“Spine Surgery Checklist”: A Step towards Perfection through Protocols
Article information
Abstract
Study Design
A retrospective study.
Purpose
This study aimed to evaluate the effectiveness of a novel checklist that was designed specifically for the “spine-surgery-subspecialty” to reduce the incidence of some common preventable human errors and major perioperative complications in spine surgery.
Overview of Literature
We propose a unique spine surgery-specific checklist that recognizes the risk factors, anticipates the possible human errors, and thus helps in preventing these errors. This checklist is associated with increased patient safety awareness, improved communication (keeps everyone updated regarding their responsibilities), reduction in the surgical claims, and reduction in the number of postoperative complications, including mortality.
Methods
This retrospective pilot study was performed at single center on 858 spine surgery patients. The patients were divided into the following two groups: the study group (after implementation of the checklist [2016–2017]) and the control group (before the implementation of the checklist [2015–2016]). The incidence of common preventable human errors and major perioperative complications in spine surgeries were recorded and compared between the two groups.
Results
The prevalence of wrong-level surgeries was 0%, and the overall prevalence of the preventable errors was 1.63% (7/428). The rate of adverse, near-miss, and no-harm events was 0.23% (1/428), 0.70% (3/428), and 0.70% (3/428), respectively. The preoperative, intraoperative, and postoperative errors were 0.70% (3/428), 0.23% (1/428), and 0.70 (3/428), respectively. The reoperation rate related to preventable errors reduced after the checklist was used. There were significant differences in the total preventable errors related to complications, such as infections, prolonged hospital stays, and unplanned hospital readmission/revision surgeries (p=0.001).
Conclusions
The authors propose the first-of-its kind spine surgery-specific checklist that is comprehensive and involves perioperative parameters. The checklist is easy to use, safe, and effective for reducing the unforgiving errors and perioperative complications. However, its broader implementation would require validation in large, multi-center, randomized control studies.
Introduction
“To err is human”; however, this statement cannot be used as an argument in a court of law in case of preventable errors [1]. To ensure safe spine surgery, human errors need to be minimized, and this requires close cooperation of the operating room (OR) personnel and every healthcare worker involved in patient care from the time of admission to the time of discharge. It is important that they know their roles and responsibilities and are capable of responding quickly and efficiently. This can be managed with ease if the personnel are prepared for their jobs. Following a protocol that helps monitor the events increases the likelihood of the recognition and thus prevention of potential errors [2]. Typical reasons for these errors include lack of training or experience, fatigue, stress, non-adherence to medical standards, lack of regulations/rules, high workload, inadequate communication (and poor relationships among health professionals), and social factors [3–5]. Haynes et al. [6] showed that the use of a checklist developed by the World Health Organization (WHO) to prevent errors during surgical procedures significantly lowers the surgical morbidity and mortality. Currently, the surgical safety guidelines and checklists are mainly generic and do not address the specialty and subspecialty-specific patient issues and risk factors. Kim et al. [2] suggested that different surgical subspecialties should develop specific guidelines in order to effectively manage their respective patients. An adverse consequence of an error can lead to not only morbidity but also mortality a well as emotional, social, and legal implications. This is especially more relevant in the context of spine surgeries where the stakes are high, considering the nature of complications and fear of spine surgery in the general public. For instance, there is a high prevalence of wrong-level surgery among spine surgeons; one in every two spine surgeons may perform at least one wrong-level surgery during his/her career [7]. In one of the national surveys conducted by Jhawar et al. [8], the neurosurgeons recognized fatigue, unusual time pressure, emergent operations, unusual patient anatomy, and failure to verify the operative site using radiography as factors that contribute to wrong-level surgeries. Although there are significant limitations to the survey-based methodology, the data suggest that for the prevention of such errors, surgeons need to recognize the risk factors. To our best knowledge, thus far, few studies have reported on the use of a surgery checklist in spine surgery than in other areas of surgery and intensive care medicine.
