicted maximum von Mises stresses on the three screw constructs. The largest predicted maximum stress for C1–C2TA was 133 MPa in FLEX on the injured FE model. The maximum stress was at the point where the screw entered C1 (Fig. 3A). The largest predicted maximum stress for C1LM–C2PD was 307 MPa in FLEX. The maximum stress was at the lateral mass screw head (Fig. 3B). In the pedicle screw, the maximum stress was 131 MPa. The largest predicted maximum stress for C1LM–C2TL was 332 MPa in FLEX. The maximum stress was at the lateral mass screw head (Fig. 3C). In the translaminar screw, the maximum stress was 123 MPa.
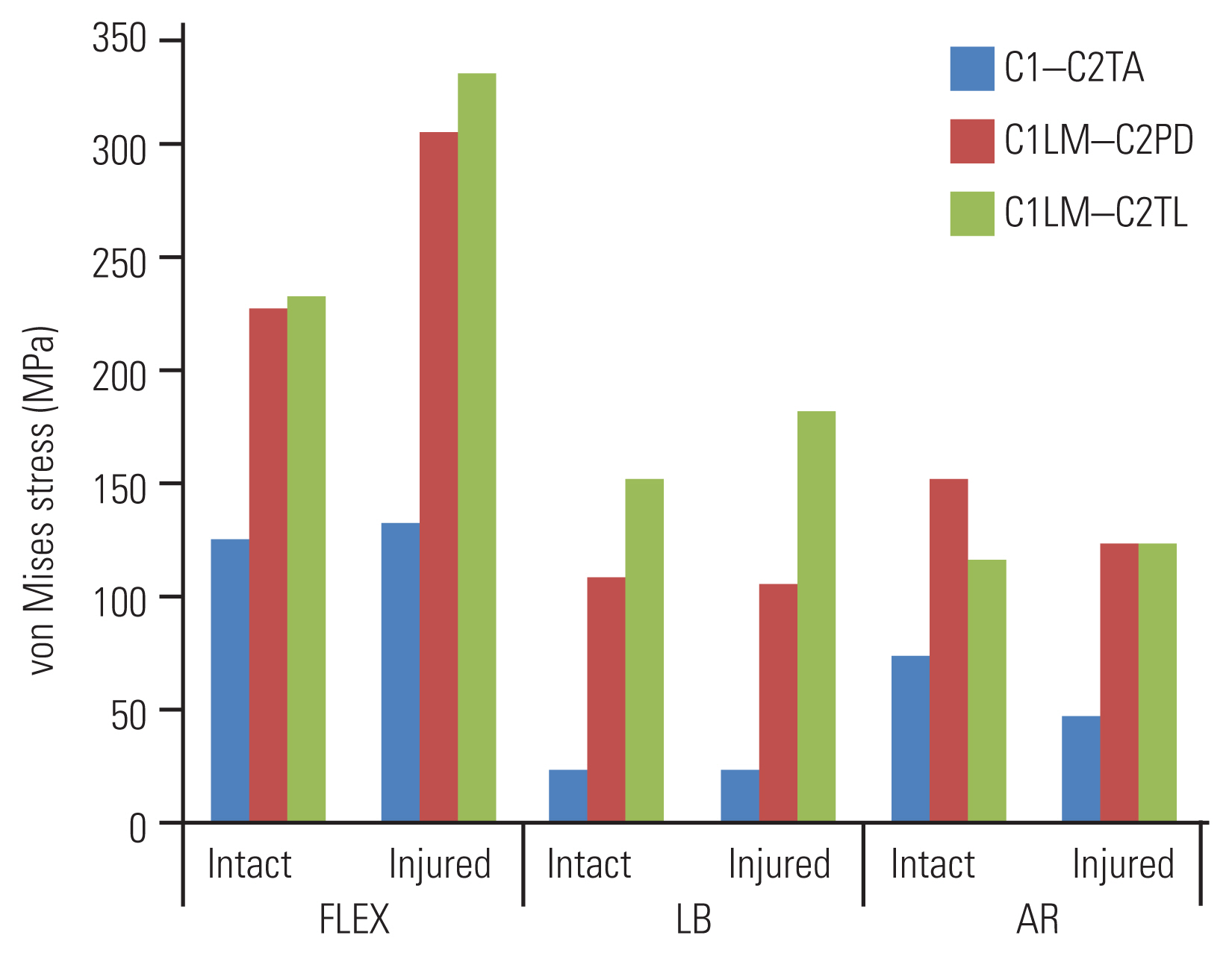
Maximum stress in the construct during flexion+extension (FLEX), lateral bending (LB), and axial rotation (AR) for both the intact and injured models. C1–C2TA, C1–C2 with a transarticular screw; C1LM–C2PD, C1 with a lateral mass screw and C2 with a pedicle screw; C1LM–C2TL, C1 with a lateral mass screw and C2 with a translaminar screw.
