Risk Factor Analysis for C5 Palsy after Double-Door Laminoplasty for Cervical Spondylotic Myelopathy
Article information
Abstract
Study Design
A retrospective comparative study.
Purpose
To clarify the risk factors related to the development of postoperative C5 palsy through radiological studies after cervical double-door laminoplasty (DDL).
Overview of Literature
Although postoperative C5 palsy is generally considered to be the result of damage to the nerve root or segmental spinal cord, the associated pathology remains controversial.
Methods
A consecutive case series of 47 patients with cervical spondylotic myelopathy treated by DDL at our institution between April 2008 and April 2015 were reviewed. Postoperative C5 palsy occurred in 5 of 47 cases after DDL. We investigated 9 radiologic factors that have been reported to be risk factors for C5 palsy in various studies, and statistically examined these between the two groups of palsy and the non-palsy patients.
Results
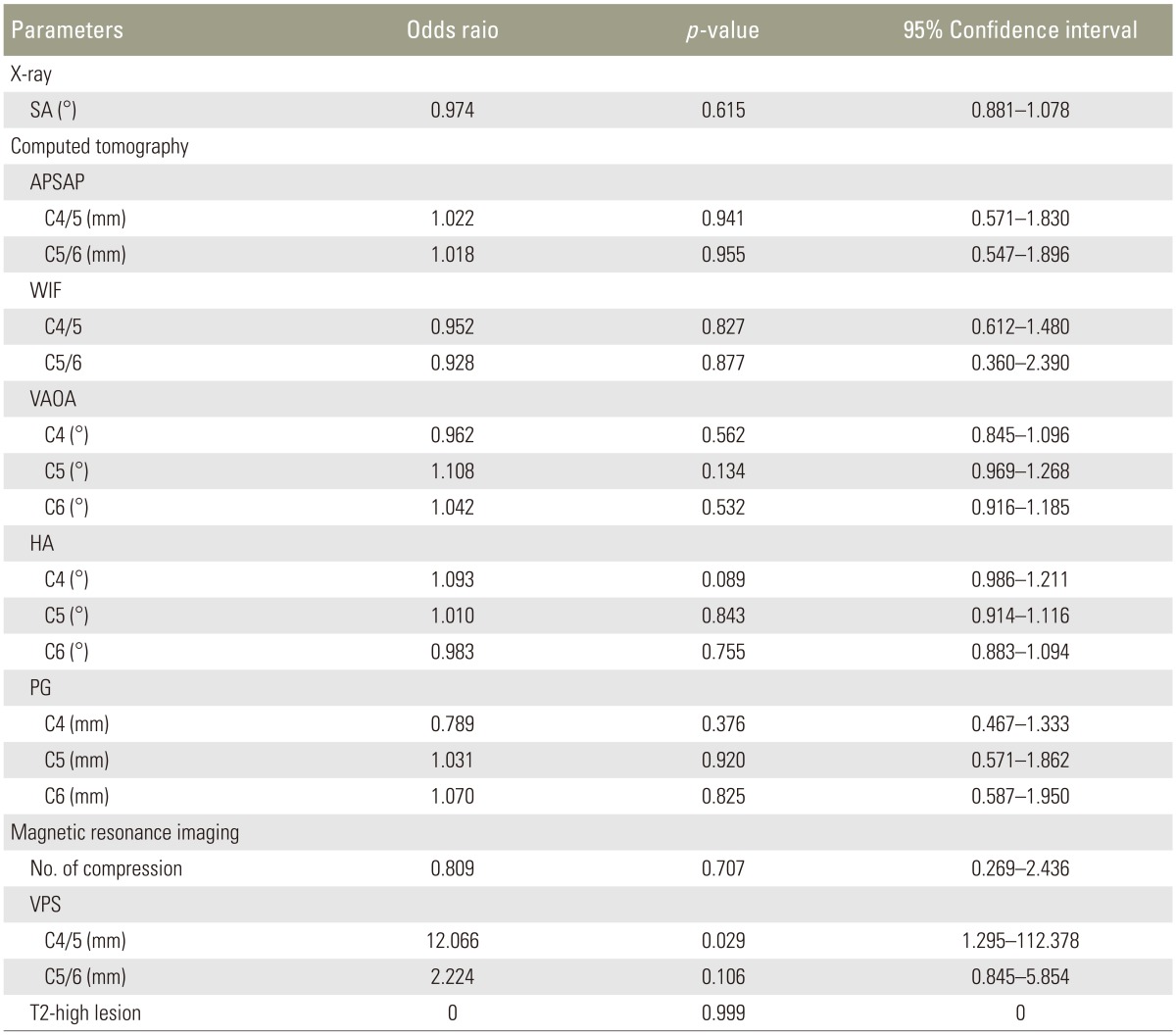
We found a significant difference between patients with and without postoperative C5 palsy with regards to the posterior shift of spinal cord at C4/5 (p=0.008). The logistic regression analyses revealed posterior shift of the spinal cord at C4/5 (odds ratio, 12.066; p=0.029; 95% confidence interval, 1.295–112.378). For the other radiologic factors, there were no statistically significant differences between the two groups.
