Extraosseous Thoracic Foraminal Osteoblastoma: Diagnostic Dilemma and Management with 3 Year Follow-Up
Article information
Abstract
Osteoblastomas are bone forming lesions arising mainly from posterior elements of the vertebra. They are commonly encountered in the cervical and lumbar regions. We present a case of a thoracic osteoblastoma which is extra osseous and is not communicating with any part of the vertebra present intraforaminally. This is a rare presentation of an osteoblastoma. Imaging studies do not accurately diagnose the osteiod lesion. The size of the lesion and cortical erosion seen on the computed tomography scan help in differentiating the osteoid osteoma and osteoblastoma, but they are less sensitive and specific. Thus a histopathology is the investigation of choice to diagnose the osteoblastoma. Early and adequate removal of mass prevents malignant transformation, metastasis, and recurrence. In our case we excised the pars interarticularis unilaterally, removed the osteoid mass intact, and performed unilateral instrumented fusion. There was no recurrence and solid fusion was seen at 3 years follow up.
Introduction
An osteoblastoma is benign bone lesion most commonly affecting the spine. The cervical and lumbar spine are commonly affected with the most common site being the posterior elements of the vertebra [1]. Imaging modalities fail to provide accurate diagnosis of the osteoblastoma [2]. Histologically, an osteoblastoma presents osteoid matrix surrounded by osteoblasts [3], and it remains the mainstay for diagnosis [4]. Since they are bone forming lesions, they tend to grow from the bone. In the thoracic spine they grow most commonly from posterior elements, but also arise from the pedicle, spinous process, vertebral body and lamina [2]. It is important to diagnose an osteoblastoma because of its ability to recur, tendency for malignant transformation, and metastasis [3].Treatment includes complete resection of a lesion to prevent recurrence [5]. We present this case of an osteoblastoma unusually present in the thoracic region within the foramen, with its clinico-radiological evaluation and surgical management, with the aim of facilitating accurate and timely diagnosis. Also the extraosseous presence of the osteoblastoma in our case is rare.
Case Report
A 27-year-old man presented himself to us with the chief complaints of pain in the mid-back from 6 months prior with no radicular symptoms or weakness. Neurological examination was normal.
The patient had been under anti-tuberculous treatment by the previous treating surgeon for thoracic tuberculosis from 5 months prior due to magnetic resonance imaging (MRI) findings. Details of the MRI films were not available. He had no relief with these medications. The radiograph of the thoracolumbar spine was performed and showed thoracic scoliosis with sclerosis around the T10 pedicle with no obvious bony destruction (Fig. 1). Haematological studies showed alkaline phosphatase was slightly raised.

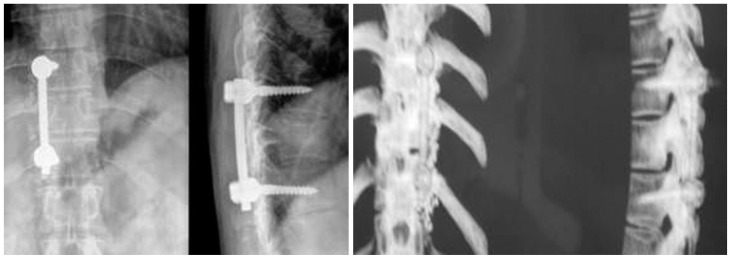
Radiograph of dorsolumbar spine showing sclerosis around the 10th thoracic vertebrae with scoliotic list.
We performed a new MRI which showed an extradural lesion in the region of the right T10-11 neural foramen with an intense marrow edema in the right half of the T10 vertebra and minimal right paravertebral edema from the T9 to T11 vertebral levels. Disc spaces were well maintained (Fig. 2). Since the findings were non-specific and a lesion was appreciated in the T 10 vertebra, we performed a computed tomography (CT) scan indicates dedicated study only at pathological level.