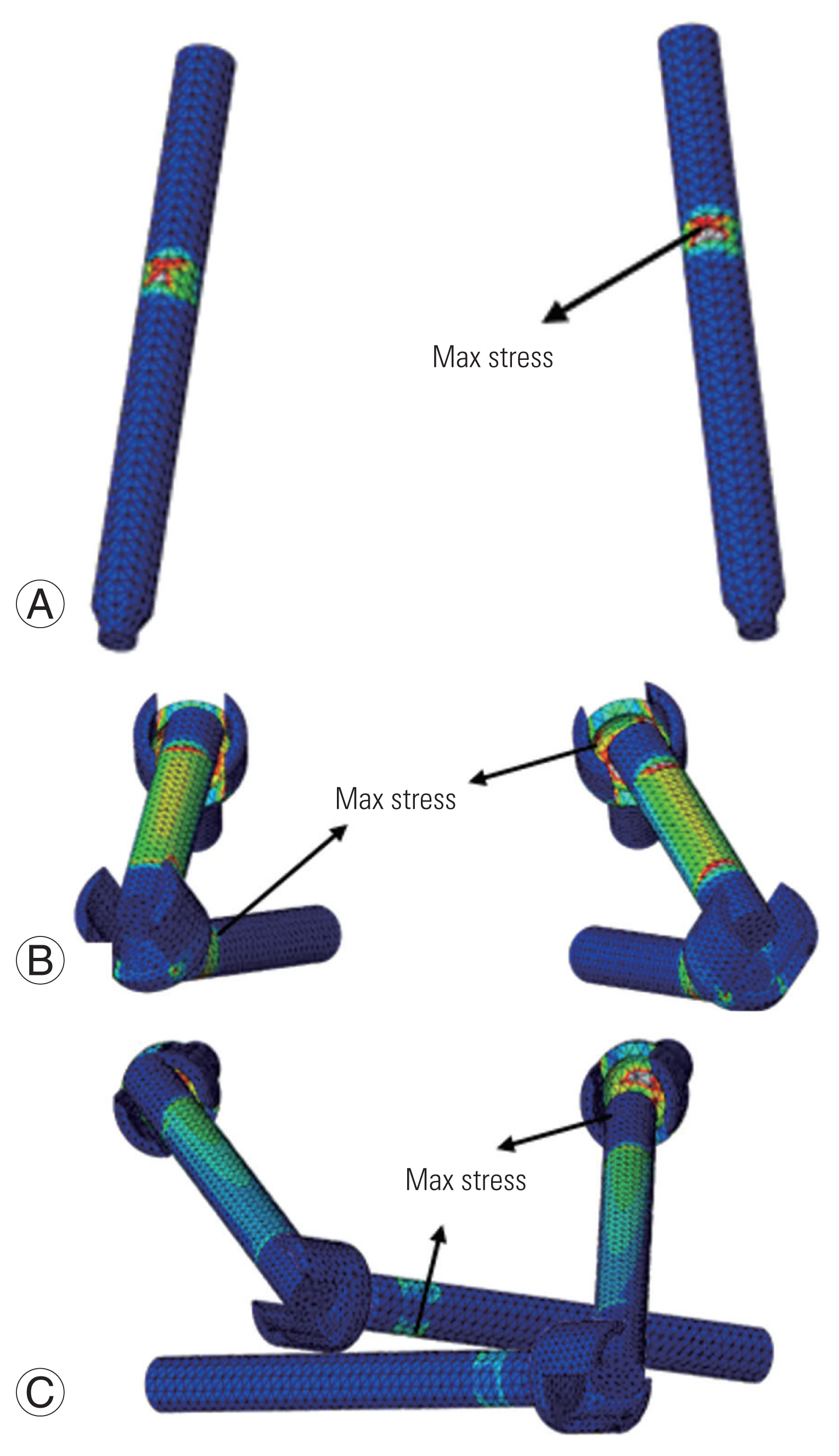
The location of the maximum (max) stress. (A) C1–C2TA construct. (B) C1LM–C2PD construct. (C) C1LM–C2TL construct. C1–C2TA, C1–C2 with a transarticular screw; C1LM–C2PD, C1 with a lateral mass screw and C2 with a pedicle screw; C1LM–C2TL, C1 with a lateral mass screw and C2 with a translaminar screw.
