Efficacy of Antibiotics Sprayed into Surgical Site for Prevention of the Contamination in the Spinal Surgery
Article information
Abstract
Study Design
Retrospective study.
Purpose
To evaluate the effect of intraoperative wound application of vancomycin on preventing surgical wound contamination during instrumented lumbar spinal surgery.
Overview of Literature
Postoperative infection is the one of the most devastating complications of lumbar surgery. There are a few reports showing the benefits of intraoperative wound application of vancomycin during spinal surgery. However, there is no report about the effectiveness of local vancomycin instillation in prevention of surgical wound contamination.
Methods
Eighty-six patients underwent instrumented lumbar spinal surgery. Mean patient age was 65.19 years (range, 23-83 years). There were 67 females and 19 males. During surgery, vancomycin powder was applied into the surgical site before closure in 43 patients (antibiotic group) and vancomycin powder was not applied into the surgical site before closure in 43 patients (control group). The tip of the surgical drain was cultured to evaluate surgical wound contamination. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were measured on the first, third, seventh, and fourteenth day after the operation.
Results
We found two patients with a positive culture from the tip of surgical drains in the antibiotic group, and one patient with a positive culture from the tip of the surgical drain in the control group. Postoperative ESR and CRP levels did not show significant differences between the two groups. On the third postoperative day, ESR in patients of the antibiotic group was more significantly decreased than that in patients of the control group, while CRP level did not show a significant difference between the two groups.
Conclusions
There was no evidence to suggest that intraoperative vancomycin application is effective in decreasing the risk of postoperative wound infection after instrumented posterior lumbar fusion surgery.
Introduction
All spinal invasive modalities carry an associated risk of postoperative infection. Infection rate after instrumented lumbar spinal fusion is reported to be about 2.2% [1]. Surgeons have made their best efforts to reduce wound contamination for preventing infection during spinal surgery. Intravenous prophylaxis with cephalosporin antibiotics has been the standard of care during spinal surgery. Directly depositing powdered antibiotics may be more effective in reducing the risk of postoperative wound infection [2,3]. Direct deposition of antibiotics ensures the highest concentration of antibiotics in the postoperative spinal wound without a systemic hazard [4]. Vancomycin is the most suitable for local delivery because it has little adverse effects on bone ingrowth even at a high concentration when compared with other antibiotics [5]. In clinical studies, intraoperative vancomycin powder application has been shown to lower the risk of infection after spinal fusion [4,6,7,8].
However, low grade infection due to contamination does not manifest with the clinical symptoms of wound infection such as fever and leukocytosis. Also, there is no report showing prevention of contamination by local vancomycin instillation. The purpose of the current study is to evaluate the effect of intraoperative wound application of vancomycin on preventing surgical site contamination during instrumented lumbar spinal surgery.
Materials and Methods
This study was approved by the Institutional Review Board at the institution of the corresponding author (IRB number: 2013-I133). Eighty-six patients who underwent instrumented lumbar spinal surgery due to degenerative lumbar spine disease from February 2006 to August 2012 at a single institute were included in the current study. The exclusion criteria included previous history of any operation due to trauma, infection, tumor, or systemic diseases. There were 67 females and 19 males. The mean patient age was 65.19 years (range, 23-83 years). The mean follow-up period was 13.1±3.4 months (range, 12-43 months). The patients were randomly divided into two groups. Powdered antibiotics were applied to the surgical site before wound closure in 43 patients (antibiotic group: mean age, 63.24 years; 28 female, 15 male), and powdered antibiotics were not applied to the surgical site before wound closure in the other 43 patients (control group: mean age, 67.14 years; 39 female, 4 male). There was no significant difference in age, sex, follow-up period, and underlying diseases between the two groups.
All operations were performed by the same orthopedic surgeon in the same operative room. The instrumented lumbar spine surgery was performed under general anesthesia. Within 1 hour before skin incision, first generation cephalosporin was given intravenously in all patients prophylactically. After spinal instrumentation, 2 g of vancomycin powder was applied under the lumbodorsal fascia and in the subcutaneous tissue of all patients in the antibiotic group. After surgery, first generation cephalosporin was regularly injected until the fifth postoperative day. The tip of the surgical drain was cultured to identify surgical wound contamination. Erythrocyte sedimentation rate (ESR; normal range, 0-26 mm/hr) and C-reactive protein (CRP; normal range, 0-5.0 mg/L) were measured on the first, third, seventh, and fourteenth day after the operation. Body temperature was checked regularly. Cultures of blood, urine, stool, and sputum were performed to identify non-surgical infection of other origin except for surgical site infection if the patient developed fever.
