The Efficacy of Intramuscular Calcitonin Injection in the Management of Lumbar Spinal Stenosis
Article information
Abstract
Study Design
A prospective, cross-sectional, non-randomized study.
Purpose
To assess the effectiveness of intramuscular calcitonin injection in the treatment of lumbar spinal stenosis (LSS).
Overview of Literature
LSS, manifesting as chronic low back pain and neurogenic claudication, is a chronic condition with an increasing incidence in the elderly population having inadequate effective conservative treatment options.
Methods
In this study, 36 patients with LSS who were diagnosed based on the clinical findings and magnetic resonance imaging were included. Patients received 100 IU of calcitonin per week for one month and were evaluated before and after treatment using the Oswestry disability index (ODI) questionnaire and visual analogue scale (VAS). Before treatment, the patients were divided into two subgroups based on their ODI results: patients with mild to moderate low back pain (disability, 0%-40%) and patients with severe or very severe low back pain (disability, 40%-100%).
Results
In patients with mild to moderate low back pain, there were no significant changes in the ODI and VAS after calcitonin injection. But in patients with severe or very severe low back pain, pain severity, personal functions, ability to lift and carry objects, time interval between standing and initiation of pain, social life, disability percentage, and VAS were significantly improved after treatment with calcitonin.
Conclusions
It seems that an intramuscular injection of low dose of calcitonin may have some beneficial effects on the pain due to LSS, especially in patients who suffer from severe or very severe low back pain.
Introduction
It is estimated that about 2% of low back pain, with a rapidly increasing incidence, is due to lumbar spinal stenosis (LSS), especially among the elderly patients [1,2]. LSS is characterized by narrowing of the spinal canal with compression of the nerve roots by surrounding soft tissues or bones, for example thickening of facet joints due to osteoarthritis or bulging of intervertebral discs [3,4]. Surgical interventions have been the treatment of choice since the recognition of LSS; however, in spite of good short-term results, the long-term outcome of surgical interventions is not encouraging. Therefore, conservative treatments including physical therapy, pharmacotherapy, and additional procedures (such as manipulation, bracing, traction, and electrical stimulation) are considered as an alternative choice, especially in the elderly patients who are more prone to developing surgical complications [5,6].
One of the studied nonsurgical management options for LSS is the calcitonin hormone, which is mainly indicated for treatment of symptomatic Paget's disease of bone, hypercalcemia caused by cancer, prevention of acute bone loss due to sudden immobilization, and also for treatment of postmenopausal osteoporosis. But, calcitonin has shown some beneficial effects for achieving control of pain and improvement of function in patients with LSS [5,7,8]. Some authors suggest that it may have a direct analgesic effect through the release of β-endorphin [5,9]. Despite these promising results, some of the published data demonstrated that there may be no advantageous effects of prescribing calcitonin in LSS [10,11,12,13]. A very recent systematic review found that there is a very low quality evidence that calcitonin is no better than placebo or paracetamol regardless of the mode of administration [14]. Our clinical observations, however, indicate some dramatic responses to even low doses of intramuscular calcitonin injection in some LSS patients. Most of the previous studies used either nasal or subcutaneous administration of high doses of calcitonin and there is also a potential bias in these studies which prohibits recommending the use of calcitonin in clinical practice [4]. Therefore, we aimed to assess the effectiveness of low dose intramuscular calcitonin injection in treatment of LSS.
Materials and Methods
1. Subjects
Thirty-six patients about 50-80 years old who had clinical signs and symptoms of LSS for a minimum period of 3 months were enrolled in this study. These patients were among those patients unresponsive to routine medical therapy. The diagnosis was confirmed by an expert radiologist through magnetic resonance imaging (MRI) based on the radiologic signs of stenosis at the lumbosacral level [12,15]. Patients who had diseases associated with low back pain, including chronic inflammation or infection, neoplasm, hematologic disorders, Paget's disease of bone, traumatic vertebral injuries, and patients who preferred surgical interventions for management of their pain were excluded from the study. Also, patients with altered mental status and those who did not have regular follow-up were excluded.