Discussion
Screw-based atlantoaxial fixation techniques have become popular because they achieve higher fusion rates than older techniques, such as wiring techniques. Transarticular, pedicle, and translaminar screw techniques are the most widely used screw-based fixation techniques for the atlantoaxial joint. The position of the vertebral artery limits the use of the transarticular screw in up to 20% of patients [9,21]. A pedicle screw is a better alternative to a transarticular screw; however, there may be complications associated with the variable location of the foramen transversarium [12]. A translaminar screw does not present these complications but may achieve lower biomechanical stability than the other techniques [22]. Many clinical and in vitro studies have evaluated the fusion rates and biomechanical stability of the joint with these techniques. Nevertheless, such studies cannot provide much information regarding the biomechanical behaviors of the atlantoaxial joint and the implants. Additionally, the reason for screw breakages has not been well investigated in many of these studies. By contrast, FE analysis permits the calculation of biomechanical values such as the stresses and strains on joints and implanted devices. In this study, a 3D FE model of the upper cervical spine was developed and used to evaluate the biomechanics of the atlantoaxial joint with the three most popular screw-based fixation techniques.
1. Range of motion
Odontoidectomy in the form of a type II dens fracture was added to the intact FE models to destabilize the joint. This fracture is among the commonest indications for atlantoaxial fixation [21]. In vitro studies have investigated the treatment of odontoidectomy with atlantoaxial fixation techniques and have observed differences in the biomechanical stability achieved in implanted intact and implanted destabilized cadavers [23,24]. Our FE results showed no difference in any direction between the implanted intact and implanted injured models when a transarticular construct was used. Moreover, a 100% ROM reduction in LB was achieved on the implanted injured model, whereas a 98% ROM reduction was achieved on the implanted intact model. The pedicle screw construct showed no difference in ROM reduction between the implanted intact and implanted injured models in AR. In LB, the ROM reduction achieved was greater in the implanted injured model than that in the implanted intact model. However, in FLEX, the ROM reduction was less in the implanted injured model than that in the implanted intact model. ROM reductions achieved in the implanted injured model with the translaminar screw construct in FLEX and LB were lower than those achieved in the implanted intact model. Our findings on the differences in ROM reduction in the implanted intact and implanted injured atlantoaxial joints confirm the findings of an in vitro study reported by Lehman et al. [24]. The translaminar screw construct was not as effective as the pedicle screw construct in reducing ROM once the odontoidectomy was added to the intact model.
Our study showed that except for C1LM–C2TL in LB, the three screw constructs reduced the C1–C2 segment ROM significantly in all loading directions. C1LM–C2TL achieved a 74% ROM reduction in LB, which was much less than the reductions achieved by the other two screw constructs. This finding is consistent with the findings of other biomechanical studies. Lehman et al. [24] found that after odontoidectomy, the translaminar screw construct did not reduce ROM in LB and AR as much as the pedicle screw construct did. Similarly, Lapsiwala et al. [25] reported the inadequate ROM reduction at the atlantoaxial joint in LB when the translaminar screw construct was used as opposed to when the transarticular and pedicle screw constructs were used. However, Gorek et al. [26] did not report a significant difference in ROM reduction between the translaminar and pedicle screw constructs. Interestingly, Dorward and Wright [23] wrote that the lower stiffness of the atlantoaxial joint after the addition of a translaminar screw construct did not prove the superiority of the transarticular and pedicle screw constructs. We, however, believe that the lower biomechanical stability of the joint that results from adding the translaminar screw construct can cause failure in the construct due to fatigue. More importantly, the high mobility of the upper cervical region subjects the construct to extensive cyclic loading.