We propose a unique spine surgery-specific checklist that recognizes the risk factors, anticipates the possible human errors, and thus can help to prevent such errors. This checklist is associated with increased patient safety awareness, improved communication (keeps everyone updated regarding their responsibilities), reduction in the surgical claims, and reduction in the number of postoperative complications, including mortality. Thus, the execution becomes relatively error free and straightforward and promotes good cooperation in the OR.
Materials and Methods
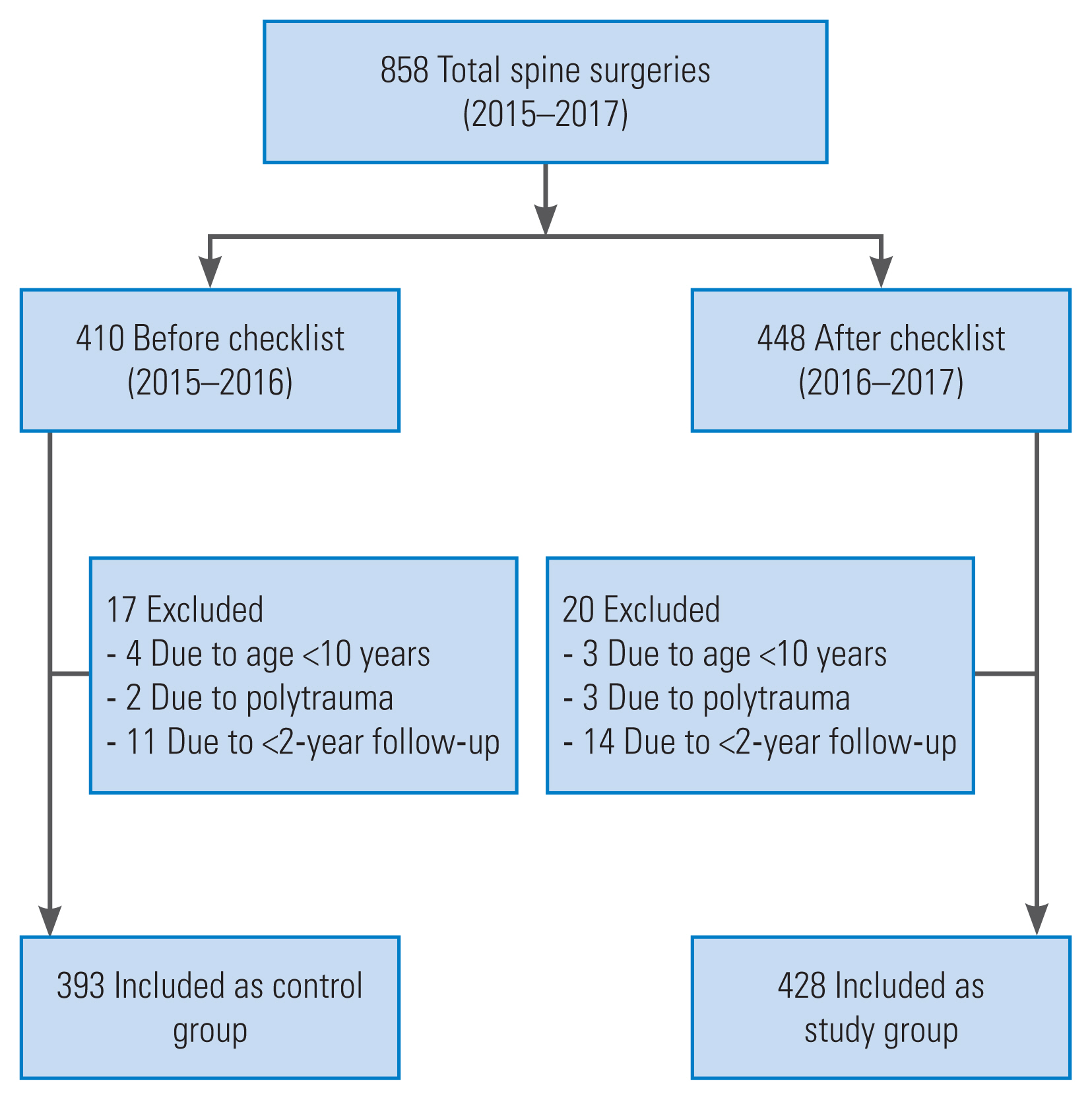
This retrospective study was performed at a tertiary care hospital in order to evaluate the efficacy of the proposed novel spine surgery checklist as per the requirements of the spinal procedures. The novel checklist was implemented from March 2016 onward after obtaining approval from the ethical and review committee of Bombay Hospital and Medical Research Centre (IRB approval no., 9120/2020–21/MRC). A total of 448 patients were scheduled to undergo various kinds of spine surgeries and were scanned using the checklist during the 1-year period from March 2016 to February 2017. Pediatric patients aged <10 years and those with polytrauma were excluded from the study (Fig. 1). The study group patients were compared with the controls (410 patients) in whom the checklist was not used in the previous year (2015–2016). The perioperative demographic and clinical data were recorded and compared between the study and control groups (Table 1).