Statistical analysis was performed using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA) Differences in continuous variables between the two groups were examined with the unpaired t-test. Power analysis was performed by G*Power ver. 3.1.5 (Franz Faul, University Kiel, Kiel, Germany). Power was 0.8 for paired t-tests with an effect size of 0.5 and an alpha error probability of 0.05. The sample size required in each group was more than 34. The statistical significance level was set at p<0.05.
Results
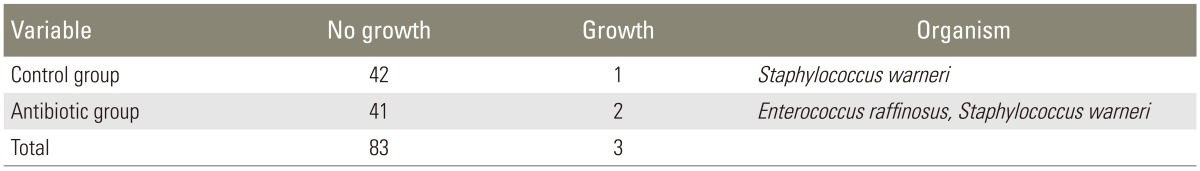
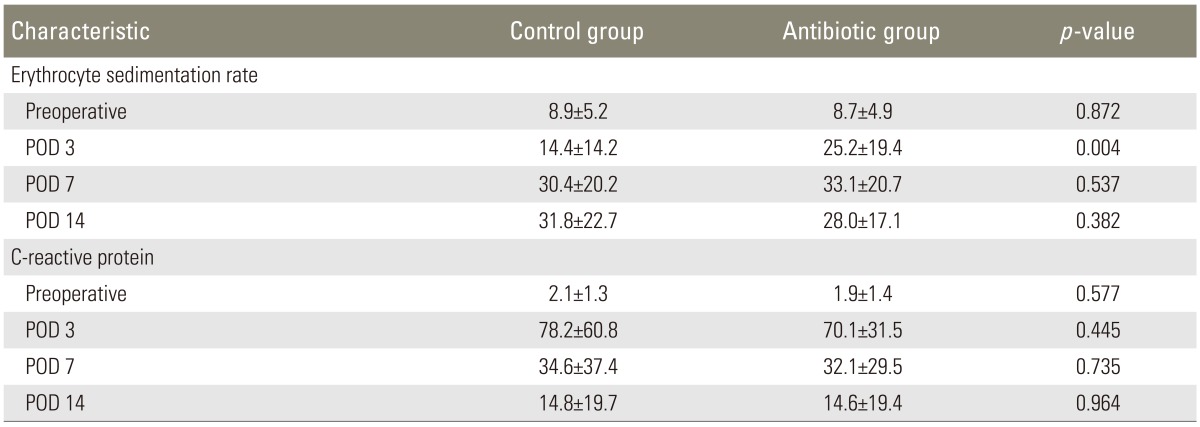
Culture results of the tip of the surgical drains showed two cases of contamination in the antibiotic group and one case of contamination in the control group (Table 1). Staphylococcus warneri was identified in one case of contamination in the antibiotic group and in one case of contamination in the control group (Table 1). Enterococcus raffinosus was identified in one case of contamination in the antibiotic group (Table 1). None of the patients had used intravenous antibiotics longer than 5 days after the surgery and had undergone additional surgery because of surgical site infection. ESR and CRP levels did not show significant differences between the two groups preoperatively, and on the first, seventh, and fourteenth postoperative day (Table 2). However, on the third postoperative day, ESR in patients of the antibiotic group was more significantly decreased than that in patients of the control group (p=0.004), while CRP level did not show a significant difference between the two groups.
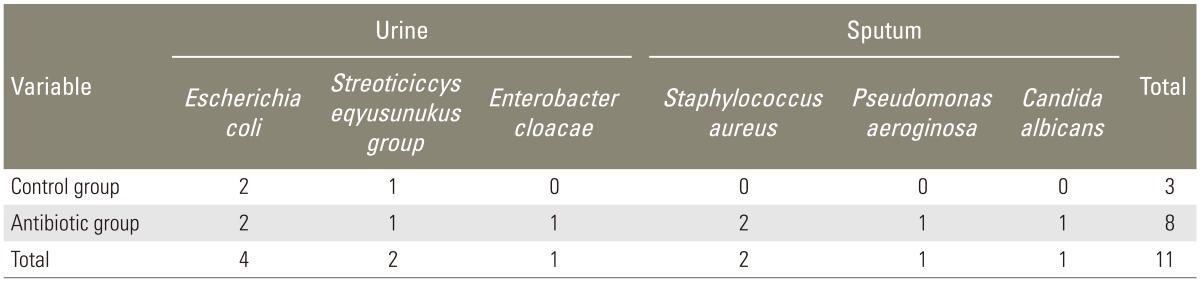
Urine culture showed Escherichia coli (E. coli) in two patients, and Streoticiccys eqyusunukus group in one patient of the control group, and E. coli in two patients (Table 3). Urine culture showed E. coli in two patients, Streoticiccys vurudabs group in one patient, and Enterobacter cloacae in one patient of the antibiotic group (Table 3). Sputum culture in patients of the control group showed no contamination (Table 3). Sputum culture in patients of the antibiotic group showed Staphylococcus aureus in two patients, Pseudomonas aeruginosa in one patient, and Candida albicans in one patient (Table 3). We did not detect delayed infection in both groups until a minimum of 12 months.
