Are Controversial Issues in Cervical Total Disc Replacement Resolved or Unresolved?: A Review of Literature and Recent Updates
Article information
Abstract
Since the launch of cervical total disc replacement (CTDR) in the early 2000s, many clinical studies have reported better outcomes of CTDR compared to those of anterior cervical discectomy and fusion. However, CTDR is still a new and innovative procedure with limited indications for clinical application in spinal surgery, particularly, for young patients presenting with soft disc herniation with radiculopathy and/or myelopathy. In addition, some controversial issues related to the assessment of clinical outcomes of CTDR remain unresolved. These issues, including surgical outcomes, adjacent segment degeneration (ASD), heterotopic ossification (HO), wear debris and tissue reaction, and multilevel total disc replacement (TDR) and hybrid surgeries are a common concern of spine surgeons and need to be resolved. Among them, the effect of CTDR on patient outcomes and ASD is theoretically and clinically important; however, this issue remains disputable. Additionally, HO, wear debris, multilevel TDR, and hybrid surgery tend to favor CTDR in terms of their effects on outcomes, but the potential of these factors for jeopardizing patients' safety postoperatively and/or to exert harmful effects on surgical outcomes in longer-term follow-up cannot be ignored. Consequently, it is too early to determine the therapeutic efficacy and cost-effectiveness of CTDR and will require considerable time and studies to provide appropriate answers regarding the same. For these reasons, CTDR requires longer-term follow-up data.
Introduction
Anterior cervical discectomy and fusion (ACDF) is a well-known gold standard surgical procedure for treating degenerative cervical spine diseases. ACDF has become the most popular surgical procedure for disorders of cervical spine. However, there have been some critical issues related to the use of ACDF that remain unresolved. Symptomatic pseudarthrosis following fusion failure is a main cause of chronic postoperative neck pain and approximately 15% of these cases require revision surgeries [1]. When an autograft is used, donor site complications including pain or infection can reduce clinical success rates. Moreover, numerous biomechanical and clinical studies have revealed the evidence of junctional degeneration adjacent to fused levels because of increased biomechanical stress [234567]. More than 5 years after cervical fusion surgery, up to 50% of patients exhibited adjacent segments degeneration (ASD) on radiographic imaging with symptomatic ASD [89].
Currently, cervical total disc replacement (CTDR) using various implants is used as a substitute for cervical fusion surgery. The rationale for CTDR is to preserve segmental motion and maintain normal physiological spinal kinematics. Unlike fusion surgery, CTDR is not associated with fusion-related complications like pseudo-arthrosis or graft-related complications. The preservation of segmental motion might prevent or delay ASD process by reducing mechanical stress. Spine surgeons anxiously await the results of various clinical trials, which could solidify the still-theoretical benefits of CTDR. Till date, most of these trials suggest that results of CTDR are favorable compared with those of ACDF [101112131415161718]. However, complete clinical adoption of CTDR requires the settling of a number of controversial issues. In this chapter, we briefly discuss the general concept of CTDR while reviewing several critical and controversial issues which need to be settled.
Implants
Artificial discs were developed for the lumbar spine; many efforts were made to develop clinically useful artificial discs. SB Charité III, which has been revised twice since 1987, is first popular implant. Since then, various other implants have been introduced and are currently in clinical use [19202122].
With the success of the lumbar prosthetic device, a new passion for development of a cervical arthroplasty device has emerged. In 1989, the department of medical engineering at Frenchay Hospital in Bristol, England initiated a project on artificial cervical joints [23]. The final product of this project was the Prestige disc comprising a two-piece steel plate that used a ball-in-socket configuration (Fig. 1). The lower plate was redesigned as a shallow ellipsoid saucer to increase the range of translation and rotation. The results of the 2-year pilot study with the Prestige disc in 15 patients revealed preserved segmental motion in 14 patients, and no device settling or migration [24]. Although there were no screw pull-outs, two incidents of screw breakage were noted.
