1. Langer R, Vacanti JP. Tissue engineering. Science 1993;260:920–926. PMID:
8493529.


2. Chiu WT, Lin HC, Lam C, Chu SF, Chiang YH, Tsai SH. Review paper: epidemiology of traumatic spinal cord injury: comparisons between developed and developing countries. Asia Pac J Public Health 2010;22:9–18. PMID:
20032030.


3. Spinal cord injury Facts and figures at a glance. J Spinal Cord Med 2008;31:357–358. PMID:
18795487.


4. Ditunno JF Jr, Young W, Donovan WH, Creasey G. The international standards booklet for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Paraplegia 1994;32:70–80. PMID:
8015848.

5. Tuszynski MH, Steeves JD, Fawcett JW, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP Panel: clinical trial inclusion/exclusion criteria and ethics. Spinal Cord 2007;45:222–231. PMID:
17179971.


6. Hamilton BB, Granger CV, Sherwin FS, et al,A uniform national data system for medical rehabilitation. Fuhrer MJ, editors. Rehabilitation outcomes: analysis and measurement. Baltimore, MD: Brookes; 1987. p.137–147.
7. Haas BM, Bergstrom E, Jamous A, Bennie A. The inter rater reliability of the original and of the modified Ashworth scale for the assessment of spasticity in patients with spinal cord injury. Spinal Cord 1996;34:560–564. PMID:
8883191.


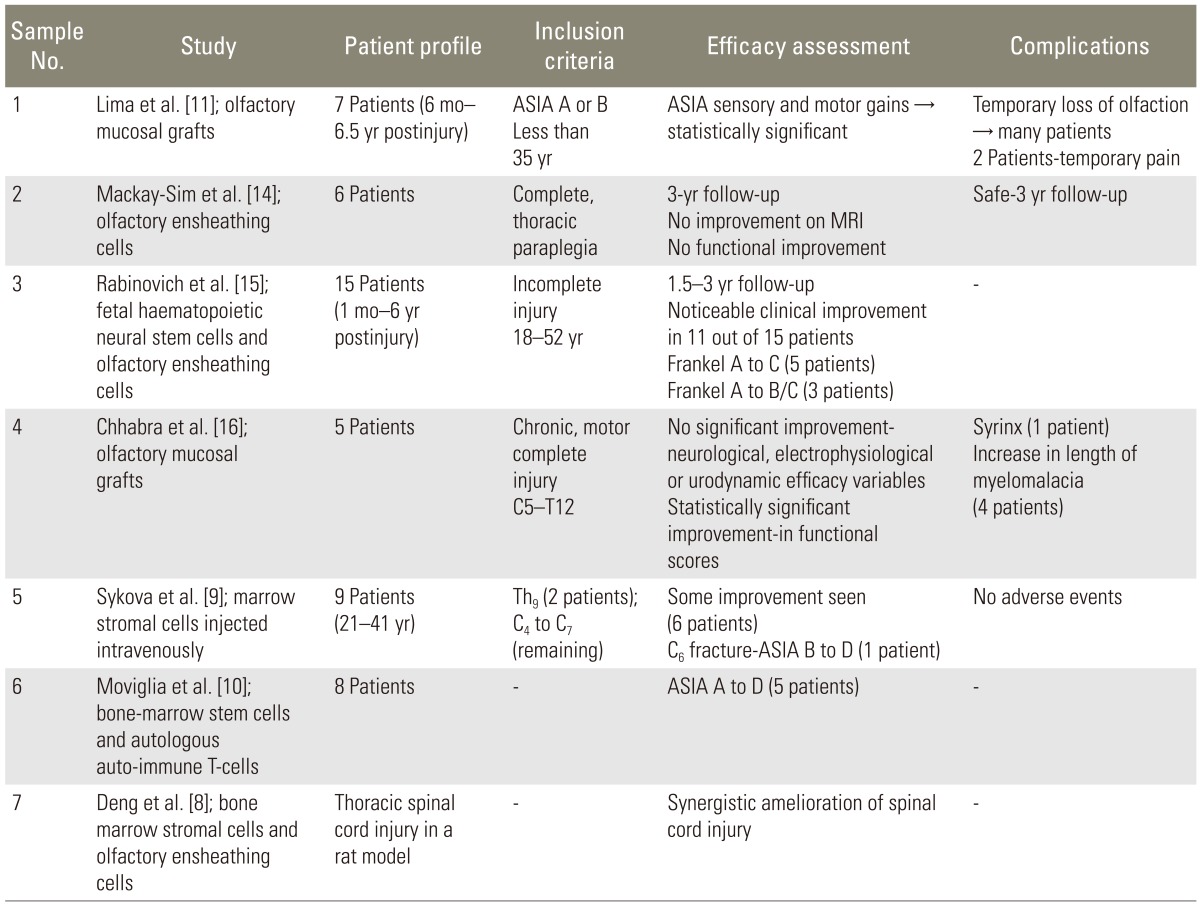
8. Deng YB, Liu Y, Zhu WB, et al. The co-transplantation of human bone marrow stromal cells and embryo olfactory ensheathing cells as a new approach to treat spinal cord injury in a rat model. Cytotherapy 2008;10:551–564. PMID:
18608352.


9. Sykova E, Homola A, Mazanec R, et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant 2006;15:675–687. PMID:
17269439.


10. Moviglia GA, Varela G, Brizuela JA, et al. Case report on the clinical results of a combined cellular therapy for chronic spinal cord injured patients. Spinal Cord 2009;47:499–503. PMID:
19223861.