Conclusions
In the present study, we showed a significant difference in the posterior shift of the spinal cord at C4/5 between the palsy and the non-palsy groups, indicating that the "tethering phenomenon" was likely a greater risk factor for postoperative C5 palsy.
Introduction
Cervical laminoplasty is a reliable surgical procedure in the treatment of cervical spondylotic myelopathy (CSM). This operation has been widely performed in place of laminectomy for multilevel posterior decompression of the spinal cord and has been considered as a relatively safe operation with low risk of complications. Among various types of laminoplasty, double door laminoplasty (DDL) is widely accepted due to its simpler and safer technical requirements [1]. However, postoperative upper extremity palsy, especially C5 palsy, is a relatively frequent complication after laminoplasty. The incidence of C5 palsy has been reported to be 4.6% but varies from 0% to 30% [2]. Although postoperative C5 palsy is generally considered to be the result of the damage to the nerve root or segmental spinal cord, the exact mechanisms underlying its pathology remain controversial, particularly as to whether the nerve is damaged during or after surgery [2]. Generally, the postoperative C5 palsy is defined as muscle paresis of deltoids and biceps corresponding to the C5 segment. However, Tamiya et al. [3] reported that there is also a case for ensuing pain from the C6 segment area, and participation of C6 segment as the cause for C5 palsy. Thus, we also investigated the possibility of C6 segment area being related to the C5 palsy.
In the past, there have been several C5 palsy-related papers, which concluded that operations involving "open door laminoplasty" and "ossification of posterior longitudinal ligament (OPLL)" carried risk factors [1]. However, there has not been much report on patients who underwent DDL for CSM, and the risk factor for C5 palsy as the risks, in general, have been reported to be relatively low. Therefore, we examined the patients who underwent the DDL for CSM patient in terms of risks for C5 palsy.
The objective of this study was to review the radiological findings in patients after DDL for CSM and the occurrence of associated adverse effects from the operation.
Materials and Methods
A consecutive case series of 76 patients with CSM treated by DDL at our institution between April 2008 and April 2015 were retrospectively reviewed. Exclusion criteria were as follows: (1) preoperatively, patients with Grade 3 or less weakness of the deltoid or biceps in a manual muscle test (MMT); (2) patients with previous cervical surgery; (3) patients who needed spinal fusion; and (4) patients who could not be examined in all radiological factors described later.
A total of 29 patients were excluded and therefore 47 patients (35 men and 12 women) were enrolled in the study. The mean age of the patients was 70.6 years (range, 37–85 years).
Postoperative C5 palsy was defined as deterioration of muscle strength by one or more grades on the manual muscle testing of the deltoid and biceps after surgery, and in absence of other neurologic symptoms. Even if the palsy developed bilaterally, or was considered to include C5 and other multi-segments, it was counted one C5 palsy case.