2. Study design
To evaluate the patients' low back pain and their related function, we used two outcome measurements before and after administration of calcitonin: Oswestry disability index (ODI) questionnaire and visual analogue scale (VAS). Based on the ODI form, each patient answered the questions in 10 tests, each consisting of six options with increasing scores from zero to five for options one to six, respectively. Using this form, each patient could obtain a score between zero and fifty. Disability percentage of each patient was estimated by multiplying this score by two. Also, using the VAS form, the severity of patients' pain was estimated (based on a score from zero to ten; the greater the mean score, the more severe the pain).
At the initial evaluation, each patient was requested to complete both outcome measures questionnaires. Based on the ODI results, the patients were divided into two subgroups [16]: (1) Mild to moderate low back pain (disability percentage: 0%-40%). (2) Severe low back pain to very severe low back pain that forces the patient to crawl instead of walk, or bed-ridden patients or those who exaggerated their pain (disability percentage: 40%-100%).
After the initial assessments, the patients received two injections of CalciHEXAL (50 international units [IU] of calcitonin per one milliliter) intramuscularly in the buttock per week for a period of one month. The patients were asked to take acetaminophen if the pain did not reduce, but not more than 1,500 milligrams per week. Patients who took more amount of acetaminophen were excluded from the study. No other medications or interventions such as physical therapy were allowed during the study period. At the end of this period, the patients were immediately evaluated again using the above-mentioned outcome measures and the results were compared to the initial assessments.
