Investigation of Efficacy of Mitomycin-C, Sodium Hyaluronate and Human Amniotic Fluid in Preventing Epidural Fibrosis and Adhesion Using a Rat Laminectomy Model
Article information
Abstract
Study Design
A retrospective study.
Purpose
The aim of this study was to evalute the effects of mitomycin-C, sodium hyaluronate and human amniotic fluid on preventing spinal epidural fibrosis.
Overview of Literature
The role of scar tissue in pain formation is not exactly known, but it is reported that scar tissue causes adhesions between anatomic structures. Intensive fibrotic tissue compresses on anatomic structures and increases the sensitivity of the nerve root for recurrent herniation and lateral spinal stenosis via limiting movements of the root. Also, neuronal atrophy and axonal degeneration occur under scar tissue.
Methods
The study design included 4 groups of rats: group 1 was the control group, groups 2, 3, and 4 receieved antifibrotic agents, mitomycin-C (group 2), sodium hyaluronate (group 3), and human amniotic fluid (group 4). Midline incision for all animals were done on L5 for total laminectomy. Four weeks after the surgery, the rats were sacrificed and specimens were stained with hematoxylin-eosin and photos of the slides were taken for quantitive assesment of the scar tissue.
Results
There was no significant scar tissue in the experimental animals of groups 2, 3, and 4. It was found that there was no significant difference between drug groups, but there was a statistically significant difference between the drug groups and the control group.
Conclusions
This experimental study shows that implantation of mitomycin-C, sodium hyaluronate and human amniotic fluid reduces epidural fibrosis and adhesions after spinal laminectomy in rat models. Further studies in humans are needed to determine the complications of the agents researched.
Introduction
Lumbago is a common health problem. The prevalence of lumbago is 80% for all ages, and it is the most common cause limiting the activity for people younger than 45 years old. Lumbar disc herniation is one of the most important etiologic causes in people with lumbago or lumbago and leg pain combined [1-4]. Lumbar spinal surgery is performed for approximately one million patients around the world every year [5]. The success ratio of lumbar disc hernia surgery is 75% to 92%. Low back pain and radiculopathy signs persist in 8% to 25% of patients. Wrong level surgery, recurrent or persistant hernia, iatrogenic instability, central or lateral stenosis, arachnoiditis, and spinal epidural fibrosis are some of the causes for persistant pain [6-8]. Spinal epidural fibrosis is a process of physiologic scar tissue formation seen in patients who have been operated for lumbar herniation. This fibrotic tissue causes the nerve root to be compressed or streched in some patients [9,10]. Because of the difficultites in reoperation and risks of complication, a lot of studies are being performed in order to minimize this physiologic period. Various membranes and materials that block the physiological course of epidural fibrosis at different stages have been used to prevent compression of this hypertrophic scar tissue on neuronal structures; however, these materials are expensive [11,12].
In this study the effects of mitomycin-C, sodium hyaluronate, and human amniotic fluid on preventing epidural fibrosis have been investigated using a rat laminectomy model.
Materials and Methods
In this study we used 40 female Wistar albino rats for the same age group (4-5 months) with the mean weight of 250 g. Routine feeding and care of the rats were obtained. All animals were kept at room temperature with 50% humidity ratio, as well as 12 hours light and 12 hours dark cycles. All processes were performed according to hygienic rules and they were under veterinarian control.
The animals were divided into four groups with ten rats per group: group 1, control group; group 2, mitomycin-C group; group 3, sodium hyaluronate group; and group 4, human amniotic fluid group.
The rats were prepared for anesthesia with fasting for 12 hours before the surgical procedure. We used intramuscular (into right quadriceps femoris muscle) 10 mg/kg Xylazine hydrocloride and 10 mg/kg Ketamine hydrochloride for anesthesia. A prophylactic of one dose ceftriaxone 50 mg/kg was administrated to rats 30 minutes before the operation. After placing the rats on the operation table and cleaning the operation fields, we made a L4-S1 midline incision, incising the thoracolumbar fascia, and we dissected paravertebral muscle groups subperiostally. For group 1, we performed only laminectomy. For group 2, we put a cotton piece which was prepared with 0.1 mg/mL mitomycine-C after laminectomy, on the operation level and waited for 3 minutes. The same process was performed with sodium hyaluronate for group 3 and with human amniotic fluid for group 4. The experiment duration was 4 weeks. We continued regular feeding and care of the rats for 4 weeks.