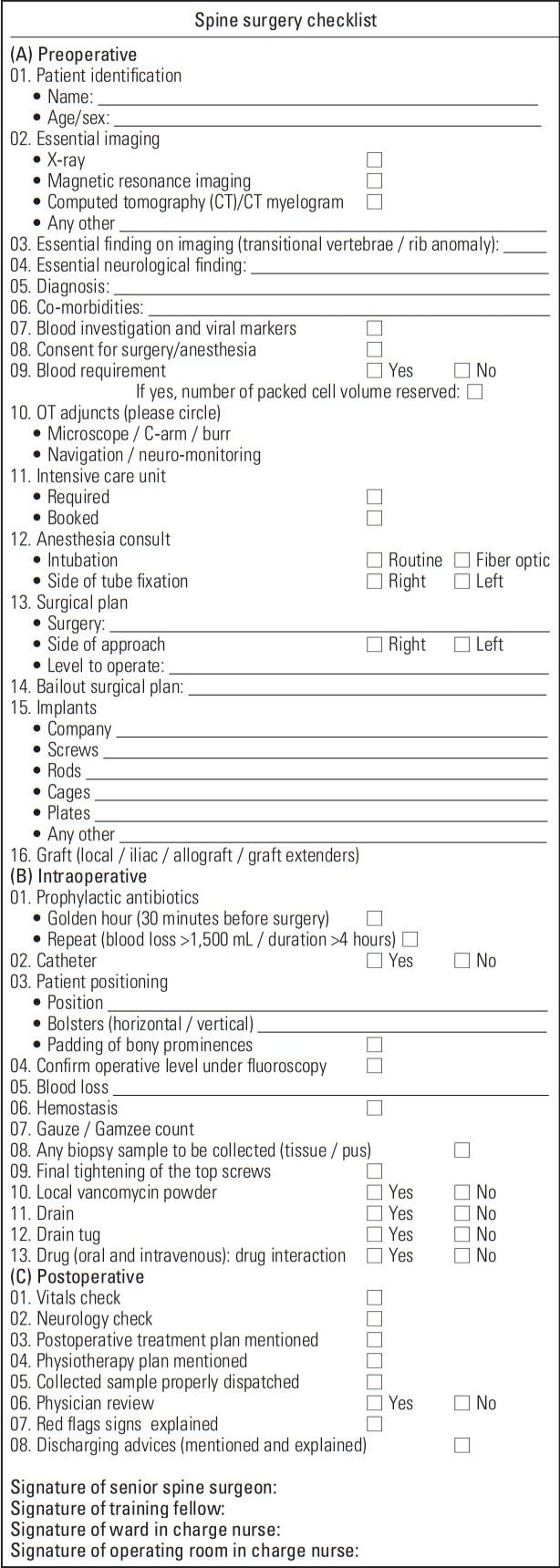
The proposed novel spine surgery checklist comprised the following three parts (Fig. 2).