Discussion
Spinal infection can be a devastating complication after spinal surgery. Locally administered antibiotics have been evaluated for their effectiveness in reducing the rate of surgical infection. Haines [2] reported that topical antibiotics may be beneficial in patients at higher risk of infection. Maguire reported that bacitracin and neomycin powder reduced surgical infection rates [3]. Vancomycin is the most suitable for local delivery. It showed the weakest inhibitory effect on osteoblast cell growth and osteogenic activity when compared with other antibiotics [5]. In addition, it has the advantages of relatively low cost, ease of use in the powdered form, and broad coverage against the organisms that typically infect deep spinal wounds such as methicillin resistant Staphylococcus aureus [4]. Intraoperative vancomycin powder application has been shown to lower the risk of infection after spinal fusion [4,6,7,8]. However, low grade infection due to contamination does not manifest with clinical symptoms of wound infection such as fever and leukocytosis. Also, there is no report showing prevention of contamination by local vancomycin instillation. The purpose of this study is to evaluate the effect of intraoperative wound application of antibiotics on preventing surgical wound contamination during instrumented lumbar spinal surgery.
In the current study, intraoperative application of powdered vancomycin into the spinal wound did not significantly decrease the risk of contamination of operative wound in the patients who underwent instrumented lumbar fusion. We found two patients with a positive culture from the tip of surgical drains in the antibiotic group, and one patient with a positive culture from the tip of the surgical drain in the control group. Postoperative ESR and CRP levels did not show significant differences between the two groups. On the third postoperative day, ESR in patients of the antibiotic group was more significantly decreased than that in patients of the control group, while CRP level did not show a significant difference between the two groups.
A study of 1,512 patients who underwent spinal surgeries showed an infection rate of 0.99% after applying 1 g of powdered vancomycin intraoperatively [4]. In the study involving 1,732 patients who underwent posterior instrumented thoracic and/or lumbar fusion, vancomycin powder application caused a reduction in the infection rate from 2.6% with intravenous cephalexin prophylaxis only to 0.2% [6]. The patients showed very a high vancomycin level in the surgical drains and low vancomycin levels in the peripheral blood, consistent with minimal systemic absorption of intraoperative vancomycin powder application [6]. In a study of 110 patients who underwent posterior spinal fusion after traumatic injuries, the wound infection rate was reduced from 13% to 0% with application of 1 g of vancomycin powder [7]. In a study of 171 patients who underwent posterior cervical fusion, the infection rate was reduced from 10.9% to 2.5% with application of 1 g of vancomycin powder [8]. However, these studies showed the effectiveness of local vancomycin instillation in preventing infection but not in preventing contamination.
In the patients who develop infection after spinal instrumentation surgery, CRP, white blood cell count, and body temperature starts increasing again 4 to 11 days after surgery [9]. The possibility of wound infection should be considered if the CRP level rises after the seventh postoperative day [9]. If there is no infection, the CRP level reaches its peak after the first-third postoperative days and starts decreasing after the fifth postoperative day [10,11], and normalizes by 2 weeks postoperatively in general [12]. Sensitivity, specificity, negative predictive value, and positive predictive value of CRP for infection has been reported to be 100%, 95%, 100%, and 48%, respectively [12]. However, postoperative levels of CRP and ESR showed no difference between the two groups with contaminated and noncontaminated culture results obtained intraoperatively [13]. CRP and ESR might not be sufficient for the evaluation of postoperative contamination, and we have checked the culture from the tip of surgical drains.
Like any other study, the present investigation too has several limitations. First, this is a retrospective study. A prospective study may yield different results. Second, our study has a relatively small sample size. Further studies with larger number of cases are necessary to confirm the effectiveness. However, this is the first study to evaluate the effectiveness of locally administered vancomycin in preventing local contamination of surgical sites.
Conclusions
We could not identify the advantages of intraoperative application of powdered vancomycin into the surgical wound during instrumented lumbar spinal fusion.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.