After 4 weeks the rats were sacrificed by C1-2 dislocation under anesthesia with Xylazine hydrochloride and Ketamin hydrochloride. Then, we opened the incisions and removed the laminectomy level along with one level above and one level below the laminectomy level together with paravertebral muscles. Tissue specimens were fixed in 10% formalin solution for two days, and then they were decalcified in 10% formic acid solution with controlling the tissue softness for two hours. Specimens were washed for three or four hours before their water was changed into alcohol (50%, 70%, 90%, 96% and absolute alcohol). They were made transparent using xylol. They were blocked in 58℃ paraffin. Five micrometer (µm) sections were taken from the blocks. Sections were stained with hematoxylin-eosin and photos of the slides were taken for quantitive assesment of the scar tissue.
The histopathologic and gross anatomic data were assessed by using Kruskal-Wallis variance analysis and the Mann-Whitney U test with Bonferrani correction.
Results
No infection was seen except in two rats during four weeks. During removal of the sutures, infection of cutaneous and subcutaneous tissue in a rat from the human amniotic fluid group and a regular shaped, soft mass lesion in paravertebral muscles in a rat from the sodium hyaluronate group were observed. Histopathologic analysis of this mass lesion was assessed as the inflammatory reaction formed from numerous neutrophils and lymphocytes. As there are publications about the role of infection in epidural fibrosis formation, these two infected rats were excluded from the study. A foreign body reaction against the drugs was not seen in any rats.
In the postoperative period, one rat from the control group and one rat from the mitomycin-C group had paraparesis and these rats died in the following days. These rats were excluded from the final analysis, so we studied with 9 rats for all four groups.
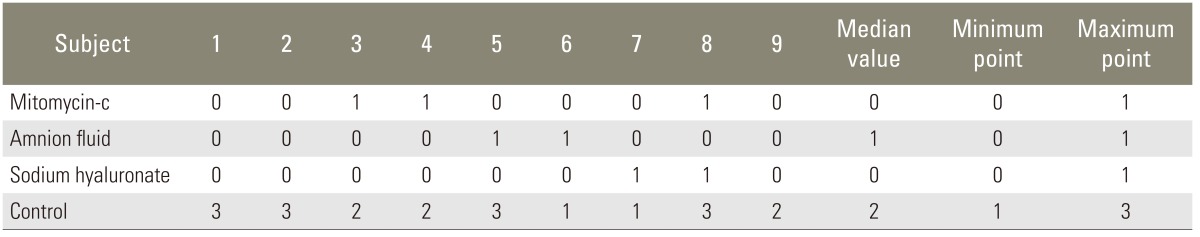
At the end of four weeks, before we had taken off the laminectomy level together with paravertebral muscles, we made a microdissection on the operation field and marked epidural fibrosis according to the Robertson pointing system (Table 1).
The microdissection findings of experimental animals were as follows: 1) There was dense scar tissue on the dura in the laminectomy field of 4 rats in the control group. This was marked as 3 points. There was reasonable scar tissue in three rats, and there were thin scar tissue and minimal adhesions in two rats. 2) Although there was no clear determination of scar tissue in other drug groups, a tolerable dural adhesion was determined in some animals. Final scores differed significantly, and these are shown on Table 2.
After using the Mann-Whitney U test with Bonferroni correction in order to determine the group or groups which show a difference, it was seen that there were no significant differences between mitomycin-C, human amniotic fluid, and sodium hyaluronate groups, but there was a significant difference between the control group and the drug groups.
In the histologic assessment of the rats, it was observed that laminectomy regions were filled up with scar tissue, and this tissue adhered to the dural sac by proceeding towards the spinal channel. In these areas, collagen fibers bundled together, and there were numerous oval shaped fibroblasts among these bundles. Moreover, it was observed that capillaries were full of erythrocytes. Most of the collagen fibers and fibroblasts were arranged as migrating from paraspinal muscles to the laminectomy region. Giant bodies and neutrophils were not observed in this area (Fig. 1).