1. Preoperative checklist
Patient identification, diagnosis, surgical plan, and procedural requirements. The checklist was completed in the wards by the training fellows under the guidance of the operating surgeon after discussion and submitted to the OR nursing staff on the day before the surgery in order to ensure the necessary preparations and identify if any of the requirements were difficult to fulfill.
2. Intraoperative checklist
This checklist involves the steps that need to be followed from the point of positioning of the patient to identify the correct operative level and other vital steps to be taken care of throughout the procedure. This was in addition to and independent of the routinely performed “time-out-system” employed by the nursing staff that is a standard practice followed in the operation theater for many years.
3. Postoperative checklist
This checklist included postoperative checks in the recovery room and the plan to be followed in the wards until patient discharge, including written and informed instructions to be followed at home. The advice regarding dressing protocol, physiotherapy, mobilization, diet, follow-up, and most importantly, red flags that would indicate a mild or severe complication was included.
At each and every step, a discussion was performed among training fellows and the operating surgeon. The operating surgeon supervised, counter-checked, and revised the details of the checklist, as needed, to resolve any compliance issues to ensure quality and patient safety. In order to determine the effectiveness and measure the outcomes of the checklist, the incidence of wrong-level surgery and the overall incidence of the errors in the parameters included in the checklist were considered. Errors and adverse events were documented and quantitatively analyzed as per the frequency of their occurrence and the severity of the possible consequences by the two fellowship trained spine surgeons. Stratification was performed by an interdisciplinary team that included a senior spine surgeon, training fellows, and OR chief nurse. The recorded adverse events were an intensive care unit (ICU) stay >24 hours, mechanical ventilation >48 hours, readmission to the ICU, unplanned reoperation, acute renal failure, sepsis, septic shock, myocardial infarction, deep-vein thrombosis, pulmonary embolism, cerebral infarction, meningitis, pneumonia, blood loss ≥500 mL during the operation or bleeding requiring blood transfusion of two or more units, cardiac arrest, wound disruption, surgical site infection, surgical site hematoma or seroma, cerebrospinal fluid (CSF) leakage from the wound, pressure ulcer, urine retention, unplanned readmission, and death.
Human errors were divided into the following three categories: (1) adverse event, where an error results in harmful consequences; (2) a near miss, when an error is realized just in the nick of time and abortive action is employed to cut short its translation; and (3) no harm, when the error is not recognized and the step is performed; however, the expected adverse event does not occur (Table 2) [5,6]. The adverse events were retrospectively analyzed and categorized as theoretically preventable and unpreventable events, based on the consensus of the senior spine surgeon, physician, and two fellowship trained spine surgeons. An infection was considered preventable if the contamination or the clinical factors that enabled the infection could have been prevented by following adequate sterile precautions or administering antibiotic prophylaxis. Other adverse events, such as bleeding, CSF leakage, and delay, were considered preventable with suboptimal human action. Infections in patients who are prone to infections or complications owing to contributory factors (co-morbidities, osteoporosis, and smoking) leading to pseudoarthrosis and implant failure were considered non-preventable. The outcome was assessed in the form of availability of the checklist, the frequency of its use in the various spinal procedures, and the incidence of any adverse events (wound complications and unplanned readmissions) in the perioperative period with regard to the various parameters suggested and implemented into the novel checklist (Table 3).
The significance of the categorical variables was tested using Pearson’s chi-square test for the normally distributed numerical variables (age) using the independent sample t-test and for the non-normally distributed numerical variables (durations and periods and average complication rate) using a nonparametric Mann-Whitney U-test. The significance of difference as per the type of operation was tested using the Kruskal-Wallis test. A p-value <0.05 was considered statistically significant. The assessment of errors and statistical analysis were performed using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA).