3. Sample size
For this study, the sample size calculation was based on detection of a 1.5 point difference after intervention for the outcome pain intensity, as measured by the VAS (estimated standard deviation of 3). For achieving a two-sided 5% significance level and a power of 70%, a sample size of 27 patients was necessary.
4. Statistical analysis
Data are presented as the mean±standard deviation or percentage as appropriate. Wilcoxon signed rank test or paired t-test was used to compare the results. A p-value less than 0.05 was considered statistically significant.
5. Ethics
The study protocol was approved by the Ethics Committee of Shiraz University of Medical Sciences. All of the patients gave their written informed consent.
Results
Of the 36 patients initially enrolled in the study, four patients preferred other therapies and two patients experienced side effects of the drug which necessitated cessation of calcitonin administration (such as severe abdominal pain and flushing); therefore finally, 30 patients were included in the study. Of these 30 patients, 17 were females and 13 were males. The mean age of the patients was 62.8 years with no significant difference between the two genders.
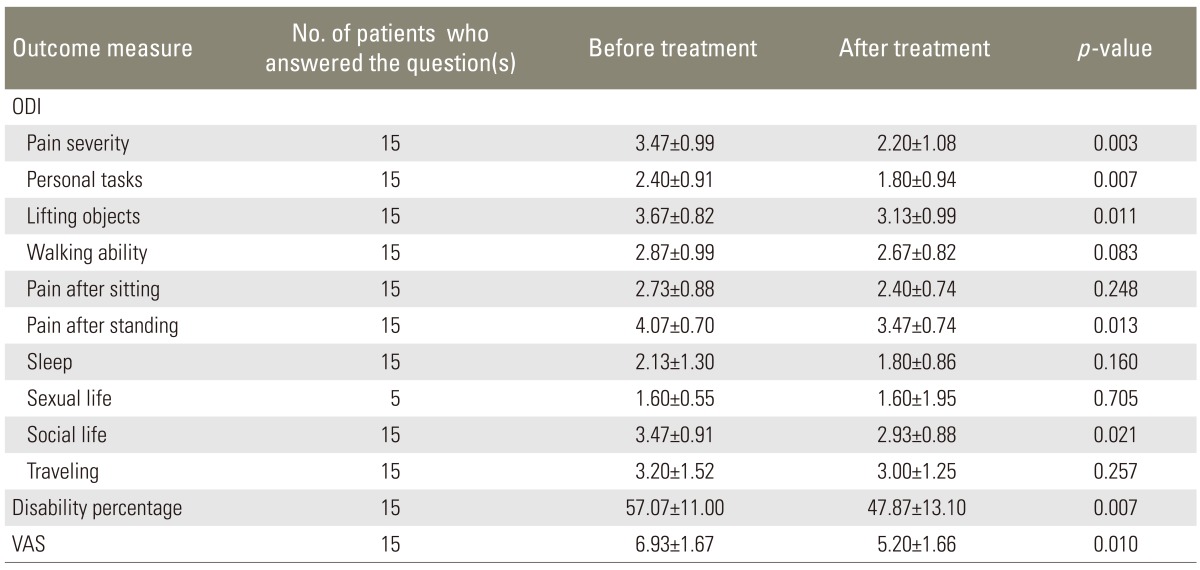
Some improvement in the ODI scores, disability percentage, and VAS score was observed after treatment with calcitonin, which was mainly attributed to treatment response in LSS patients with severe or very severe low back pain (Tables 1,2,3).
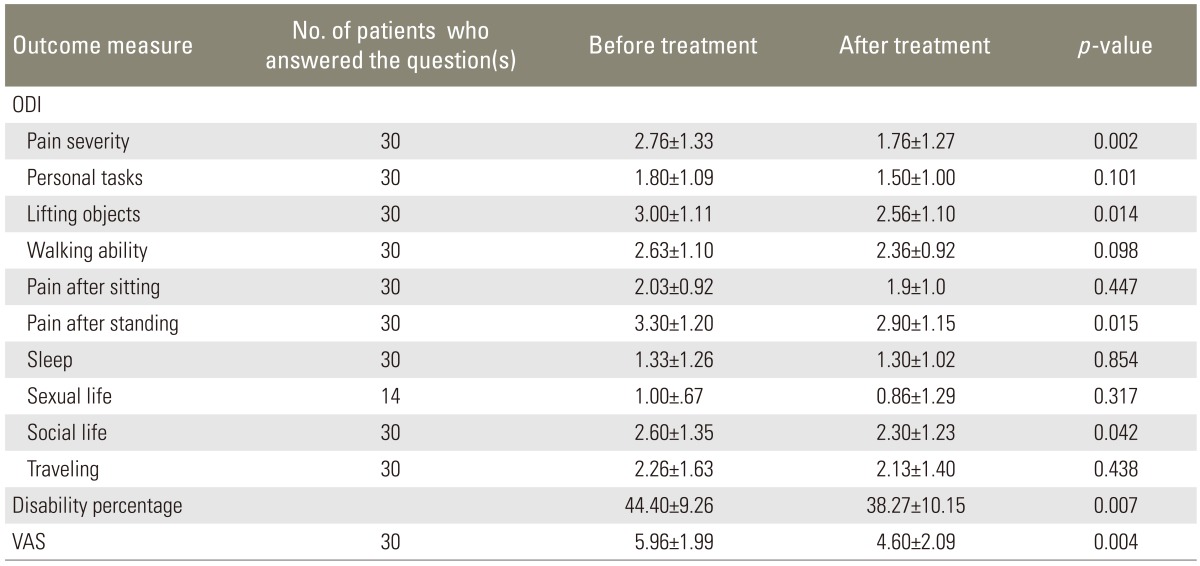
ODI, disability percentage, and VAS before and after treatment with intramuscular injection of calcitonin in LSS patients