A metal-on-plastic design called the Bryan disc was introduced in the late 1990s. The Bryan disc is a single-piece unconstrained device with a completely variable instantaneous axis of rotation, comprising a polyurethane core that articulates between two titanium alloy shells (Fig. 2). It provides physiological coupled translation-flexion-extension, and allows for shock absorption. Early research reports of the Bryan disc have demonstrated good results. In 2002, Goffin et al. [25] reported a study on a European prospective multicenter trial using the Bryan disc for treating single-level cervical disc disease. The clinical success rate was 86% in 60 patients at 6 months and 90% in 30 patients at 1 year, with motion preservation in all patients and no evidence of device migration. Although many subsequent studies have reported good results over time, long-term follow-up results have exposed various problems. Postoperative segmental kyphosis is frequently reported, and is caused by segmental malalignment of the functional spinal unit and prosthetic shell angle of the Bryan prosthesis [26272829]. Prosthesis loosening, migration, and subsidence of the implant have been problematic [3031].
In 2002, the ProDisc-C implant was developed based on the same design principles as that of the ProDisc-L implant for the lumbar spine. The ProDisc-C is a metal polyethylene (PE) ball-in-socket design with two metal fins (Fig. 3). The unit comprises two cobalt-chrome endplates with an intervening polyurethane inlay. This semiconstrained device does not allow sagittal translation in order to prevent excessive motion. After a multicenter randomized prospective clinical trial, it was approved by the Food and Drug Administration (FDA) for use in the United States in December 2007 [32].
When cervical artificial discs were introduced in early 2000s, it was not easy for surgeons to assemble the devices and/or to insert the unforgiving prostheses. Nowadays, numerous cervical artificial discs have been introduced in the market, and CTDR has been popular worldwide for the surgical treatment of degenerative cervical spine diseases. Unlike early types of artificial discs, these new prostheses featured designs that were simple and easy to manage. Additionally, the devices themselves were more forgiving during surgical procedures, similar to cervical interbody cages. These changes in prosthesis design and procedure were driven by the continuous demand by surgeons coupled with industrial efforts to improve the implants.
Surgical Techniques
The surgical technique used to implant a cervical artificial disc is similar in approach and technique to conventional ACD, with or without fusion. The target vertebra is anteriorly accessed and the affected disc and all material compressing neural structures are removed. Osteophytes are removed to maximize the potential for normal range of motion (ROM) [33]. Whether the posterior longitudinal ligament (PLL) is divided or not remains controversial. One theory is that the removal of the PLL ensures complete decompression of the disc space and helps to restore intervertebral height [33]. Another theory is that preservation of the PLL, as long as it is undamaged, positively influences maintenance of ROM after CTDR [34].
Many biomechanical and clinical studies demonstrated that total uncinectomy (even unilateral), causes hypermobility of the segment and increases facet loads [35]. Thus, the hypertrophied uncinate processes should be removed. Once the uncinate processes are cleaned out bilaterally, the midline of the disc space can be determined. The center of the disc is defined as the midline of line between both sides of uncinate processes on the axial view, and between the anterior and posterior marginal line on the sagittal axis. This allows for accurate placement of the artificial disc in the center of the disc space. The endplates are prepared by removing the cartilaginous endplate and repositioning the surfaces until they are parallel to ensure even insertion of the prosthesis. Although the implantation techniques differ according to each artificial disc type, the basic principles remain similar. The size of the implant should be selected so that the endplate is able to completely fill up the empty space. A wider implant can prevent postoperative subsidence of the implant. The height of implant should be similar to normal disc height. The over-distraction of disc space and too tight positioning increases the load on facet joints and can cause postoperative neck pain or limited motion within the artificial disc. On the other hand, in case with too loose disc space or too low-height implant, foraminal stenosis and poor device function occurs. Final placement of the device is then confirmed using a fluoroscope.
North America Spine Society has proposed the guidelines for CTDR [36]. The indications and contraindications are listed in Table 1.