Results
The spine surgery checklist was proposed by the authors and implemented at the institute after providing formal introduction and basic education about the checklist to the training fellows, ward, and OR nurses under the supervision of the senior spine surgeon. The pilot project included 448 patients of which 20 who did not fulfill the inclusion criteria were excluded; thus, the final study sample comprised 428 patients (Fig. 1). The control group included 393 patients (2015–2016) who were analyzed before the implementation of the checklist. There were no significant differences in the average age (p=0.283) and male-to-female ratio between the two groups (Table 1). The surgical divisions and etiologies included in the study have been detailed in Table 1. The most commonly performed surgery was lumbar microtubular decompression for degenerative lumbar diseases. There were no significant differences in the surgical indications and the anatomical region-wise surgical procedures between the two groups (Table 1). The mean hospital stay was marginally higher in the study group (p=0.015), and the average follow-up duration of the two groups was comparable. In the pre-checklist cohorts, at three instances, errors occurred owing to the following oversights in preoperative vigilance: (1) blood products were not reserved preoperatively for a patient posted for a two-level transforaminal lumbar interbody fusion, and this was realized when the patient was about to be positioned on the operating table; (2) rescue pedicle screws (7.5-mm diameter) were not kept handy, and the 6.5-mm-diameter screws failed to obtain adequate purchase at S1 during an L5–S1 interbody fusion (Table 2); and (3) the preoperative antiplatelet medications were not stopped preoperatively, and the fellows and surgeons were unaware of this, resulting in increased surgical blood loss. In the first case, the blood sample was sent immediately from the OR to our blood bank for grouping and cross-matching; any possible harm was prevented because sufficient blood of the same group was available, if required. In the second case, the anesthesia time for the patient was more, and there was prolonged prone posturing until arrangements for the bigger diameter rescue screws could be made, although the extubation and postoperative periods were uneventful. In the third case, the increased blood loss was managed by the transfusion of two units of blood, and the patient became hemodynamically stable. With regard to the intraoperative errors in the pre-checklist phase, the incidence of wrong-level surgery was 0%. A wrong side procedure of root block was performed in one patient; however, this was soon realized and corrected before turning the patient back to the supine position. In terms of postoperative errors during the pre-checklist phase, at two instances, the patients were discharged without being provided an explanation regarding the appropriate postoperative physiotherapy plan. This oversight was detected at their first follow-up visit after 1 week of discharge. However, neither of the two patients suffered or experienced any adverse consequences. In one major adverse event in the pre-checklist group, following an uneventful minimally invasive lumbar decompression surgery, the patient was discharged without postoperative advice, especially regarding the red flags. The patient developed delayed CSF leak through the surgical wound, ignored it initially, and presented to us with florid wound infection and meningitis. The patient underwent immediate wound wash, sealing of the tear with fibrin glue, and administration of intravenous (IV) antibiotics sensitive to Escherichia coli that the culture revealed. After a supervised hospital stay and multiple cross-consultations with the associated specialists who included neurologists and infection disease specialists for approximately 1 month, the patient died. Compliance failure for the preoperative and postoperative plans occurred when there was a change in the training fellows and the workload was at its peak. The rate of adverse, near-miss and no-harm events was 0.23% (1/428), 0.70% (3/428) and 0.70% (3/428), respectively. The rate of preoperative, intraoperative, and postoperative errors was 0.70% (3/428), 0.23% (1/428), and 0.70% (3/428) respectively (Table 2). The percentage of preventable adverse events was significantly higher before the institution of the checklist as compared with that after the institution of the checklist (14.24% versus and 3.50%) (Table 3). The rate of unpreventable adverse events before (14.5%) and after (10.28%) the execution of the checklist was not significantly different (Table 3). Thus, although the checklist was effective in significantly reducing the preventable adverse events and contributed toward marginal reduction of unpreventable adverse events via early recognition and prompt treatment during the perioperative period.