2. Stress
The maximum stresses in C1LM–C2PD and C1LM–C2TL were higher than that in C1–C2TA by 56% and 59%, respectively. The reason for these significant differences between the screw constructs may be related to the cantilever concept on which the designs of C1LM–C2PD and C1LM–C2TL are based. Although these two constructs are surgically less challenging atlantoaxial fixation techniques than C1–C2TA, they are more prone to failure because of the higher stresses induced.
Failures in atlantoaxial fixation have been reported, usually due to breakage of the screw. Lateral mass screws are generally considered safe for use in the treatment of cervical spine trauma [27]. Nonetheless, there have been a few reports of breakage of lateral mass screws, the breakage rate being 0.6% [28]. For transarticular screws, the breakage rate is reported to be 4% [9], although the screw breakages were attributed to malpositioning. The breakage rates of translaminar screws are reported to be 12.5% and 6.7% [15,22]. No reports of screw breakage were found in literature for the pedicle screw. Based on these clinical reports, translaminar screws have the lowest durability and are most prone to breakage. By contrast, our FE analysis results showed that the lateral mass screw is more vulnerable to breakage because it experiences higher maximum stress (332 MPa) than the other screws experience. This inconsistency between the clinical results and our FE findings can be explained by the lower biomechanical stability of the translaminar screw construct when compared with those of the other two constructs. The high mobility of the upper cervical region results in high cyclic loading on atlantoaxial fixation constructs. This is particularly a problem for the translaminar screw construct because of its low biomechanical stability, mainly in LB. Additionally, our FE analysis showed that the maximum stress in the translaminar screw was at the screw neck, which is consistent with the screw breakages in the clinical reports.
3. Finite element model
In contrast to other recently developed FE models [17], our FE model was developed on the basis of the exact geometry of the upper cervical spine and took the asymmetry in the sagittal plane into consideration. The asymmetry in the sagittal plane causes coupled motion, which is dramatic in the cervical spine, especially the upper cervical region. Hence, our FE model could predict the kinematic behavior of the upper cervical spine more realistically than symmetric models. Our FE model used hexahedral elements to simulate the tectorial membrane and transverse ligament. In other FE models [17,29,30], spring, shell, and tetrahedral elements were used. Stress in the thickness direction cannot be measured in spring and shell elements, and tetrahedral elements are limited in their ability to represent material properties accurately.
4. Limitations
As with any numerical study, the present FE study had certain limitations such as variations in the geometry. The current model was based on a CT scan of a 35-year-old male, whereas in vitro studies are usually conducted on specimens from elderly patients. The effect of sex and the variations in material properties were also not considered in this study. Moreover, implants were assumed to be rigidly fixed to the bone.
Conclusions
In summary, our FE model predicted that the translaminar screw construct provides lower biomechanical stability, specifically in LB, than the transarticular and pedicle screw constructs. The translaminar screw construct also experiences higher maximum stresses than the transarticular and pedicle screw constructs. Therefore, translaminar screw constructs in atlantoaxial fixation may fail because of the high number of cyclic loadings that occur in the upper cervical spine. However, these results cannot be generalized as muscle damage during surgery may alter the biomechanics of the simulated implants. Furthermore, the alignment of the cervical spine was not considered in this study. These aspects should be explored in future studies and could be beneficial for surgeons during clinical decision making.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
D. U. Erbulut: conceptualization, data analysis, manuscript overview; Muzammil Mumtaz: modelling, data collection, data analyses, manuscript draft; Iman Zafarparandeh: modelling, conceptualization, manuscript draft; and A. F. Ozer: conceptualization, manuscript preparation.