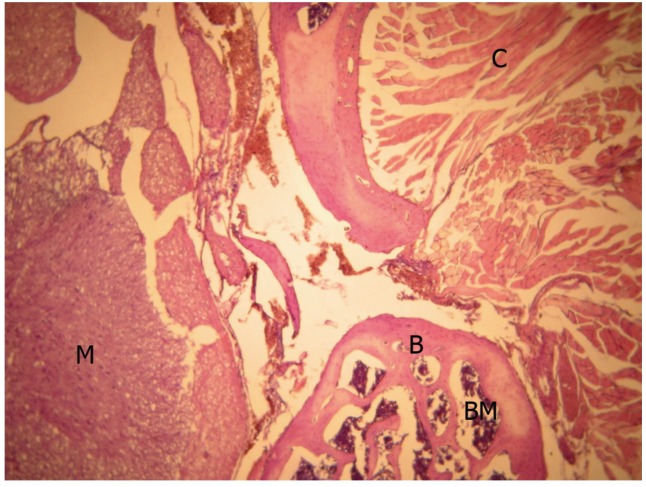
Control group, light microscopic view (H&E, ×40). Bone tissue and medulla spinalis were in normal structure. M, medulla spinalis; B, bone tissue; BM, bone marrow; C, collagen fiber bundles.
There was no significant scar tissue in the human amniotic fluid group. There was only grade 1 scar tissue in 2 animals. These tissues which adhered to the dura had a loose structure, and collagen fibers did not show any bundle formation (Fig. 2).
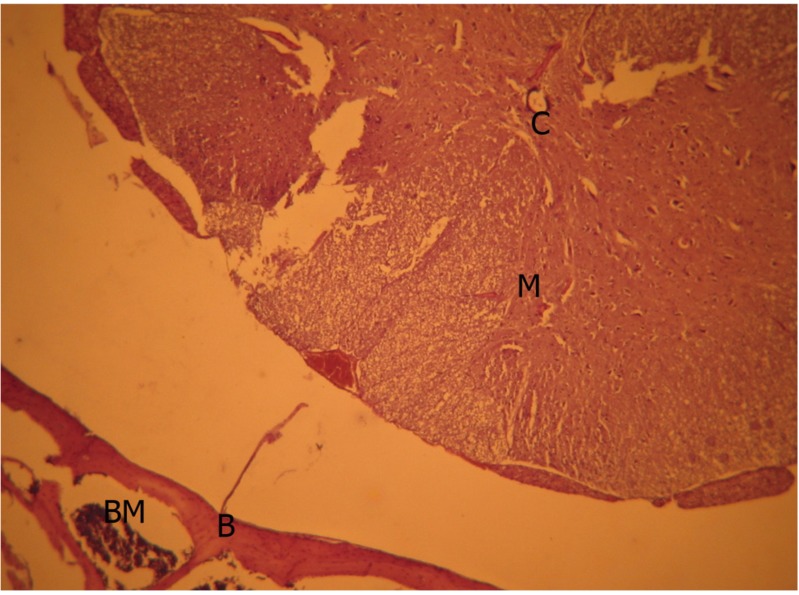
Human amnionfluid group, light microscopic view (H&E, ×40). M, medulla spinalis; B, bone tissue; BM, bone marrow; C, collagen fiber bundles.
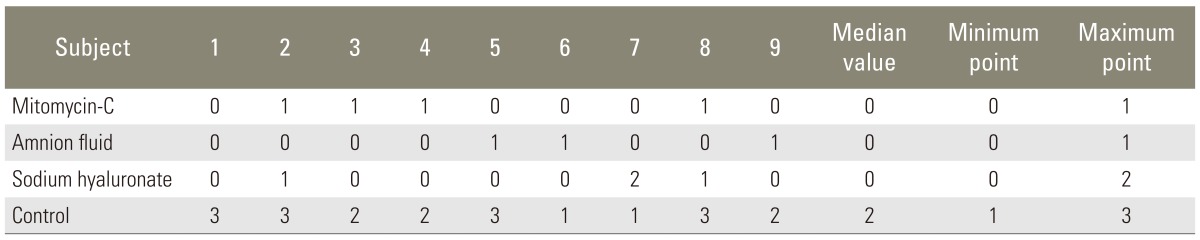
Although there was no remarkable scar tissue in mitomycin-C (Fig. 3) and sodium hyaluronate groups (Fig. 4), grade 1, thin and loose fibrous tissue adhered to the dura in the laminectomy region was determined in 3 animals in the mitomycin-C group and 2 animals in the sodium hyaluronate group. Histopathologic findings were also significant which are shown on Table 3 and the points for groups in table 4 demonstrated a significant difference (Tables 3, 4).