Discussion
Various potential complications may occur during spinal surgery; few of these are preventable, whereas most are non-preventable. “Preventable adverse events” refer to those that involve an adverse consequence resulting from improper application of a medical care protocol rather than an underlying disease that occurred [1,7]. The institution of a “checklist” is one of the key critical strategies deployed to reduce preventable human errors. Previous studies have shown the efficacy of using a checklist for reducing the overall rate of adverse events, even mortality, and its positive impact on communication and teamwork [6,9–12]. A study on WHO checklist implementation and the SURPASS study have shown a reduction in the mortality from 1.5% to 0.8% and a reduction in the overall complication rate from 15.4% to 10.6% with the implementation of a perioperative checklist [6,13]. In a similar manner, a recent study has demonstrated that the successful implementation of the WHO Surgical Safety Checklist with the provision of pulse oximeters in a resource-limited setting led to a reduction in the overall postoperative complications from 21.5% to 8.8% [14]. However, current surgical safety guidelines and checklists are generic, and there is a recommendation for different surgical subspecialties for the development of specific guidelines for their set of patients [2]. This is essential because each specialty involves a unique set of complications; further, some of these potential complications assume high priority and have profound medico-legal implications. Wrong-level surgery is one such important example that is unique to spine surgery. In a landmark study by Mody et al. [7] that included 415 surgeons, 15% reported having prepared the incorrect spine level at least once during their professional experience; however, in each case, they had detected the error before making the incision, and 50% of them admitted that they had performed wrong-level surgery once or more during their career. Although various recommendations have been given by various authoritative bodies [8,15,16], the common drawback is that these protocols uniquely stress on avoiding “wrong-level surgery” that, although, is a top-priority adverse event, is just one potential complication that is associated with spine surgery and is not part of a comprehensive “checklist”. On the basis of their experience, the authors of the current study attempted to bridge this gap in the current surgical setting and instituted a comprehensive 37-point checklist to introduce safety measures during the perioperative care of a patient requiring spine surgery. The results of this study showed a reduction in the occurrence of preventable adverse events from 14.24% to 3.50% after the checklist was implemented. The authors noticed that all the adverse events, including surgical site infections, especially the infections categorized as preventable, prolonged hospital stay, and unplanned readmissions significantly reduced after the checklist was implemented. Dependence on imaging is integral to spine surgery; moreover, it is extremely important that radiographs, magnetic resonance imaging, computed tomography (CT), and CT myelogram (in indicated situations) are available for the patient preoperatively, and this is one of the many reasons for subdividing the checklist into a preoperative component. Omission on the part of the patient to bring all the imaging documents to the hospital and misplacement of these documents in the radiology department, wards, or clinical meeting rooms are potential reasons for the unpleasant postponement or delay in some surgeries; such incidences enhance the anxiety of the patient, relatives, and the surgical team. Although this issue can be resolved via the adoption of a digital platform, the infrastructure may not be available universally. Furthermore, spine surgeries and many other orthopedic/neurosurgical procedures are high demand and gadget intensive, and most operation theater complexes have limited resources with respect to high-end infrastructure, such as navigation, microscope, high-speed drill, and neuro-monitoring. Thus, it is important to check beforehand (preoperatively) if the required gadgets are available and are not being used by some other surgeon so that surgical time can be determined, or in case the required gadget is unavailable or needs repair, the necessary action can be taken. The spectrum of spinal implants is enormous, and a vast majority of operation theater complexes do not have the space to stock up all spinal implants, and thus, the inventory is generally ordered by the surgical team and made available by the implant company. This aspect is very critical in scheduling and planning because occasionally, there may be omissions by the ordering or supplying team, and this communication gap may result in disasters, as was observed in one case where rescue screws with a bigger diameter were needed. The current checklist designed by the authors allows the surgical team to think and recall certain important aspects related to the patient’s individual biology and its impact on surgical success. The need to plan for a bailout and the need for bone-graft substitutes prompt the surgical team in their busy practice to pay attention to the preoperative bone mineral density of the patient and take the required