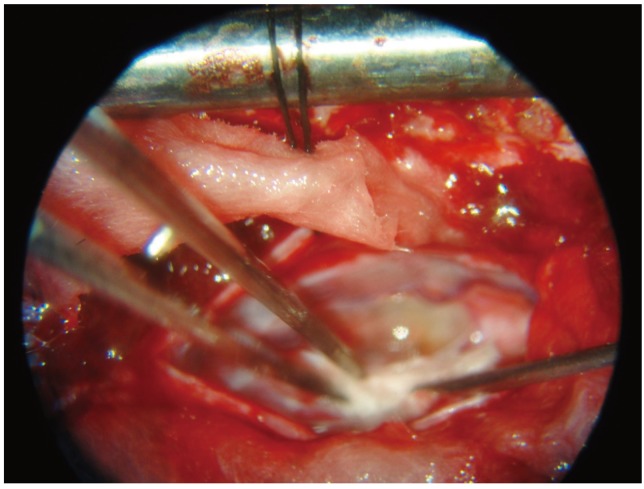
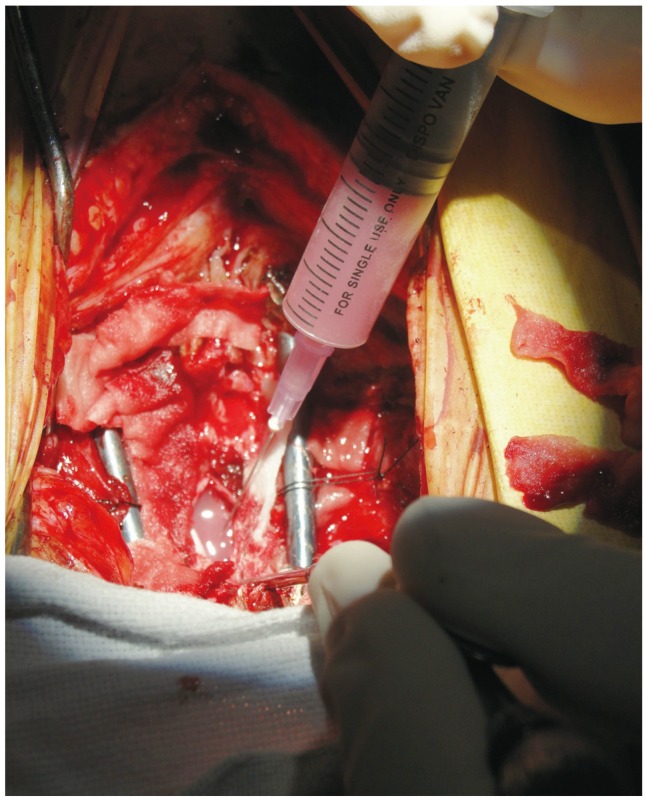
11. Lima C, Pratas-Vital J, Escada P, Hasse-Ferreira A, Capucho C, Peduzzi JD. Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. J Spinal Cord Med 2006;29:191–203. PMID:
16859223.



12. Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 1996;139:244–256. PMID:
8654527.


13. Tator CH. Biology of neurological recovery and functional restoration after spinal cord injury. Neurosurgery 1998;42:696–707. PMID:
9574633.


14. Mackay-Sim A, Feron F, Cochrane J, et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain 2008;131:2376–2386. PMID:
18689435.



15. Rabinovich SS, Seledtsov VI, Poveschenko OV, et al. Transplantation treatment of spinal cord injury patients. Biomed Pharmacother 2003;57:428–433. PMID:
14652169.


16. Chhabra HS, Lima C, Sachdeva S, et al. Autologous olfactory [corrected] mucosal transplant in chronic spinal cord injury: an Indian Pilot Study. Spinal Cord 2009;47:887–895. PMID:
19488051.


17. Burns AS, Ditunno JF. Establishing prognosis and maximizing functional outcomes after spinal cord injury: a review of current and future directions in rehabilitation management. Spine (Phila Pa 1976) 2001;26(24 Suppl): S137–S145. PMID:
11805621.


18. Nandoe Tewarie RS, Hurtado A, Bartels RH, Grotenhuis A, Oudega M. Stem cell-based therapies for spinal cord injury. J Spinal Cord Med 2009;32:105–114. PMID:
19569457.



19. Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol 1998;400:469–486. PMID:
9786409.


20. Roisen FJ, Klueber KM, Lu CL, et al. Adult human olfactory stem cells. Brain Res 2001;890:11–22. PMID:
11164764.


21. Murrell W, Feron F, Wetzig A, et al. Multipotent stem cells from adult olfactory mucosa. Dev Dyn 2005;233:496–515. PMID:
15782416.


22. Geffner LF, Santacruz P, Izurieta M, et al. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: comprehensive case studies. Cell Transplant 2008;17:1277–1293. PMID:
19364066.


23. Chernykh ER, Stupak VV, Muradov GM, et al. Application of autologous bone marrow stem cells in the therapy of spinal cord injury patients. Bull Exp Biol Med 2007;143:543–547. PMID:
18214319.


24. Fouad K, Pearse DD, Tetzlaff W, Vavrek R. Transplantation and repair: combined cell implantation and chondroitinase delivery prevents deterioration of bladder function in rats with complete spinal cord injury. Spinal Cord 2009;47:727–732. PMID:
19255587.


25. Zhang C, He X, Lan B, Li H. Study on repair of subacute spinal cord injury by transplantation of olfactory ensheathing cells combined with chondroitinase ABC in adult rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2009;23:8–13. PMID:
19192870.