Controversial Issues
1. Surgical outcomes
There have been many clinical studies dealing with CTDR for treating degenerative cervical spine diseases. The summarized results of prospective clinical studies are listed in Table 2 [2527373839404142]. Mummaneni et al. [37] presented the clinical results of a prospective, randomized comparison of ACDF versus CTDR, using the Prestige ST, with 3–5 years of follow-up. The study cohort included 347 patients who reached 3 years of follow-up and 111 who reached 5 years of follow-up. The Neck Disability Index (NDI) and visual analogue scale (VAS) scores were significantly better in the total disc replacement (TDR) group at 3 years that those of ACDF group (p=0.015 and p=0.044, respectively), but were similar at 5 years (p=0.214 and p=0.895, respectively). There was no statistical difference between the groups for the 36-item short form (SF-36) physical component summary (PCS), SF-36 mental component summary, or VAS arm pain scores at 3 or 5 years. Latest follow-up evaluation revealed that the Prestige devices maintained a mean of 7.1 degrees of motion on flexion and extension X-rays. There were 7 TDR group removed versus 12 ACDFs removed. The TDR group maintained segmental motion up to 5 years after implantation.
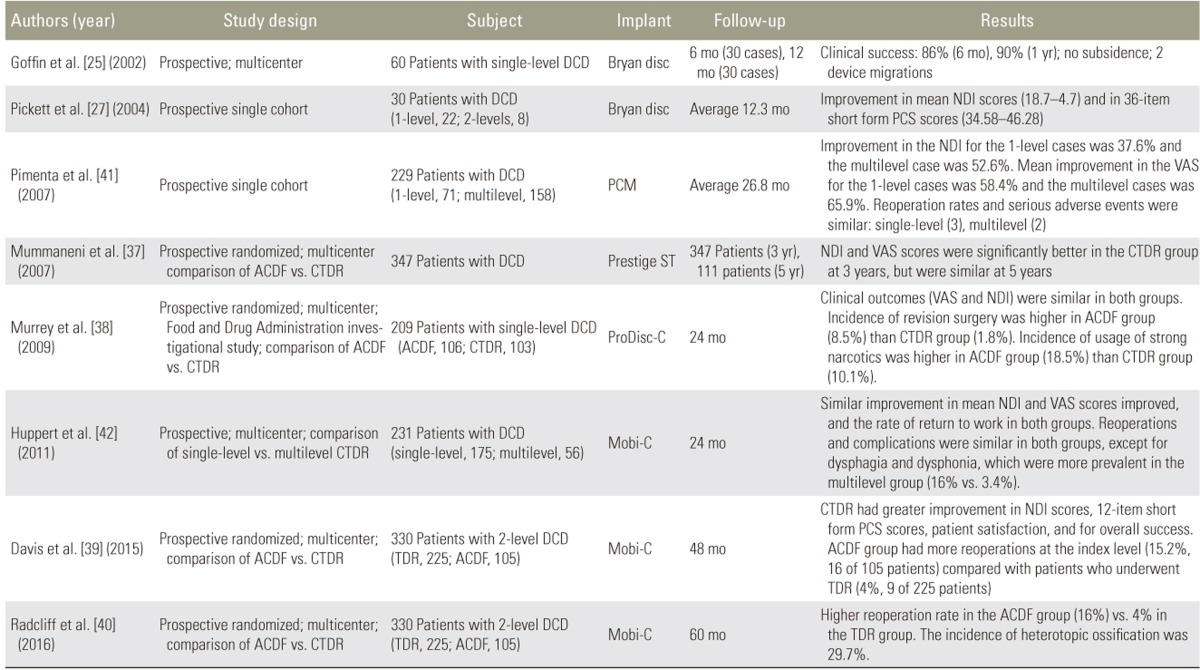
Summary of prospective clinical studies discussing surgical outcomes of cervical total disc replacement surgeries
Murrey et al. [38] reported the multicenter FDA investigational device exemption (IDE) study on the ProDisc-C TDR versus anterior discectomy and fusion for the treatment of one-level symptomatic cervical disc disease. A total of 209 patients were enrolled in the study (ACDF, 106; TDR, 103). According to their results, both the groups experienced similar positive clinical outcomes; however, there was a statistically significant difference in the number of secondary surgeries with 8.5% of fusion patients requiring reoperation, revision, or supplemental fixation within the 24-month postoperative period, compared with 1.8% of TDR patients (p=0.033). At 24 months, there was a statistically significant difference in the use of medication with 89.9% of TDR patients requiring no strong narcotics or muscle relaxants, compared with 81.5% of fusion patients. Thus, they concluded that TDR using the ProDisc-C is a safe and effective surgical treatment for patients with disabling cervical radiculopathy because of single-level disease. After evaluating primary and secondary measures, it was clear that clinical outcomes obtained after CTDR with ProDisc-C were either equivalent or superior to those obtained after fusion surgery.