There were a total of 5 patients (3 men and 2 women, average age 69.2 years) with C5 palsy, and clinical details of each patient are shown in Table 1. The MMT score of the deltoid at the onset of palsy was between 1 and 3, and that of the biceps brachii was between 1 and 5. Paralysis was noted after a mean of 2.6 days after surgery (range, 8 hours–5 days), and one patient experienced right side paralysis, 2 patients on the left side, and in 2 patients paralysis was on both sides. Upper extremity pain appeared upon the development of the palsy in 3 patients. Of the 5 patients, one patient required additional foraminotomy because there was a possibility of kinking intervertebral foramen from postoperative CT images; other patients, however, were treated conservatively. All of the 5 patients fully recovered within 11 months. We categorized these 5 patients as the P-group (as having palsy), and the remaining 42 patients (32 men and 10 women, average age 70.7 years) were categorized as the N-group (no palsy).
According to patient demographics, there were no significant differences between the two groups (Table 2). We then statistically performed a comparison between the P-group and the N-group, and also determined the associated risk factors occurring with the onset of postoperative palsy.
1. Radiological examination
We investigated 9 factors that have been reported to be risk factors for C5 palsy in various studies. We investigated cervical alignment [14], anterior protrusion of the superior articular process [145], width of the intervertebral foramen [45], and number of the compressed segment of the cord [4] as preoperative risk factors. We also considered vertebral arch opening angle [6], hinge angle [6], position of the gutters [2], value of posterior shift of the spinal cord [1245], and appearance of a high intensity area in the cord [24] as preoperativ and postoperative risk factors.
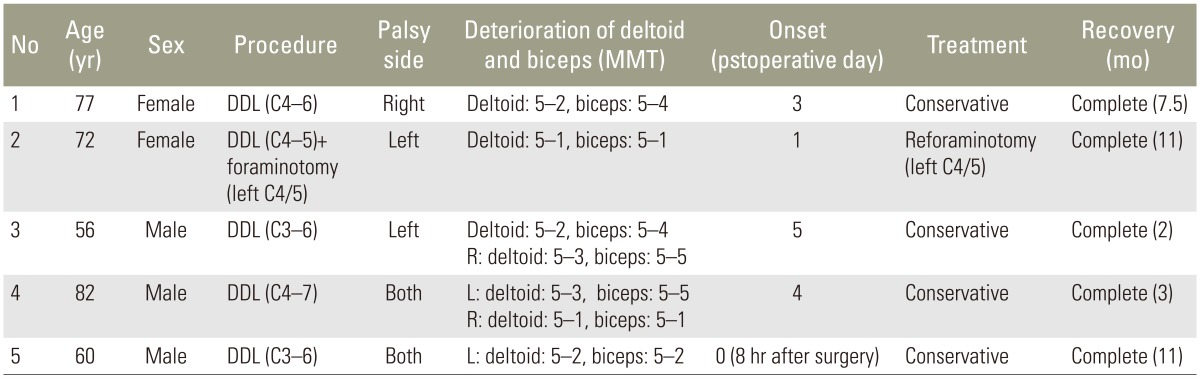
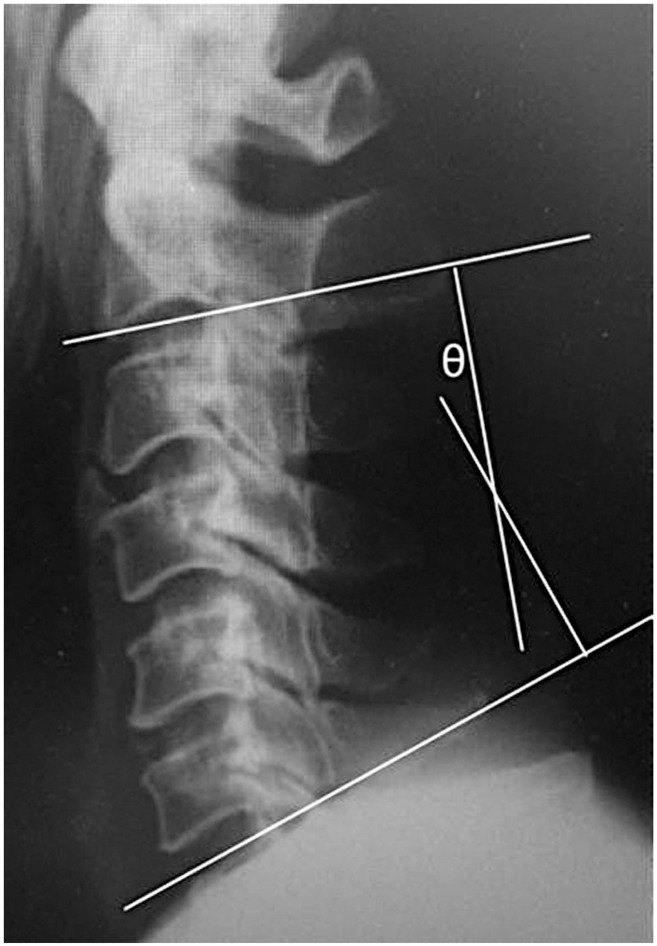
With preoperative X-ray images, cervical alignment was measured from C2–C7 sagittal angle (Fig. 1). Using preoperative computed tomography (CT) images, the width of the intervertebral foramen at C4/5 and C5/6 levels, and the anterior protrusion of the superior articular process of C5 and C6 were measured according to the method described by Sunago et al. (Fig. 2) [7]. The width of the intervertebral foramen and the anterior protrusion of the superior articular process were both measured at their narrowest and the most prominent points, respectively.