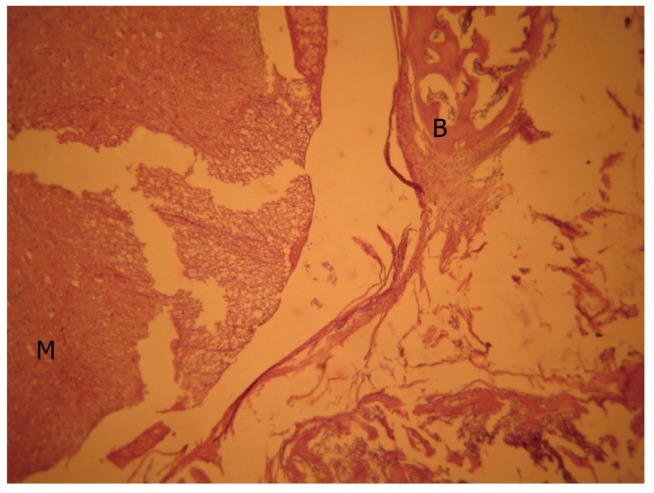
Mitomycin group, light microscopic view (H&E, ×40). Bone tissue and medulla spinalis appeared normal. M, medulla spinalis; B, bone tissue.
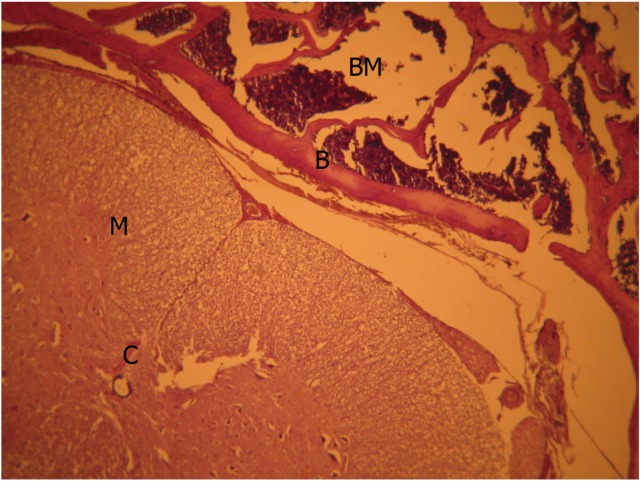
Sodium hyaluronate group, light microscopic view (H&E, ×40). M, medulla spinalis; B, bone tissue; BM, bone marrow; C, collagen fiber bundles.
After the Mann-Whitney U test with Bonferroni correction, it was found that there was no significant difference between the drug groups, but there was a significant difference between the drug groups and the control group.
Percentage of scar tissue in laminectomy regions of all animals were also assessed one by one. For the control group, 4 animals had more than 75%, 3 animals had 50% to 75% and 2 animals had 25% to 50% scar tissue. No significant scar tissue was seen in the drug groups, but adhesions of the dura were observed which were also shown histopathologically.
Discussion
Despite the fact that lumbar disc surgery is one of the most successful neurosurgical operations, 8% to 25% of the patients have recurrent symptoms. One of its causes is the scar tissue that occurs as a result of the natural healing period in the surgical field [6,11].
The role of scar tissue in pain formation is not exactly known, but it is reported that scar tissue causes adhesions between anatomic structures. Intensive fibrotic tissue compresses on anatomic structures, and increases the sensitivity of the nerve root for recurrent herniation and lateral spinal stenosis via limiting movements of the root. Also, neuronal atrophy and axonal degeneration occur under scar tissue [13-16].
It has been mentioned in various reports that epidural scar tissue is not the only reason for postoperative pain, and excluding other reasons regarding failed back surgery syndrome is very important. Due to the difficulties of reoperation and high risk of complications, the importance of this exclusion has been confirmed by various studies [7,8,17-19].