Davis et al. [39] reported the results of a prospective randomized study evaluating the safety and efficacy of two-level TDR using a Mobi-C artificial disc compared with that of ACDF after a minimum 48 months of follow-up (TDR, 225 patients; ACDF, 105 patients). After 48 months, the follow-up rate was 89% in the TDR group and 81.2% in the ACDF group. They reported that both groups demonstrated significant clinical improvement, but the patients treated with CTDR showed greater improvement in NDI scores, 12-item short form (SF-12) PCS scores, patient satisfaction, and measures of overall success. The patients in the ACDF group underwent more reoperations at the index level (15.2%; 16 of 105 patients, with a total of 18 reoperations) compared with patients who underwent TDR (4%, 9 of 225 patients) (p<0.001). Fusion failure was the most common cause of reoperations in the ACDF group.
Radcliff et al. [40] reported unique prospective randomized clinical study with 60 months follow-up in the 225 patients who received TDR using Mobi-C versus the 105 patients who underwent ACDF. Three independent authors critically reviewed the data and the methodology. They noted that patients who underwent TDR exhibited more improvement compared with those who underwent ACDF when considering the NDI, SF-12 PCS, and overall satisfaction. These authors also reported a higher reoperation rate in the ACDF group (16%) than in the TDR group (4%) (p=0.003), without differences in the incidence of adverse events. ROM was preserved in the group with arthroplasty, despite a 29.7% rate of heterotopic ossification (HO).
Recently, Mehren et al. [43] presented the results of a nonrandomized prospective study which determined clinical and radiological outcomes of 50 patients at 10 years of follow-up after CTDR using the ProDisc-C. They observed that significant clinical improvements were maintained at last follow-up (VAS arm, 6.3–2.1; VAS neck, 6.4–1.9; NDI, 2.1–6). The incidence and the extent of HO were found to be increased with a significant influence on the prosthesis mobility; however, there was no relationship with clinical symptoms. Segmental motion of the index level declined from 9.0° preoperatively and 9.1° at 1 year to 7.7° and 7.6° at the 5- and 10-year examinations, respectively. Radiological signs of ASD were detected in 13/38 (35.7%), and 3/38 (7.9%) patients showing these radiological changes exhibited clinical symptoms requiring conservative treatment. In two cases, intraoperative technical failure required interbody fusion with a cage (2/50). One patient (1/48, 2.1%) required revision surgery at the index level.
1) Authors' series
The authors performed a comparison study between the Bryan disc and the ProDisc-C [44]. After final follow-up visits, mean VAS and NDI scores were found to be significantly lower than their preoperative values; mean VAS score reduced from 7.2±2.1 to 1.5±1.6, and mean NDI score reduced from 41.8%±21.6% to 9.6%±13.2%. The authors' other study focused on degenerative changes of the facet joints and the incidence of HO after CTDR [45]. At the index level, progression of facet arthrosis (PFA) was observed in 7 of 36 levels (1 level with the Bryan disc, 6 with the ProDisc-C). At adjacent levels, PFA was minimally observed. The HO was observed at 19 levels (11 with the Bryan disc, 8 with ProDisc-C). PFA at the index segments was positively related to prosthesis malposition on the frontal plane, and decreased postoperative functional spinal unit ROM at the index level. Occurrence of the HO was correlated with preoperative calcification of the PLL at the operative level, regardless of prosthesis type. Clinical outcomes and the occurrence of PFA or HO were unrelated. According to the authors' experiences, CTDR is a safe and effective treatment modality; however, various undesirable postoperative changes occur in operated and adjacent segments including HO or PFA following CTDR using both unconstrained and semiconstrained devices. Although these changes were unrelated to clinical outcomes and recent studies have revealed relatively favorable results of CTDR, preventive measures should be taken to avoid these postoperative changes because they may lead to potentially threatening outcomes in a long-term follow-up.