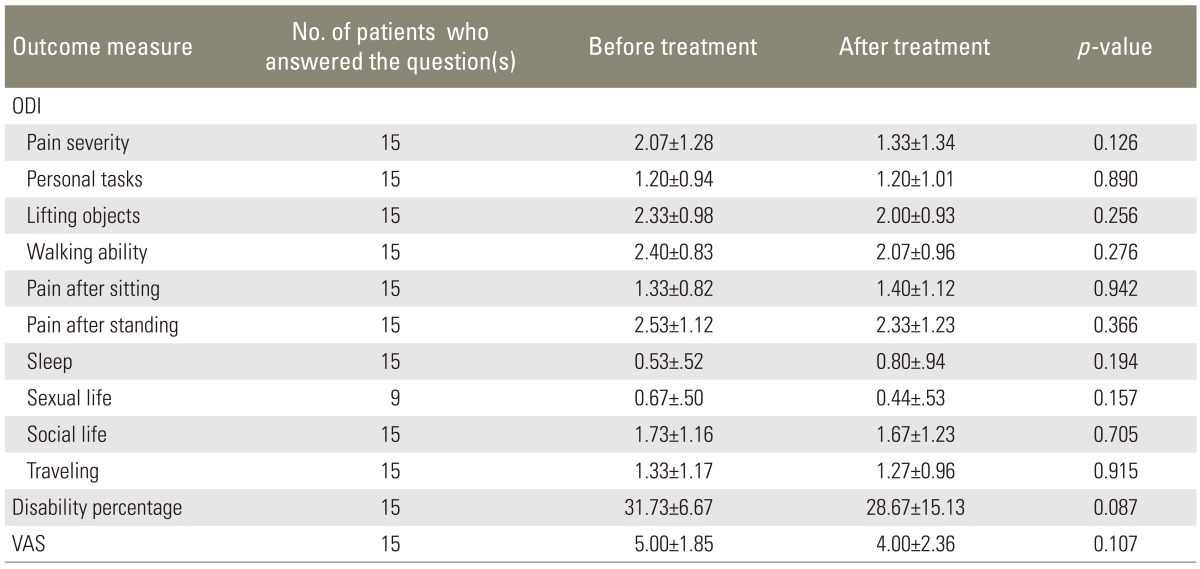
ODI, disability percentage, and VAS before and after treatment with intramuscular injection of calcitonin in LSS patients with mild to moderate low back pain
Discussion
Lumbar spinal stenosis predominantly affects the elderly population in whom surgical interventions are associated with a high degree of risk [1,5]. Therefore, conservative treatment strategies are desirable in these patients and they are the accepted treatments for LSS in mild and moderately symptomatic patients, while surgical interventions are preferred in patients with severe symptoms or in whom conservative treatment has failed [17,18,19,20].
Our study investigated the efficacy of conservative treatment with intramuscular calcitonin injection for LSS and demonstrated that calcitonin may provide significant benefits especially in those who suffer from severe pain. According to our findings, in patients with mild to moderate low back pain, there were no significant changes in the ODI and VAS after the period of calcitonin injection; however, in patients with severe or very severe pain, five out of 10 tests in the ODI (including pain severity, personal functions, ability to lift and carry objects, time interval between standing and initiation of pain, and social life), disability percentage, and VAS were significantly improved after treatment with calcitonin. Concurrent to our findings, some studies have previously shown that calcitonin may improve the outcome in patients with LSS. On the other hand, some studies failed to show any therapeutic advantages of calcitonin in LSS patients, regardless of the severity of the symptoms of LSS (Table 4).
