Introduction
Presently, non-compound muscle action potential specific criteria, such as somatosensory evoked potential (SSEP) criteria (an amplitude decrease ≥50% and ≥10% latency), are often used as the criteria for the warning point of compound muscle action potential (CMAP) [12]. Several studies have addressed the definition of alarms, with no consensus reached. We have reviewed conventional CMAP alarm points and classified CMAP waveform changes into four grades as novel criteria. Grade 0 is defined as a normal waveform, grade 1 as an amplitude decrease ≥50% and ≥10% latency, grade 2 as multi-phasing of waveform, and grade 3 as loss of amplitude [3]. The waveform changes from grade 1 to grade 3 in proportion to the severity of injury. The following reports our basic review of the waveform changes using Sprague-Dawley rats.
Materials and Methods
1. Modeling of spinal injuries
Forty-one 8-week-old Sprague-Dawley rats (200-230 g) were used. After anesthesia from ether inhalation and intraperitoneal administration of ketamine (100 mg/kg) and xylazine (10 mg/kg), T10 of the spine of each rat was laminectomized to expose the dura mater, which was pressed with a tension gage (Fig. 1). To create single crush and sustained crush models, stepwise compressions were performed at intensities of 2-140 g for 1-150 seconds. The bladders of the rats were manually voided twice a day for a week after the injury. To prevent infection, 1.0 mL of Bactramin (Roche, Basel, Switzerland) was mixed in 500 mL of bottled water provided for hydration for 2 weeks following spinal cord injury. Food was provided on the cage floor, and the rats had no difficulty reaching their water bottles. All animals were treated and cared for in accordance with the Nagoya University School of Medicine Guidelines pertaining to the treatment of experimental animals.
2. Measurement of control waveform
The cranial bones were exposed and bores were drilled 3 mm lateral and 2 mm posterior to the bregma where bipolar stimulating needles were inserted. A Nihon Koden Neuropack 8 (Nihon Koden Corp., Tokyo, Japan) was used as the stimulator. Except for the stimulus intensity, the stimulus conditions were approximately the same as actual spinal surgery (a train of four pulses at 2-ms intervals, stimulus intensities of 10-60 mA, and 20 additions with a phase switch after 10 additions). A Nihon Koden Neuropack (MEB-2200, ver. 04.02) used in spinal surgery was used to derive the control waveform by inserting bipolar needles in both perifemoral muscles (Fig. 1). The ground electrode was placed subcutaneously between the coil and the recording electrodes.
3. Derivation and review of CMAP waveform
Derivation was started immediately after the infliction of injury at every 30 seconds for up to 15 minutes under the same conditions as the foregoing waveform measurement. We reviewed amplitude decrease and morphology change. Morphology change was having occurred in the event of any of the following: change in the number of waves in the waveform, prolonged duration, and shift in the location of the peak latency. For convenience, integrated intensity was defined as compression time multiplied by compression intensity.
4. Evaluation of the motor function of the lower limbs
The locomotor performance of 16 animals was analyzed using the Basso, Beattie and Bresnahan (BBB, 0-21 pts) open-field score for 4 weeks (1 day, 3 days, 5 days, 1 week, 2 weeks, 3 weeks, and 4 weeks) [4]. The evaluations were made by two blind observers for all analyzed rats.
5. Statistical analyses
Statistical analyses were performed with an unpaired two-tailed Student's t-test for single comparisons and one-way analysis of variance (ANOVA) for multiple comparisons. For the locomotor performance scores, repeated measures ANOVA and the Mann-Whitney U-test were used. In all statistical analyses, values of p<0.05 were considered to indicate significance. To obtain the data for statistical analyses, the investigators were blinded to the genotypes in all procedures.
Results
1. Types of waveform change
Four different types of waveforms were obtained: no change, amplitude decrease only, morphology change only, and amplitude and morphology change. In the 50 limbs of 25 animals, type 1 accounted for 14% (7/50 limbs), type 2 for 36% (18/50 limbs), type 3 for 18% (9/50 limbs), and type 4 for 32% (16/50 limbs) (Fig. 2). No change of latency was seen in any rat.