2. Adjacent segment degeneration
ACDF is the most frequently used cervical spine surgical procedure. However, increases in the number of fusion surgeries as well as in the duration of follow-up have raised serious concerns about ASD. Since early 2000s, motion preservation technology has been used in clinical practice; ever since, ASD has become a popular issue of debate in the community of spine surgeons because this new technology was developed with the expectation of minimizing the effects on ASD. The clinical significance of this expectation is a popular topic of discussion in scientific meetings.
ASD is defined as a newly-developed degeneration at the operative level, or levels adjacent to the operative level in the spine. ASD has two subtypes: one is symptomatic ASD, accompanied with relevant clinical findings such as myelopathy, radiculopathy, or instability; the other is radiographic ASD, representing the radiographic changes and without relevant symptoms. For the symptomatic ASD, the rates of incidence reported by Hilibrand and Robbins [9] are frequently cited and generally accepted. They indicate that approximately 3% cases per year were asymptomatic and 25.6% were asymptomatic at 10 years after ACDF. Recently Xia et al. [46] reported that the occurrence of radiographic ASD was 32.8% and that of symptomatic ASD was 6.3% in their systematic review and meta-analysis of 94 published studies involving 34,716 patients with follow-ups that ranged from fewer than half a year to more than 20 years. According to these clinical observations, the discrepancy in incidence between radiographic and symptomatic ASDs is an indisputable fact. However, it is still unknown if radiographic degeneration can be a precursor for symptomatic spondylosis [47]. The etiology of ASD is multifactorial and depends on (1) the natural history of the adjacent disc, (2) biomechanical stress on the adjacent level caused by the fusion, and (3) anatomical disruption at the adjacent level during the initial surgery [484950]. The identity of the major factor contributing to the development of ASD remains undetermined, although biomechanical and clinical studies suggest that ACDF influences may accelerate the natural degenerative processes [85152].
TDR may reduce the incidence of ASD. However, the association of CTDR is still a subject of debate [853]. Recently, Nunley et al. [54] presented an annual incidence of symptomatic ASD of 3.1% after CTDR in 173 patients using data of four different prospective randomized trials with a median follow-up of 51 months. Kelly et al. [55] compared 100 CTDR and 99 ACDF cases from the radiographical IDE studies at the 2-year follow-up point and observed no statistical difference in ROM at adjacent levels between the two groups. Maldonado et al. [56] assessed radiological ASD in CTDR and ACDF groups after 36 months follow-up; 8.8% patients presented with ASD after CTDR; 10.5% patients presented with ASD after ACDF. This difference was not statistically significant. Meanwhile, Coric et al. [57] published the results of the IDE trial, where improved radiological outcomes were observed in patients with CTDR versus those with ACDF. Another report of the IDE trial studying the superior outcomes and inferior occurrence rate of radiographic ASD in patients with CTDR to those in patients with ACDF at two contiguous cervical degenerative lesions has been published [39]. Very recently, Zhu et al. [58] reported the results of meta-analysis of 14 randomized controlled trials. Here they evaluated the reported rate of symptomatic ASD in patients with CTDR compared with that in patients with ACDF. They found that CTDR was superior to ACDF, exhibits less ASD and requires few reoperations; they determined that CTDR was a better surgical procedure for reducing the incidence of ASD. In biomechanical models, CTDR was found to be better than ACDF in mimicking the native cervical ROM at adjacent levels [59606162]. No studies have reported evidence of the effects of ACDF on the occurrence of symptomatic ASD in the spine [63]. Nowadays, CTDR might be more recognized as an alternative treatment modality to ACDF than before, because many favorable results in preventive effects of CTDR have been published as mentioned above. However, Helgeson et al. [8] argued in their review article on ASD that, although recent studies provide a high level of evidence, conclusions about the effects of motion preservation on ASD cannot be drawn because of the necessity for long-term follow-up.