action. Apart from marking and confirming the correct operative level and following the golden hour concept of administering pre-incision IV antibiotics that can critically minimize the most concerning complications, the intraoperative component of the checklist has provisions for critical steps related to spine surgery, such as marking the side of the incision (the spine being a symmetrical structure, the surgeon prefers to generally choose a particular side of approach) and final tightening of the inner screws (torque–counter–torque), which can have disastrous complications if forgotten. In comparison with the WHO checklist, the current checklist might appear elaborate. However, spine surgery involves several complexities, such as the presence of various levels, sides, different regions, and approaches and need for intraoperative imaging, the issue of implants, and the liability of any complication, that can result in a considerable degree of morbidity and even mortality. The surgical team is solely responsible for the patient’s care and the continuum of care from the operation theater to discharge and beyond. In this respect, the checklist extends to a postoperative component that, in addition to routine postoperative advice, ensures a discussion regarding red flags. Postoperative CSF leaks that are not detected during the primary surgery [17] or manifest later [18] are not uncommon following surgery for spinal stenosis. Thus, lack of awareness in the patient regarding this issue could considerably raise the chances of developing meningitis or death, as noted in one of our patients. Our experience showed that in the initial period, there were teething problems, and it was time-consuming to ensure that all the tick boxes were accounted. This was most common at the time of completion of the postoperative protocol because the concerned individuals were either involved in the surgery or in outpatient clinics when the patients were getting discharged. However, this was quickly corrected by reorganizing the available resources. Thus, effective implementation of surgical checklists is challenging; however, it is necessary to optimize patient safety [19]. The authors agree that successful implementation requires local resources, education, effective leadership, and commitment at all levels, particularly from the senior clinician [20–22]. The creation of a comprehensive subspecialty-specific surgical checklist is in itself a large task with applications across several work systems.
The proposed checklist is comprehensive and assumes its intended goals only if the surgeons, residents, fellows, and nursing staff integrate and work as a team. It is noteworthy that the checklist involves unprecedented integration of all the healthcare workers like never before. Teamwork and communication are known to be pivotal for preventing and managing complications. The majority of the reviews found that a checklist is a useful, simple, and cost-effective intervention that helps in enhancing communication and patient safety in surgical patients [9–12,23]. Ryan et al. [23] noted a decrease in the spinal wound infection rate from 5.8% to 2.2% after the checklist was implemented and proper communication was maintained among the healthcare workers. Thus, the introduction of any new procedure or practice that deviates from the known routines of the OR working environment may hinder teamwork and communication; thus, close collaboration with various OR professionals is encouraged.
This single-center study has certain limitations, such as the retrospective nature, small sample size, and short follow-up duration. Although this is the first checklist that has been designed to include all the aspects of perioperative management of spine surgery patients, this checklist was proposed as per the author’s best knowledge and experience. Moreover, the checklist was contemplated to tie the manifest and potential loose ends the surgeon felt were contributing to complications. Its larger implementation in the field of spine surgery would require its validation via a large multi-centric and randomized study. This study is based on a large amount of information from a single center, and the results were beneficial in reducing the risk of human errors in various spine surgeries. It provides a framework and template that other spine surgeons can modify as per their local situations. Finally, the lack of assessment of compliance with the proposed checklist in this study could be a limitation that could be mitigated with the use of a large sample size and long-term follow-up to obtain more consistent results and outcome.
Conclusions
The proposed spine surgery checklist is an efficient, reliable, cost-effective, and time-saving tool that helps lower the rate of preventable adverse events and increases the surgeon’s confidence with perioperative safety culture in spine surgery. With an increase in the incidence of medical litigations, the development of a subspecialty-specific, comprehensive checklist that emphasizes on human error reduction is necessary. The proposed checklist helps in reducing the problem of lack of communication among healthcare personnel that is more often seen with close coordination between the primary surgeon, the training fellows, and the OR staff.
Notes
No potential conflict of interest relevant to this article was reported.