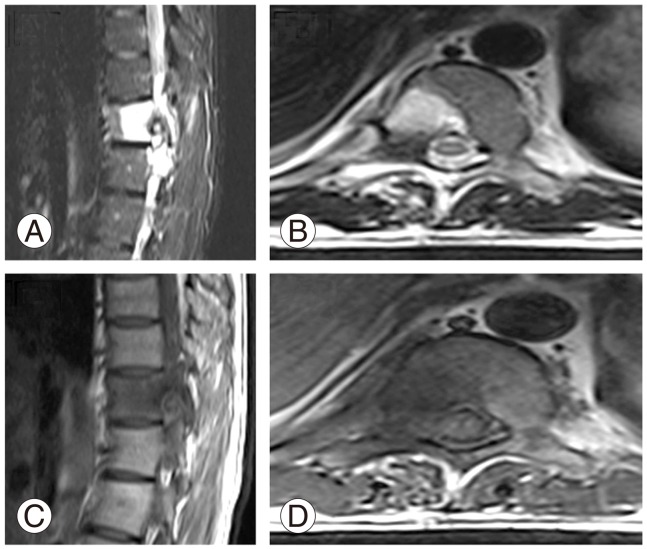
(A-D) Magnetic resonance imaging scan. T2 and T1 weighted images show perilesional edema and reaction.
The CT scan showed a well-defined round lesion with ossified rim, majority lytic component, and a speck of calcification below the right pedicle and underneath the right pars interarticularis (it commented to be nidus by the radiologist, or it was a sequestrum as rare possibility due to a history of long-standing infection). There was subtle scalloping of the posterior cortex of the T10 vertebral body anteriorly and of the superior articular facet posteriorly. There was sclerosis of the adjacent vertebral body and pedicle. No obvious communication with the vertebral body/pedicle was seen. No evidence of bony destruction/erosion was seen. The lesion did not arise from any bony tissue and was extraosseous (Fig. 3).

Computed tomography scan. (A-C) Show the location of the osteoblastoma in the axial, sagittal, and coronal planes.
The bone scan was available (done by one of the previous treating surgeons) and showed normal findings in the 1st phase. At 3 hours, the delayed phase showed focal, increased uptake of the tracer in the right T10 vertebral pedicle with no paravertebral soft-tissue component and maintained disc space with scoliosis. No multiple lesions were present.
After all these investigations, surgical excision with right-sided instrumented fusion of the lesion was planned, keeping osteiod osteoma as the most probable diagnosis.
1. Surgical plan
On the day of the surgery, CT guided marking of the T10 pedicle was done to find out the exact location of the tumour and to plan the incision (Fig. 4A).
2. Procedure
A standard posterior minimal approach with unilateral right side of T9-11 exposed. The pedicles were identified, and pedical screws fixed on the right T9 and T11 level. With the high speed burr after confirming the exact level in the image intensifier, the pars interarticularis was excised, and the facet was removed partially. Approaching the undersurface of the pars, we identified the lesion and the mass was removed intact (Fig. 4B, C) and sent for histopathology. Unilateral, instrumented fusion with allografts was done to maintain spinal stability. (Fig. 4D, E). Postoperatively, the patient was significantly relieved of pain, and neurologic signs were intact.
The histopathology report suggested the presence of an osteoblastoma (Fig. 5). Since osteoblastomas are known for malignant transformation and subsequent metastasis, a CT scan of the thorax was done to rule out any additional lesions. It was within normal limits. Follow up radiographs, CT scan, and MRI scan show no signs of recurrence (Fig. 6). Presently, the patient is asymptomatic without recurrence with a three-year follow up.

Histopathology (H&E). (A) Shows irregular anastomosing-woven tiny bony bits (40×). (B) Shows irregular bony bits that are lined by regular osteoblasts (100×). (C) Shows lining osteoblasts that do not show any cytological atypia (400×).
Discussion
An osteoblastoma and osteiod osteoma usually present themselved in the second decade and are more common in males than in females [2,5]. An osteiod osteoma is common in long bones whereas an osteoblastoma is common in the axial skeleton [3,5]. Clinically, an osteoblastoma is usually presents with dull aching pain, scoliotic deformity, and stiffness [6]. Pain of an osteoblastoma, unlike that of an osteiod osteoma, does not have diurnal variation and is not relieved by nonsteroidal anti-inflammatory drugs [2]. Radiculopathy and neurological deficit occurs mostly in an osteoblastoma with rare occurrence in an osteiod osteoma [7]. In laboratory studies, alkaline phosphatase may be slightly raised. No other findings are observed [4].