3. Heterotopic ossification
Many investigators have reported the occurrence of HO in the follow-up studies of the patients who underwent CTDR (Fig. 4). HO is a well-known and unexplained phenomenon after large joint replacement surgery and has become an unexplained phenomenon following CTDR. HO appears to have a wide range of incidence. In the authors' series, a high incidence rate of 53% was observed during a 25-month follow-up cohort study of CTDR [45]. Such high incidence rates of HO can also be found in both the short- and long-term follow-up periods of other studies; 40.6% incidence rate in 170 patients in an average of 19.9 months of follow-up [64] and 37% incidence rate in 24 consecutive patients in an average of 7.7 years of follow-up [65]. In the meantime, some reports revealed relatively lower incidence rate; 2.9% in 2-year of follow-up [38] and 5.8% of 103 patients in 5-year of follow-up [66].

Sagittal computed tomography scan shows heterotopic ossification at the same level after 26 months of arthroplasty using a Bryan disc placement. The abnormal ossification behind the implant indicates it is McAfee Class II (arrow).
Recently, several articles dealing with pathogenesis of HO have emerged. The authors have reported that HO could be caused by iatrogenic factors as a normal defense mechanism against the non-physiological motion of artificial discs and biomechanical stress related to motion of artificial discs. HO may also result from constitutional factors such as preoperative calcification, osteophytes, or ossification of the surrounding structures [67]. Recently published articles studying the mechanism of HO formation following CTDR tended to focus on the postoperative biomechanical influence of the artificial disc on HO. Kim and Heo [68] presented postoperative biomechanical changes, such as overcorrection of disc space height by use of a CTDR level that is too high, could influence the severity of HO. Tian et al. [69] also reported that HO did not occur in the soft tissue (like typical HO) but rather occurred in the bony vertebral body (96.2% of HO). Based on this result, they insisted that the ossification associated with CTDR should be called ‘paravertebral ossification’ rather than HO. HO might result from postoperative development of preoperatively existing osteophytes and improper motion of the artificial disc. Jin et al. [70] proposed a new classification for HO, in which HO was divided into three types (endplate, tear-drop, and traction spur types) according to the morphologic features of HO formed by relevant biomechanical influence. These three articles appear to have observed HO from different points of view, but with a common viewpoint, specifically, the importance of postoperative biomechanical factors in the formation of HO after CTDR. Considering these biomechanical factors, we can emphasize the importance of selecting a biomechanically-relevant motion device to avoid HO formation.
Nowadays, there exist various suggested countermeasures for avoiding HO formation. These include selecting proper candidates, gentle handling of soft tissues, strict hemostasis (particularly, using bone wax during surgery), avoiding too much dissection of the longus coli, washing the operation field thoroughly, and administering nonsteroidal anti-inflammatory drugs (NSAIDs). Considering biomechanical factors in the formation of HO, more physiological devices can be added as new countermeasures. Among these countermeasures, selecting proper candidates is important. Proper candidates are the patients who have preoperative soft cervical disc herniation only but no ossified PLL, bony spurs, collapsed disc space or instability. The prophylactic use of NSAIDs clearly decreased the incidence of postoperative HO in patients with hip arthroplasty [71]. However, whether HO in CTDR can be prevented by postoperative use of NSAIDs is yet to be determined. Tu et al. [72] evaluated the efficacy of NSAIDs in preventing HO following CTDR. They concluded that less HO formation was observed in patients who used postoperative NSAIDs following CTDR than those who did not, but this difference was not statistically significant. In the authors' series, even if we routinely gave postoperative NSAIDs to all registered cases until postoperative day 7, the occurrence rate of HO was still higher than those reported in other HO studies without NSAID usage [45]. Considering the issue of bleeding, and the cardiovascular or gastrointestinal complications of NSAIDs and low clinical significance of HO altogether, routine use of prophylactic NSAIDS for HO after CTDR cannot be justified [73]. Further studies are required to assess the role of NSAIDs in the development of HO following CTDR [74].