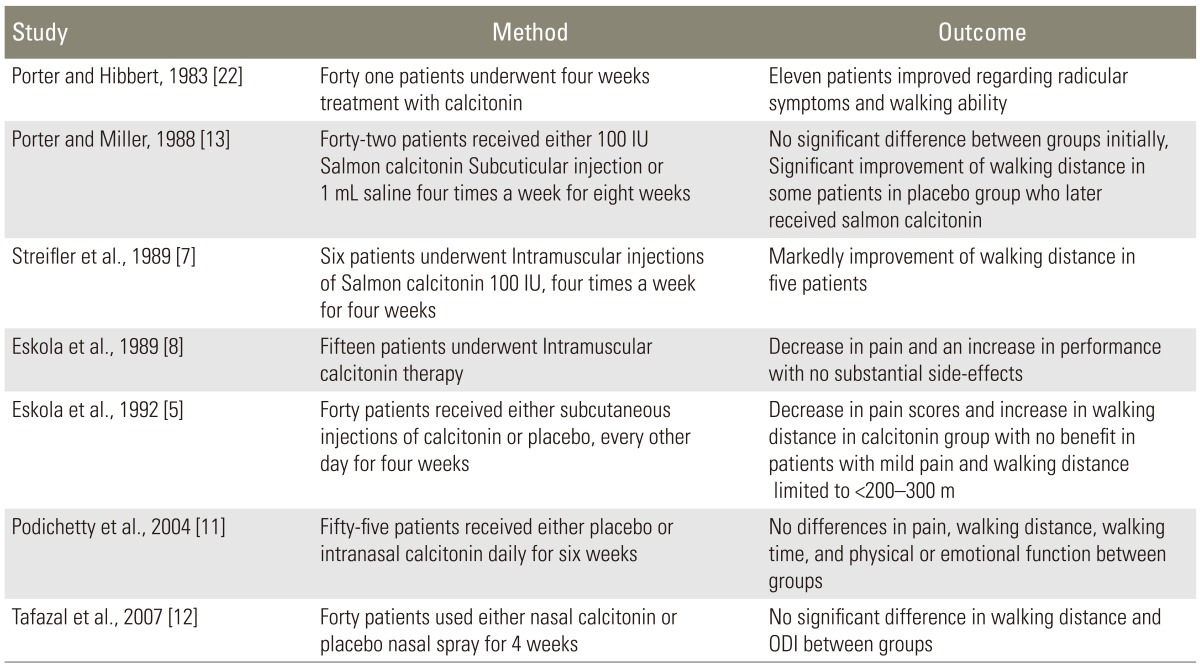
Different outcomes of studies investigating the effect of calcitonin in patients with lumbar spinal stenosis
Some other studies investigated the combined effect of calcitonin and physical therapy. In a prospective study conducted by Onel et al. [21], 145 patients with LSS received physical therapy (infrared heating, ultrasonic diathermy, and active lumbar exercises) and calcitonin injection for one month. Clinical parameters such as pain on motion, lumbar range of motion, straight leg raising test, deep tendon reflexes, dermatomal sensation, motor function, and neurogenic claudication distance were assessed on admission and were compared after conservative therapies. Except for reflex deficits, all of the assessed parameters improved, particularly pain on motion (in 91% of patients), lumbar spine functional capacity (in 55% of patients), and walking distance (in 89% of patients). However, it should be noted that despite these promising results, in this study the definite effects of calcitonin on some of the significant confounding variables, including infrared heating, ultrasonic diathermy, and active lumbar exercises, are unclear. Sahin et al. [10] randomized 45 patients between the intranasal calcitonin 200 U/day and paracetamol 1,500 mg/day treatment groups. Both groups received the same physical therapy including interferential current, hot pack, short wave diathermy, and exercise protocol. They concluded that although pain, physical examination findings, walking distance, and functional parameters in the patients improved significantly in both groups, these improvements were only due to physical therapies, and not due to the addition of calcitonin.
One of the possible reasons for the difference observed between our study and those that used intranasal formulation of calcitonin may be the lower bioavailability of the nasal formulation of calcitonin in comparison to the intramuscular injection formulation (only around 3%, ranging from 0.3% to 30.6%) [12], and therefore when a lesser amount of calcitonin was absorbed into the systemic circulation, less benefits of calcitonin were observed. It should also be noted that although the studies conducted by Podichetty et al. [11], Tafazal et al. [12], and Sahin et al. [10] were randomized control trials, it is not clear whether the randomization method was adequate or not; it is also not clear whether the treatment allocation was blinded or not. Therefore, these studies may have a potential bias and also a type II error, and thus, an insufficient sample size to detect the difference between the groups.
