Analgesic Effect of Intravenous Nefopam for Postoperative Pain in Minimally Invasive Spine Surgery: A Randomized Prospective Study
Article information
Abstract
Study Design
Randomized double-blind control study.
Purpose
To evaluate the effects of nefopam on reducing morphine consumption and postoperative pain in patients undergoing minimally invasive spine surgery (MISS) and to evaluate its effects on enhanced recovery after spine surgery.
Overview of Literature
Enhanced recovery after surgery (ERAS) has become a major goal for spine surgery. Multimodal pain management combining non-opioid analgesics is a key element of this. However, there is little evidence regarding the use of nefopam in spine surgery as part of an ERAS protocol.
Methods
One hundred patients undergoing MISS were randomized into two groups. Patients in the nefopam group received 20 mg of intravenous nefopam diluted in 100 mL of normal saline intraoperatively, followed by 80 mg of nefopam diluted in 500 mL of normal saline, given as a continuous infusion postoperatively for 24 hours. The control group received an identical volume of normal saline. Postoperative pain was managed by patient-controlled analgesia in the form of intravenous morphine. Morphine consumption in the first 24 hours was recorded as a primary outcome. Secondary outcomes regarding ERAS were also collected.
Results
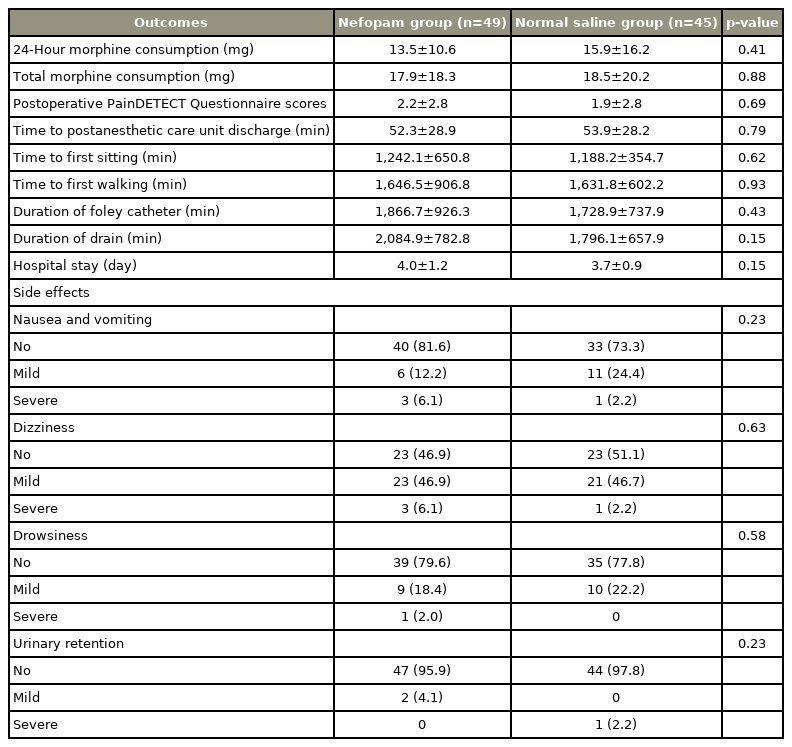
There were no significant differences in either total morphine consumption or postoperative pain score in the first 24 hours postoperatively between patients receiving nefopam and the control group. Morphine consumption in patients receiving nefopam was 13.54±10.64 mg compared with 15.86±16.2 mg in the control group (p=0.41). Time to postanesthetic care unit discharge, times to first sitting and walking, length of hospital stay, as well as duration of Foley catheter use and time until drain removal were also similar. There were no serious adverse effects of nefopam compared with normal saline.
Conclusions
Nefopam did not significantly reduce opioid consumption or postoperative pain score. Adding nefopam as part of multimodal analgesia did not show beneficial effects for enhancing recovery after spine surgery.
Introduction
Enhanced recovery after surgery (ERAS) has become a major goal for spine surgery, resulting in improvement in surgical outcomes and cost savings. This concept requires a multidisciplinary approach, including a minimally invasive surgical technique, multimodal pain management, early mobilization, early nutrition, and patient education. Although there is still no definite protocol for ERAS in spine surgery, recommendations focus on opioid-sparing multimodal analgesia [1]. The paradigm of perioperative pain management in spine surgery has shifted from opioid-based analgesia toward non-opioid analgesics. Adverse effects of opioids (e.g., drowsiness, nausea/vomiting, dizziness, and bowel ileus) can potentially delay postoperative mobilization and nutrition. Non-opioid analgesics that have various mechanisms of action provide optimal pain relief as well as reduce overall opioid consumption and opioid-related adverse effects. Acetaminophen, gabapentin, lidocaine, ketamine, magnesium, dexmedetomidine, and dexamethasone have demonstrated opioid-sparing effects and the ability to significantly reduce postoperative pain in several clinical studies, including in major spine surgery [2].