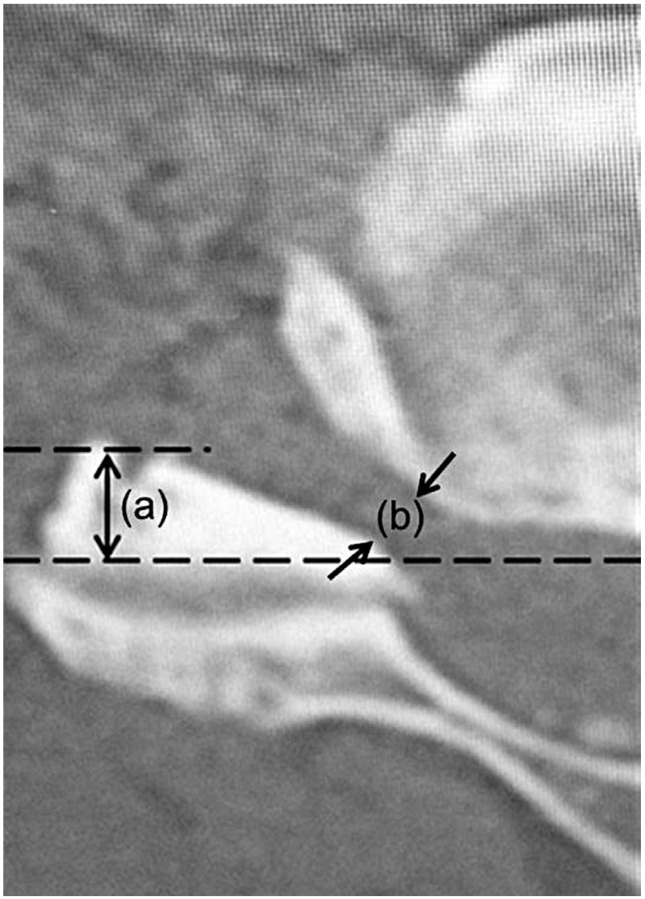
The position of the superior articular process was measured by the method of Sunago et al. as illustrated (a). The black arrows (b) indicate the width of the intervertebral foramen of C4–5 at the narrowest point.
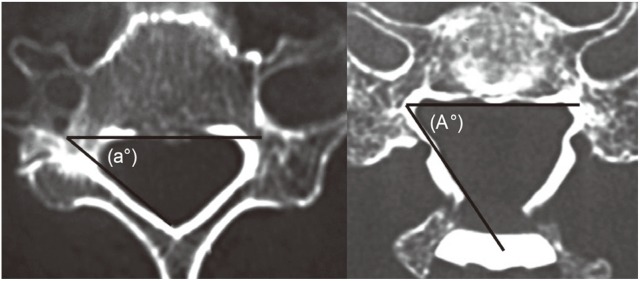
Using postoperative CT images, the vertebral arch opening angle, the hinge angle, and the position of the gutters were measured. The vertebral arch opening angle was measured at C4, C5, and C6 by the method in Fig. 3, and the hinge angle was measured at C4, C5 and C6 by the method in Fig. 4. The position of the gutter was measured the distance to the gutter from the vertebral body center line portion at C4, C5, and C6 by the method in Fig. 4. With the CT images, the P-group was evaluated by measuring the palsy side, and the N-group was evaluated by measuring both sides.