2. Stimulus intensity and waveform change
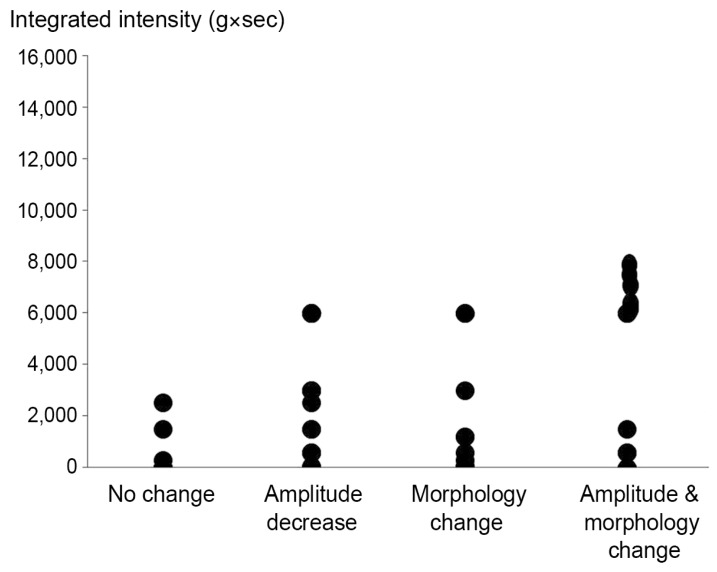
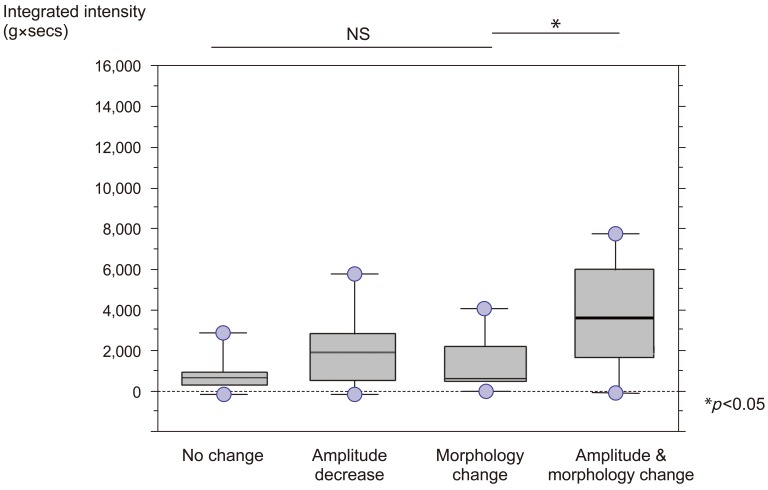
While the integrated intensity was naturally low in the group with no change, the amplitude group and the morphology group produced their respective waveforms at approximately the same level of intensity. At high intensities, numerous waveforms were derived that exhibited amplitude decrease and morphology change (Fig. 3). In a box plot, the amplitude group and the morphology group were significantly correlated with high intensities (p<0.05) (Fig. 4).
1) Evaluation of lower limb motor function
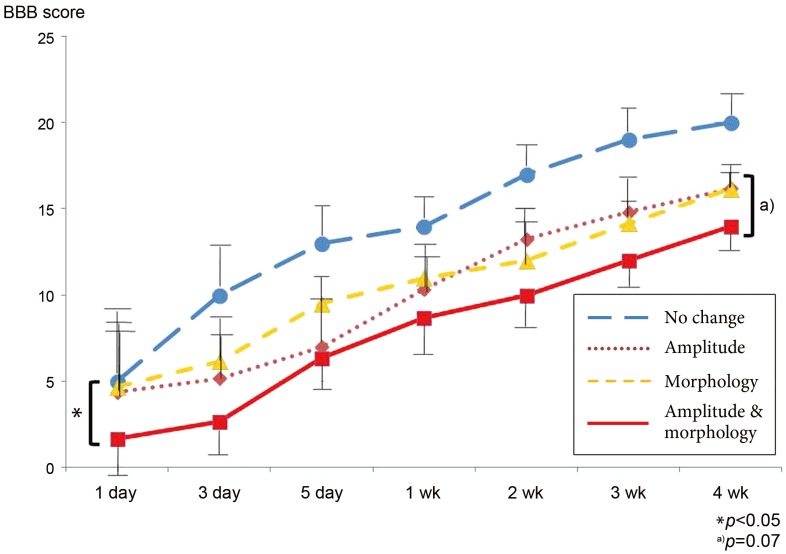
At 4 weeks after spinal cord injury, the best result was found in the no change group according to the BBB score (20). While the amplitude and morphology group (BBB, 13.5) exhibited the strongest degree of paralysis due to its highest integral intensity, there was no significant difference between that group and the amplitude group (BBB, 16.1) or the morphology group (BBB, 16.5) using repeated measure ANOVA (p=0.07). However, the amplitude and morphology group exhibited a significantly higher degree of paralysis up to the third day (Fig. 5).
Discussion
The CMAP alarm point for stopping surgery remains equivocal [5678]. Luk et al. [2] applied the same criteria as those for SSEP (amplitude decrease ≥50% and ≥10% latency), Langeloo et al. [9] defined an amplitude decrease ≥50% or more in any muscle as an alarm point, and Sala et al. [1011] defined waveform loss as an alarm point. Quinones-Hinojosa et al. [12] discussed morphological change, defining waveform change from a biphase to a monophase as an alarm point. We proposed defining morphology change as an alarm point [3]. Some authors also reported on a basic study of electrophysiology. However, the data only concerned the stimulation method, SSEP alarm point, and electromyography potential [131415]. So, it is clinically unclear and difficult to demonstrate what degree of injury and paralysis of the spinal cord causes morphology change or amplitude decrease. In this study, we used an animal testing model to demonstrate the foregoing.
We were able to classify the waveform into four types. Eighteen percent of the animals exhibited morphology change only without amplitude change. These waveforms were caused by approximately the same force causing the amplitude decrease. This is a novel observation and we believe morphology change should be considered when discussing CMAP alarm points. Additionally, relatively severe injuries tend to be accompanied by amplitude decrease and also morphology change, indicating more critical conditions. Presently, particularly severe paralysis was evident immediately after the injury in the amplitude and morphology groups. This indicates that the concurrence of morphology change and amplitude decrease should be interpreted as a higher level of alarm.
A limitation of this study is the difficulty to explain the mechanism of each waveform. More electrophysiological experiments are needed before clinical applications can be contemplated.