Nefopam is a non-opioid, non-steroidal anti-inflammatory drugs, and centrally acting analgesic. Its mechanism of actions for pain relief involves the inhibition of serotonin, norepinephrine, and dopamine reuptake in descending pain modulation as well as the inhibition of sodium channels and calcium channels at the synaptic area of the dorsal horn of the spinal cord, resulting in activities against nociceptive and neuropathic pain [3,4]. The inclusion of nefopam in a multimodal analgesia regimen for major surgery was reported to reduce postoperative pain intensity and the consumption of 13 mg of morphine [5–7]. In addition, nefopam provided a greater analgesic effect in patients with severe preoperative pain in hip arthroplasty [5]. A recent prospective study in patients who had undergone percutaneous endoscopic lumbar discectomy demonstrated that nefopam significantly decreased neuropathic pain symptoms, including paresthesia and dysesthesia [8]. However, there is little evidence regarding nefopam in spine surgery as part of an ERAS protocol. The advantages of nefopam for spine surgery over other analgesics include preserved platelet function, no sedative effect, and reductions of both nociceptive and neuropathic pain.
This prospective study aimed to determine the analgesic effects of nefopam on morphine consumption, postoperative pain score, and time to recovery in patients undergoing minimally invasive spine surgery (MISS).
Materials and Methods
1. Study design
The protocol for this study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB approval no., 377/62), and complied with the Declaration of Helsinki guidelines. This study was registered prior to patient enrollment at clinicaltrials.in.th (Thai Clinical Trials Registry no., TCTR20190525001). It was a randomized, double-blind, placebo-control study. This manuscript adheres to the applicable CONSORT (Consolidated Standards of Reporting Trials) guidelines. Informed consent was obtained from 100 patients aged between 20 and 80 years old with the American Society of Anesthesiologists physical status I and III undergoing elective minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF) under general anesthesia at King Chulalongkorn Memorial Hospital, Bangkok, Thailand. The exclusion criteria were patients with a history of chronic pain, epilepsy, coronary artery disease, glaucoma, severe liver, and kidney dysfunction, use of monoamine oxidase inhibitors, allergic to medications, and impaired cognitive function. The operations were performed by two surgeons (W.S. and W.Y.) with the same surgical technique. Their levels of experience and skill in MISS were similar.
After obtaining informed consent, the patients were asked to complete the painDETECT Questionnaire (PDQ) to assess preoperative neuropathic pain [9]. This questionnaire classifies lower back and leg pain into three groups: neuropathic pain, unclear, and non-neuropathic pain. Patient education, including a rehabilitation program, was performed by a spine nurse and a physiotherapist perioperatively. The patients were randomized using computer-generated blocked randomization with randomly selected block sizes into two groups: a nefopam group and a control group. Sealed envelopes were prepared by a research assistant and were opened on the day of surgery by a nurse anesthetist, who prepared both nefopam and normal saline. The solutions of nefopam and normal saline were identical in volume and color. Both solutions were labeled as a study drug so that the anesthesiologist, the surgeon, and the pain assessor could not identify the solutions. In the operating room, the patients were not premedicated and received standard monitoring. All patients were given 2–3 mg/kg propofol and 0.15–0.2 mg/kg cisatracurium intravenously for the induction of anesthesia, followed by desflurane for the maintenance of anesthesia. After the induction of anesthesia, 10 mg of dexamethasone was given intravenously to all patients for antiemetic, anti-inflammatory, and analgesic purposes. Intravenous fentanyl (no more than 5 μg/kg) was allowed during intubation and throughout the surgery. The last dose of fentanyl was given 45 minutes prior to extubation, according to the judgment of the anesthesiologist. At the end of the surgery, patients in the nefopam group received 20 mg of intravenous nefopam diluted in 100 mL of normal saline intraoperatively [10]. The control group received an identical solution of normal saline in the same manner. Anesthesiologists, surgeons, pain assessors, and patients were blinded to the study groups. All patients received 40 mg of intravenous parecoxib (or 30 mg of ketorolac in sulfa allergic patients), 8 mg of ondansetron for antiemetic prophylaxis, and local infiltration of 10 mL of 0.25% bupivacaine immediately prior to skin closure. Normothermia and normovolemia were maintained throughout the perioperative period. After extubation, all patients were transferred to the postanesthetic care unit (PACU).