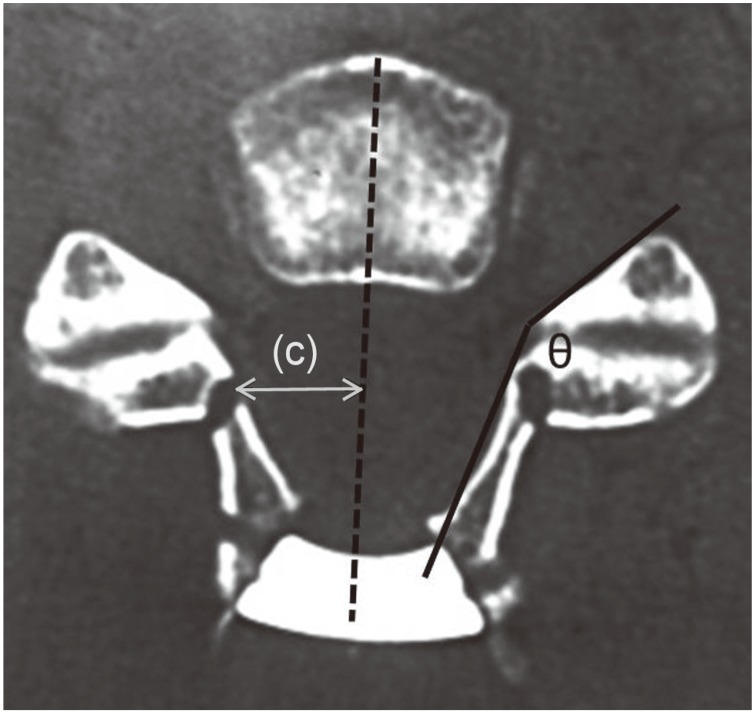
The hinge angle (theta) was measured as illustrated. The position of the gutter was the distance to the gutter from the vertebral body centerline portion as illustrated (c).
Using preoperative magnetic resonance imaging (MRI), the number and the position of the compressed segments of the cord were evaluated. Using postoperative MRI, the value of posterior shift of the spinal cord, and appearance of a high intensity area in the cord were evaluated. The posterior shift of spinal cord at level C4/5 and C5/6 was measured from the posterior edge of the compressive mass to the midpoint of the spinal cord in the mid-sagittal plane of T2-weighted MRI (Fig. 5). The midpoint of the spinal cord was selected because it is believed that this measurement is less influenced by postoperative spinal cord expansion and thus provides a more accurate determination of spinal cord displacement. Appearance of a high signal intensity lesion in the cord at the C3–5 level was evaluated using T2-weighted sagittal and axial MRI and any new high intensity lesion was defined as positive (Fig. 5).
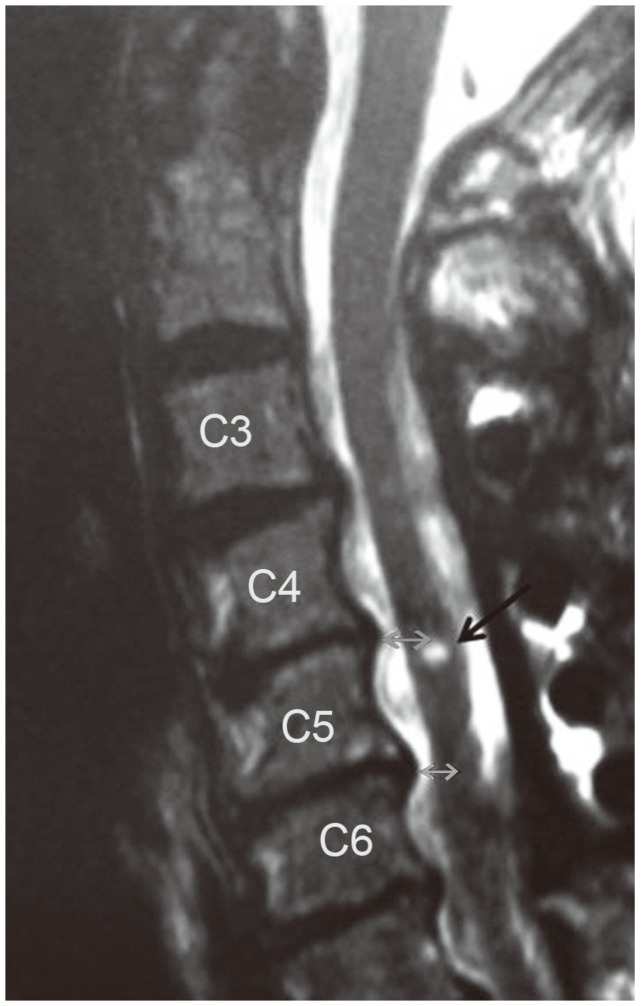
Posterior shift of the spinal cord at the level of C4/5 and C5/6 was measured from the posterior compressive mass to the preoperative and postoperative midpoint of the spinal cord on T2-weighted midsagittal magnetic resonance imaging (grey arrows). The preoperative value was then subtracted from the postoperative value for the measurement of the spinal cord shift. The appearance of new high signal intensity lesion in the cord at the level of C3–C5 was evaluated before and after surgery as illustrated (black arrow).