An osteoblastoma is an expansile lytic lesion with sclerotic (changed) margins on plain radiographs [8]. However it is difficult to interpret because of overlapping shadows as lesions are common in posterior elements [9]. A bone scan is helpful for early diagnosis and in localizing the tumour, but it is non-specific in giving accurate diagnosis [5].
MRI scans are usually the initial investigation modality for spinal pathology. MRI scans show peritumoral inflammation in case of the presence of an osteoblastoma. Peritumoral inflammation is common in benign lesions like osteoblastoma, osteiod osteoma, chondroblastoma, etc. but can also be seen in malignant conditions like osteosarcoma [10]. We believe the presence of edema and soft tissue reaction may have prompted the previous treating surgeon to feel a diagnosis of infectious aetiology. Since tuberculosis is endemic in developing nations [11], the patient was started on anti-tuberculous drugs. However, MRI scans help poorly in differentiating between an osteoblastoma and an osteiod osteoma but help by delineating the involvement of the spinal cord and adjacent soft tissues [8].
A CT scan gives a better idea about the origin and extent of the tumour, matrix mineralization, and surrounding bony shell [8]. Benign findings on the CT scan help to negate the malignant differentials seen through an MRI. In our case, the findings of the CT scan narrowed down our differential to osteiod osteoma or osteoblastoma.
The main radiological differentiating points between osteiod osteoma and osteoblastoma are the size of the lesion and cortical breech. Classification of an osteoblastoma according to the size of the lesion is defined by various authors to be a lesion greater than 1 cm, 1.5 cm, or 2 cm [12]. However differentiating an osteoblastoma from an osteiod osteoma based on the size of the lesion is not recommended [13]. Cortical breech is hallmark of an osteoblastoma but is only seen in up to 62% of cases [14]. In our case, the size of the mass was 9 mm, and it was showing no cortical erosions. Unfortunately, there is nothing specific in the plain radiograph, CT, MRI, or bone scan to differentiate between an osteiod osteoma or osteoblastoma.
Histopathologically, it was once considered to be two different expression of same tumour, but now it is clear that they are separate entities [13]. Both are vascular and bone forming tumours. However, an osteoblastoma has scanty reactive sclerosis and contains fibro-vascular stroma with interlacing woven bone [3]. Commonly, it can present itself with more than one nidus [15]. In our case, the presence of osteoblasts with scanty sclerosis pointed towards the diagnosis of an osteoblastoma. The absence of cellular atypia and mitotic activity indicated a benign lesion.
A complete resection of the mass is important to prevent recurrence [5]. Intra-foraminal location of an osteoblastoma is challenging in terms of the adequacy of tumour removal and of maintaining spinal stability. In our case, the mass was present intraforaminally on the right side. Therefore, after resecting pars and hemi facets, only unilateral fusion was done.
Nemoto et al. [2] reviewed 75 cases of spinal osteoblastomas and showed that osteoblastomas of the spine arise from various parts of the vertebra. In our case, the osteoblastoma was extra osseous and did not communicate with any part of the vertebra. This is a rare presentation of an osteoblastoma, and that fact should be borne in mind.
Thus, the diagnosis of an osteoblastoma depends mainly on the histopathology. One should be highly vigilant in analysing clinical features and imaging characteristics, especially in patients with chronic symptoms. A CT scan is the modality of choice for diagnosing an osteiod lesion. It gives a better idea about the size of the lesion and of cortical erosions. Treating surgeons should be familiar with the clinico-radiological evaluation of an osteoid lesion in the spine since osteoblastomas are most common in the spine. It is important to differentiate an osteoblastoma from an osteoid osteoma to explain the prognosis of the lesion in terms of recurrence and malignant transformation.
Notes
No potential conflict of interest relevant to this article was reported.