In this study performed on rats, the difference of epidural fibrosis formation, described as the postlaminectomy physiologic period and the formation of fibrous tissue instead of epidural fat tissue in the surgical field, between the control group and 3 different drug groups has been assessed with the help of literature [17,20-24].
Mitomycin-C is an anti-tumor antibiotic agent isolated from Streptomyces caespitosus. The anti-tumor effect of mitomycin-C is attributed to its inhibition of DNA replication, leading to cell death [20,21]. It has been demonstrated to inhibit fibroblastic proliferation in vitro and in vivo. Khaw et al. [22] showed that a single application of mitomycin-C has a measurable effect on cell proliferation and morphology for up to 36 days in vitro. It has been shown in vitro and in vivo that mitomycin-C primarily has an antifibroblastic proliferation yet allows for epithelial growth to occur. Beside the tumoral pathologies, mitomycin-C is also used in non-oncological pathologies like proliferative vitroretinopathy, pterygium, glaucome, nasolacrimal obstruction, subglottic stenosis, bronchial stenosis, and pericardiofibrosis [23]. In our study we aimed to show the property of mitomycin-C on fibroblastic proliferation inhibition in postoperative epidural fibrosis. Liu et al. [24] demonstrated that the treatment of postlaminectomy wounds with mitomycin C-polyethylene glycol film reduces the severity of adhesion by decreasing the concentration of hydroxyproline and increasing the apoptosis of fibroblasts [23]. Su et al. [25] mentioned in their study that mitomycin-C can be beneficial in reducing epidural scar adhesion and that locally applying mitomycin-C in a concentration of 0.5 mg/mL and 0.7 mg/mL may be the optimal concentration in preventing postlaminectomy epidural adhesion [24,25]. A recently published study states that topical application of mitomycin-C above the concentration of 0.3 mg/mL could affect all steps of the wound healing process via inhibiting the angiogenesis and fibroblast proliferation, thus delaying wound healing after laminectomy [26].
Sodium hyaluronate is a connective tissue polysaccharide. sodium hyaluronate creates a dense solution which covers serosal surfaces and prevents lymphocyte migration, proliferation and chemotaxis. As a result of these processes the formation of scar tissue is blocked [27,28]. Songer et al. [11] reported that viscous hyaluronate solution with its semifluid properties coats the nerve roots and the dura anteriorly and posteriorly and reduces peridural fibrosis in the critical anterior region where adhesions form between the nerve root and annulus fibrosus.
Human amniotic fluid includes high concentrations of hyaluronic acid, extracellular macromolecules like fibronectin, laminin, and multiple growth factors when it is taken with amniocentesis in the second trimester [16]. We preferred human amniotic fluid in our study because the literature concerning applications of rat amnion fluid along with its results are not sufficient and no drug reactions were found in human amniotic fluid studies. Its content of high concentration of hyaluronic acid also has significant importance. The amniotic membrane has been used in two studies. Tao and Fan [29] demonstrated that the cross-linked amniotic membrane is effective in reducing epidural fibrosis and scar adhesion after laminectomy in a canine model. They mentioned that it is a promising biomaterial for future clinical applications [26]. The experimental study by Choi et al. [30] showed that implantation of the irradiated frozen-dried human amniotic membrane reduced epidural fibrosis and adhesion after spinal laminectomy in a rat model.
Hyaluronic acid is a naturally occurring glycosaminoglycan found in soft tissues, skin, synovial fluid, and subcutaneous and connective tissues. It is a prototype of the glycosaminoglycan family including chondroitin, keratan and heparan. It is formed of repetitive (1-4)-D-glucuronic acid-beta-(1-3)-D-N-acetylglucosamine disaccharide units. It forms a dense solution that covers surfaces and prevents scar tissue formation via preventing lymphocyte migration, proliferation and chemotaxis [17-19]. Animal studies have also shown hyaluronic acid solutions to be effective in preventing adhesions.
Conclusions
In light of our study's findings, we suggest that mitomycin-C, sodium hyaluronate and human amniotic fluid can be used for the prevention of epidural fibrosis as they are effective agents and inexpensive. However, our results require further clinical research for clinical practice.
Notes
No potential conflict of interest relevant to this article was reported.