2. Statistical analysis
The comparison of continuous variables between two groups was Mann-Whitney U-test or chi-square test. In addition, we conducted logistic regression analysis in each parameter. In the logistic regression model, P-group and N-group were treated as dependent variables.
All p-value less than 0.05 were considered statistically significant. Logistic regression analysis was performed with SPSS software ver. 21.0 (SPSS Inc., Chicago, IL, USA). Each parameter was measured 2 times by the same person and the median was adopted.
Results
Details of the radiological parameters of the P-group are shown in Table 3, and comparison of radiological parameters for the P- and N-groups is shown in Table 4.
1. Comparison of radiological parameters
On radiographic studies, the C2–7 sagittal angle had a mean of 15.4°±6.0° in the P-group and 17.9°±10.7° in the N-group (p=0.849), and there was no significant difference.
On CT images evaluations, there were no significant differences between the two groups with regard to the anterior protrusion of the superior articular process at C4/5 and C5/6 (p=0.846 and 0.783, respectively) along with the width of the intervertebral foramen at C4/5 and C5/6 (p=0.946 and 0.917, respectively), the vertebral arch opening angle at C4, C5 and C6 (p=0.793, 0.293, and 0.413, respectively), the hinge angle at C4, C5, and C6 (p=0.064, 0.858, and 0.783, respectively) and the position of the gutters at C4, C5 and C6 (p=0.098, 0.536, and 0.770, respectively).
On MRI evaluations, there was significant difference between the two groups with regards to the amount of the posterior shift of the spinal cord at C4/5 (p=0.008). The posterior shift of the spinal cord at C5/6 presented no statistical significant difference (p=0.261) between the groups. The number of the compressed segments of the cord (p=0.824), and the appearance of an intramedullary T2-weighted high signal lesion after surgery at C3–5 (p=0.537) revealed no significant differences between the two groups. Thus, with the 9 radiological factors, there was only significant difference of the posterior shift of the spinal cord at C4/5 between the two groups (p=0.008 as above).
2. Risk factor analysis
Logistic regression analyses revealed posterior shift of the spinal cord at C4/5 (odds ratio, 12.066; p-value=0.029; 95% confidence interval, 1.295–112.378) was significantly involved in C5 palsy, whereas other radiologic examinations did not significantly affect the occurrence of postoperative C5 palsy (Table 5).
Odds ratio of posterior shift of the spinal cord at C4/5 was 1 or more, and it became a risk factor for C5 palsy in this study. For the T2-high lesion, because there were too few cases of any new high intensity lesions, a statistical analysis was not possible.
Discussion
In 1961, Stoops and King [8] and Scoville [9] reported postoperative paresis of the upper extremity as a neurologic complication following cervical laminectomy. Although the occurrence of C5 palsy after laminoplasty has been reported in many studies, its detailed mechanism remains poorly understood. There have been several hypotheses regarding the etiology of C5 palsy, including direct injury to nerve root during surgery, tethering of the nerve root, segmental spinal cord disorder, and reperfusion injury to the spinal cord, but none of these hypotheses have been completely established.