Although some previously published data indicated that calcitonin is no better than placebo or paracetamol regardless of the mode of administration or outcome assessed, based on our results and evidence from the studies by Porter and Hibbert [22], Eskola et al. [8], Streifler et al. [7], and Onel et al. [21], it seems that if calcitonin is administered as an intramuscular formulation, it may have some benefits in patients with LSS. Although all of the authors who used calcitonin as an intramuscular formulation reported an improvement in walking distance in patients with LSS, our results did not demonstrate any association between calcitonin and walking distance. One of the possible reasons for this finding is that our patients were older than those in previous studies, and therefore, the risk of comorbidities such as osteoarthritis was higher in our study. Also, we used a lower dosage of calcitonin compared to that used in previous studies, and this can explain the lower percentage of benefits of calcitonin in our study.
There are certain limitations to our study. First, it was a cross-sectional study with a small number of patients and there was no control group; therefore, causality cannot be established by this study and only an association can be determined by this study. Hence, any conclusion derived from such a study must be considered preliminary and such a study can be considered to be hypothesis-generating rather than hypothesis-proving. Second, all of the variables assessed in our study were subjective, and not objective; therefore, there is a possibility of potential biases among both interviewers and interviewees. Also, due to the cultural belief in our society, most of the patients, especially women, ignored the questions about their sex life or answered them very briefly. Therefore, the effect of calcitonin on patients' sex life and the actual ODI may have been underestimated. Furthermore, despite the efficacy of calcitonin injection and good tolerance in patients during the short term, the long-term effect of calcitonin and also the long-term acceptance by patients are unclear. It is noteworthy that although we investigated the patients with spinal claudication, we did not use a specific claudication score system or a walking distance measure. Also, we did not measure the spinal canal diameter in patients, which could add more value to the results if its relation to the severity of low back pain and ODI was investigated.
Moreover, it should be noted that the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) has recently reported that long-term usage of calcitonin-containing medicines may be associated with an increased risk of cancer, and hence, it should only be used for short-term treatment. CHMP has stated that the risk of developing cancer was 0.7% to 2.4% higher in patients receiving calcitonin-containing medicines compared to the patients receiving placebo in long-term clinical trials, especially in those who used intranasal calcitonin [23]. But, it is still a matter of debate and the concern regarding cancer risk does not appear to be valid with the short-term use. Recently, Health Canada has stated that the increased risk of cancer has not been confirmed to be caused by calcitonin and may simply be an association [24]. Also, it should be kept in mind that cancerous effects of calcitonin-containing medicines were only observed after long term treatment with oral or nasal formulation, and not after short-term treatment and with a low dose injectable formulation of calcitonin. However, the increased incidence of pituitary adenomas which was observed in one-year toxicity studies in Sprague-Dawley rats administered calcitonin-salmon at dosages of 20 IU/kg/day and 80 IU/kg/day and in Fischer 344 rats given 80 IU/kg/day has demonstrated that the relevance of these findings in humans is still unknown. Furthermore, calcitonin-salmon was not mutagenic in tests using Salmonella typhimurium, Escherichia coli, and Chinese Hamster V79 cells [25].
Conclusions
Intramuscular injection of calcitonin even at a low dosage seems to have some beneficial effects on the pain and function in patients with LSS, especially in those who suffer from severe or very severe low back pain. It is necessary to perform further well-designed clinical trials to help in making clear recommendations for its use in clinical practice.
Acknowledgments
This study was extracted from the postgraduate thesis of Dr. Amin Sadraei (no: 2393) and was supported by a grant from Shiraz University of Medical Sciences.
Notes
No potential conflict of interest relevant to this article was reported.