Patients were discharged from the PACU when a modified Aldrete score of ≥9 was achieved. At the ward, patients in the nefopam group received 80 mg of nefopam diluted in 500 mL of normal saline infused in 24 hours postoperatively [10], while the control group received an identical solution of normal saline. Both groups received 1,000 mg of paracetamol orally every 6 hours, 90 mg of etoricoxib once daily in the morning, and 75 mg of pregabalin at bedtime. Rescue pain was managed by patient-controlled analgesia with morphine (1 mg bolus, 6-minute lockout, no continuous infusion). A numerical rating scale of 0–10 was used to assess the severity of pain both at rest and upon movement at the PACU and on postoperative days 1, 2, and 3. Pain upon movement was defined as pain upon changing position, sitting, or walking. The PDQ was repeated on postoperative day 1. All patients were encouraged to have early mobilization and nutrition. Morphine consumption in the first 24 hours and during the postoperative period was recorded as the primary outcome. Secondary outcomes regarding ERAS were collected, including the time to PACU discharge, first sitting (defined as the interval from the end of surgery to sitting), first walking (defined as the interval from the end of surgery to walking), time to discontinuing Foley catheter use and drain removal, and length of hospital stay (defined as the time between the end of surgery until readiness for discharge from hospital). The adverse effects of pain medications, including nausea/vomiting, dizziness, drowsiness, tachycardia, and hypertension, were closely observed.
2. Statistical analysis
The sample size was calculated using 17 mg of morphine, as the average opioid consumption in patients undergoing spine surgery. The power analysis was performed using 9 mg of morphine as the minimum clinical difference and 90% power. It was hypothesized that adding nefopam perioperatively would reduce morphine intake to 8 mg. On this basis, a sample size of 46 patients per group was required; after accounting for a 10% dropout rate, a total of 100 patients were required. Categorical data were compared using chi-square test or Fisher’s exact test. Continuous data were compared between groups by unpaired t-test or Mann-Whitney test using Stata ver. 14.0 (Stata Corp., College Station, TX, USA). A p-value of <0.05 was considered to indicate a statistically significant difference.
Results
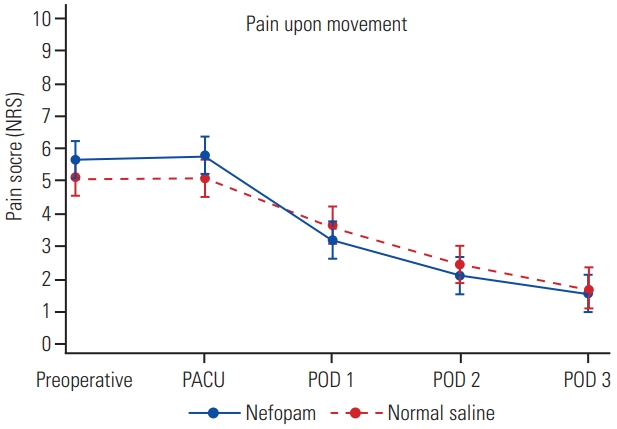
One hundred surgical patients were prospectively randomized in this study between July 2019 and September 2020 (Fig. 1). Six patients were excluded: because of deviation from the study protocol (one patient in the normal saline group), changing operation from minimally invasive surgery to open surgery (three patients in the normal saline group and one patient in the nefopam group), or falling unconscious and subsequently dying due to a brain tumor (one patient). As such, data were obtained from 49 and 45 patients receiving perioperative nefopam and normal saline, respectively. Demographic data and patient characteristics are shown in Table 1. There were no significant differences in 24-hour morphine consumption and total morphine consumption between the two study groups (Table 2). Patients receiving nefopam required 13.5±10.6 mg of morphine, compared with 15.9±16.2 mg of morphine in patients in the control group in the first 24 hours postoperatively (p=0.41). In addition, postoperative pain scores for both resting pain (Fig. 2) and pain upon movement (Fig. 3) were similar (nefopam group 2.3±2.8, 4.0±2.6, 1.5±1.6, 1.1±1.4 versus control group 2.1±2.3, 3.7±2.8, 1.8±1.9, 1.1±1.6 at PACU, and on postoperative days 1, 2, and 3, respectively). There were no differences in time to PACU discharge, and times to first sitting and walking or in the duration of Foley catheter use and time until drain removal. The hospital stays were comparable in the two groups. The PDQ assessing neuropathic pain showed significant improvement postoperatively in both groups. There were no serious adverse effects (nausea/vomiting, dizziness, and urinary retention) of nefopam compared with the findings with normal saline.