1. Direct injury to nerve root during surgery
Tamiya et al. [3] reported that direct injury or heat injury has become a cause of damage during surgery. However, Sasai et al. [10] suggested that additional microcervical foraminotomy could prevent postoperative C5 palsy, although during foraminotomy high-speed drills come much closer to nerve root than any other procedure, so that frictional heat reaching the roots must be the highest. It is inconsistent with injury due to heat if the injury is preventable by a procedure that generates even more heat.
Hirabayashi et al. [11] along with Iwamoto et al. [12] reported that if damage occurs during surgery by direct injury, C5 palsy becomes immediately evident after surgery. However, none of our cases immediately developed C5 palsy after surgery, so we thought the possibility of damage during surgery, as the cause of palsy, was low in our study. There were, however, only 5 cases of C5 palsy in our study, so we could not definitively conclude that direct injury to nerve root during surgery did not induce C5 palsy in our study. We thought that future study should examine more cases.
2. Tethering of the nerve root
Nerve root traction may be caused by posterior shift of the spinal cord after laminoplasty, and the so-called "tethering effect" has been considered as one of the more acceptable pathologic mechanisms of C5 palsy. We showed a significant difference between the two groups with regards to the posterior shift of spinal cord at C4/5. The extent of posterior shift of the spinal cord after laminoplasty was measured by various methods and the reports indicate a 1 to 3 mm displacement [1314]. Yamashita et al. [15] reported that the posterior shift averaged 5 mm at C4 or C5 in patients with C5 palsy; this was significantly greater than in patients without clinical signs of palsy. In this study, we indicated that the posterior shift of the spinal cord averaged 3.6 mm at C4/5 in patients with C5 palsy. There was a significant difference in the posterior shift between the two groups with almost the same results as those of the previous studies.
Radiological examinations, however, may some problems with respect to the time of imagings performed postoperatively. Shiozaki et al. [16] reported that the posterior shift of the spinal cord at 24 hours postoperation had a tendency to shift more posteriorly than that observed at 2 weeks after cervical laminoplasty. In this study, the P-group had their MRI recorded immediately upon occurrence of their palsy. Most of the N-group, however, had the MRI more than one month after their surgery. There is a possibility that difference of time in taking the MRI caused a significant difference in the posterior shift of the spinal cord between the two groups. It is difficult to simply compare this data with those of previous reports, because many of reports with the exception of Shiozaki et al. [16] do not mention the time of the MRI postoperation. Posterior shift of the spinal cord at C4/5 in this study was the risk factor of C5 palsy by logistic regression analysis. But for the reasons mentioned above, this risk factor has this confounding factor in intrepretation. However we thought that the posterior shift of the spinal cord at C4/5 is likely to be the largest factor of C5 palsy from this study and that in a future study, a greater number of cases postoperation with matched timing of their MRI should be examined.
There are other causes of nerve root lesion that have been thought to play a part in C5 palsy; these include kinking by the superior articular process, pressuring and impinging the nerve root by an osteophyte, excessive tethering on the nerve roots to one side caused by deviation of the vertebral arch opening angle, and excessive stress on the nerve roots caused by edge of too steep a hinge, and kinking caused by excessively lateral bony gutter [246]. For preoperative risk factors, there are the anterior protrusion of the superior articular process and the width of the intervertebral foramen that are thought to play a role [17].
Most posterior shifts occurred at the C5 vertebral level because C5 is the apex of cervical lordosis. In addition, the superior articular process of C5 protrudes in a more anterior direction and as the root of C5 is shorter compared with other levels, a posterior shift might create a tension on the C5 nerve root, causing C5 palsy. However, there was no significant difference between the two groups with regards to the anterior protrusion of the superior articular process in this study.