The graph showed postoperative pain score at rest between the study groups. NRS, Numerical Rating Scale; PACU, postanesthetic care unit; POD, postoperative day.
Discussion
This study showed that the addition of 24-hour intravenous nefopam in a multimodal analgesic regimen provided no beneficial effects on morphine consumption, postoperative pain, or functional outcomes in MISS. The clinical outcomes regarding early ambulation and the hospital stay in patients receiving nefopam were similar to those in the control group. The adverse effects of nefopam did not differ compared with the findings with normal saline. Nefopam is a non-opioid analgesic that may be included in multimodal analgesic regimens during the perioperative period. It has activities against both nociceptive and neuropathic pain without affecting platelet aggregation and has no sedative effect [4]. In previous studies, nefopam was found to reduce postoperative pain intensity and morphine consumption by up to 13 mg in major surgery [5–7]. The results of this study did not reveal the same outcomes as those studies focusing on orthopedic and hip surgeries. Hip replacement surgery involves muscle splitting, capsulotomy, and the removal of damaged bone, whereas MISS, which utilizes a smaller surgical incision, is associated with less spinal muscle and bony injury, resulting in less postoperative pain. Adding nefopam in patients undergoing an operation with a low amount of postoperative pain might thus be less beneficial than using it for more painful operations.
Regarding spine surgery, a recent study in patients undergoing open spine surgery receiving intravenous nefopam administered before skin incision and before the end of the operation demonstrated similar morphine consumption and postoperative pain compared to those in the placebo group [11]. Our study showed the same results, namely, there was no additional analgesic effect of nefopam despite its continuous infusion for 24 hours after the operation.
The activity of nefopam against neuropathic pain in MISS was limited and unclear. The results of this study contradict a previous study on patients who underwent percutaneous endoscopic lumbar discectomy, which demonstrated that nefopam significantly decreased neuropathic pain symptoms, including paresthesia and dysesthesia [8]. MIS-TLIF provided spinal decompression following fixation and stabilization of the spine. As a result, there was immediate alleviation of static and dynamic neuropathic pain symptoms. In this study, the PDQ showed a significant decrease in neuropathic pain scores between the preoperative and postoperative periods in both study groups, indicating successful surgery. The administration of additional nefopam in patients undergoing MISS might be less beneficial for postoperative neuropathic pain in this setting.
The strength of this study was the secondary outcomes focusing on recovery-related parameters, including ambulation function, tube removal, and the length of hospital stay. This is the first study of nefopam regarding its analgesic effects on postsurgical function as part of an ERAS protocol. Unfortunately, adding nefopam did not demonstrate any beneficial impact on enhanced recovery after spine surgery. The limitations of this study include the exclusion of patients with coronary artery disease, for whom COX-2 inhibitors are contraindicated in the study protocol. Second, the study was performed in a university hospital in which ward rounds are mainly performed by residents, which may affect the time to ambulation and tubing removal. In addition, the hospital stay appeared to be longer than the standard care, which could be attributable to Asian culture. The power analysis was a concern because of the drop out of five participants (five out of 50) in the normal saline group. For 90% power, 46 patients per group were required. However, the analysis of 45 patients in the normal saline group ensured more than 80% power of this study. Future study could focus on the role of nefopam in treating preoperative neuropathic pain due to degenerative spine or its analgesic effect in open spine surgery.
Conclusions
Nefopam did not significantly reduce opioid consumption or postoperative pain score in MISS. Adding nefopam as part of multimodal analgesia did not show a beneficial effect on enhanced recovery after spine surgery.
Notes
No potential conflict of interest relevant to this article was reported.
Author Contributions
Pornpan Chalermkitpanit: conceptualization, writing (original draft); Worawat Limthongkul: supervision, resources; Wicharn Yingsakmongkol: methodology, resources; Marvin Thepsoparn: data collection, resources; Patt Pannangpetch: data analysis, methodology; Nattapat Tangchitcharoen: data collection; Teerachat Tanasansomboon: writing (review and editing); and Weerasak Singhatanadgige: writing (review and editing), supervision, resources.
Funding
This research project is supported by Ratchadapiseksompotch Fund Chulalongkorn University (Funding no., CU_GR_63_100_30_07).
Acknowledgements
We thank the anesthesiologists Prof. Supranee Niruthisard, Dr. Danita Weeracharoenkul, and Dr. Pongkwan Jinaworn; nurse anesthetists Ms. Panatchakorn Pitugchaiyawong and Ms. Supreeda Dowkrajang; and research assistants Mr. Jirat Chantakhat and Ms. Chayanit Apibanmongkol.