Preexisting foraminal stenosis has been suggested to be associated with C5 palsy in several studies. Imagama et al. [17] presented radiographic evidence of nerve root impingement from CT in a retrospective study of 1,858 patients who underwent a cervical laminoplasty. They found that the width of the C5 intervertebral foramen was significantly smaller, and the anterior protrusion of C5 superior articular process was significantly greater in patients with C5 palsy. In this study, there was no significant difference between the two groups with regards to the width of the intervertebral foramen. Some researchers reported that patients who have narrow foramen should have prophylactic foraminotomy to prevent C5 palsy. Komagata et al. [18] reported that prophylactic bilateral partial foraminotomy could reduce the incidence of C5 palsy after open-door laminoplasty from 4.0% to 0.6%. In contrast, Tamiya et al. [3] reported that foraminotomy was not necessary to prevent dysfunction of the cervical nerve root. Thus, whether foraminotomy is beneficial as a prophylactic measure on the nerve root is not yet clear. Foraminotomy may be preventative when the cause of C5 palsy is tethering of the nerve root. However, we didn't perform foraminotomy to prevent C5 palsy in all cases when we performed the laminoplasty. We only investigated respective risk factors in this study. But we also thought that the risk factors might also increase when performing additional procedures. As a future study, it may be necessary to consider the case of overlapping risk factors.
For additional postoperative risk factors, there were the vertebral arch opening angle, the hinge angle, and the position of the gutters to consider. Matsunaga et al. [6] have reported that the occurrence of C5 palsy was more common in the cases where the vertebral arch opening angle was large and the hinge angle was acute [5]. They reported that excessive tethering to one side of the nerve root by the vertebral arch opening angle deviation and excessive stress on the nerve root by the edge of the sharp hinge were causes of C5 palsy. As a precautionary measure against C5 palsy, Matsunaga et al. proposed that the vertebral arch opening angle should be 15°–20° and the hinge angle should not be too acute. The authors also reported that this insight during surgery could reduce the incidence of C5 palsy from 35.3% to 16.4%. In our study, there were no significant differences between the two groups with regards to the vertebral arch opening angle and the hinge angle.
Creating the bony gutter too laterally or too medially might also result in kinking of the nerve root after posterior shifting of the cord. Some researchers recommend making the gutter on the medial aspect of the zygapophyseal joint to prevent C5 palsy [1920], whereas others reported that the incidence of C5 palsy did not correlate with the position of the bony gutter [1321]. In this study, there was no significant difference between the two groups with regards to the position of the gutter.
3. Segmental spinal cord disorder and reperfusion injury
Some researchers have reported that reperfusion injury occurs when the spinal nerve is depressurized. Chiba et al. [22] and Hasegawa et al. [23] proposed that local reperfusion injury in the spinal cord could cause the deterioration of gray matter and could lead to postoperative segmental motor palsy. The lesions identified as T2 high-signal intensity zone in the spinal cord newly arose or increased in intensity after surgery. Kaneko et al. [24] also suggested the association of gray matter lesion with the palsy as a high signal change on T2-weighted MRI in spinal cord at the relevant segment was shown in all patients who developed postoperative palsy. However, Seichi et al. [25] reported that the expansion of intramedullary high intensity lesion on T2-weighted MRI was only minimally associated with postoperative segmental motor palsy. In our study, none of C5 palsy cases had new high signal intensity lesions in the spinal cord at the level of C3–C5 after surgery; therefore, this measure could not predict C5 palsy occurrence.
4. Involvement of C6 segment
In the present study, we showed a significant difference of the posterior shift of the spinal cord at C4/5 between palsy group and non-palsy group. If the shift of the spinal cord at C4/5 is large, there is a possibility that there is a tethering effect also to C6 ventral root. But in this study, an involvement of the C6 segment was not recognized with various radiological examinations (Table 4). In addition, the strength of the deltoid muscle was not lower than the biceps muscle (Table 1) for all cases. Thus, we could not establish an involvement of C6 segment in the C5 palsy cases.
The limitations of this study were the difference in the average time postoperation for the MRI between the P- and N-groups and the relatively small number of research cases studied.
Conclusions
In the present study, the large amount of postoperative posterior shift of the spinal cord at the C4/5 level was the only factor that correlated with occurrence of postoperative C5 palsy. From this study, it may be reasonable that the tethering phenomenon might be the most significant contributing cause to C5 palsy. Since the present study had some limitations, additional studies with a greater number of cases of DDL for CSM may better address the causes of postoperative C5 palsy in this population.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.