Although HO induced artificial discs to lose the most important function of motion preservation at the end, no difference was observed in clinical outcomes of the patients with HO compared with those without HO in 5-year follow-up in a US FDA IDE study with ProDisc-C [66]. Zhou et al. [75] conducted a meta-analysis of nine cohort studies with more than 2 years of follow-up and reported that the presence of HO was not associated with clinical outcomes after CTDR. If a patient with a well-fused index segment following ACDF has relatively good outcomes, loss of segmental motion by HO may not have a significant negative impact. HO-related effects on long-term outcomes have not been verified. Trials with even longer follow-up periods are required to understand the definite prognostic value of HO.
4. Wear debris and tissue reaction
Globally, there are numerous varieties of implants for CTDR, and there are various ways to classify these prostheses. Considering the materials used for the bearing surface, CTDRs in the market could be largely divided into two kinds: metal-on-metal (MoM) and metal-on-polyethylene (MoP).
Concerns about metallic wear follow use of MoM TDR. One example is Maverick, which was withdrawn from the US market. Some investigators have cited a threshold serum ion level for predicting complications with MoM prostheses, with below-threshold serum ion levels associated with increased safety [76]. However, the correlation (if any) between serum ion levels and clinical problems remains undetermined. Gornet et al. [77] reported elevated postoperative serum ion levels, even in patients with well-functioning MoM TDR in a prospective study of 24 patients. The authors insisted that no reliable threshold values were currently available for circulating serum metallic debris following arthroplasty surgery. Considering these observations, clinical implications of local tissue and systemic ionic concentrations remain uncertain. Therefore, even when serum ion levels are lower than the threshold, a patient with MoM TDR cannot be free from the potential risk of hypersensitivity and other biologic responses. Importantly, released ionic compounds can trigger an immune reaction anytime, potentially producing osteolysis and prosthesis failure [7879] as consequences of cell-mediated hypersensitivity, which has been well documented for hip and knee arthroplasty.
Until recently, PE wear was not a clinically relevant issue for spine TDR as there is no synovial joint in the intervertebral disc and the limitation of motion between the lower lumbar segments. These days, it is well known that PE particles may induce osteolysis and aseptic loosening. Punt et al. [80] reported that they could observe PE and chronic inflammatory reactions in periprosthetic tissue collected during reoperation in 15 of 16 patients with MoP. Among the 15 who underwent reoperation, two patients had received prosthesis only 3 years prior, and the investigators observed PE and macrophages in their collected peri-prosthetic tissues. Kurtz et al. [81] reported the results of quantitatively analyzing long-term mechanisms of PE damage in contemporary TDRs stating that increasing wear with implantation time might be accompanied with a potential risk for osteolysis in the spine during long-term follow-up. Recently, the use of highly cross-linked PE has reduced the wear rate. This evolution came from an increased understanding of PE over the last 20 years [82]. MoP using cobalt-chromium-molybdenum and modern ultrahigh molecular weight polyethylene (UHMWPE) is regarded as a reference standard based on its extensive clinical use as a bearing surface. However, development of new materials engineering may not fully protect TDR from wear-related issues. A debris-induced tissue reaction that caused peri-prosthetic osteolysis in a patient with UHMWPE TDR has been reported [83].
Most of the patients who receive TDR are relatively young and have active life styles. Consequently, wear and local tissue responses may emerge as major issues in these patients at long-term follow-up [84]. Small number of wear-related complications have been reported which represent an overall low rate of wear-related complications. Nevertheless, we cannot know if this overall low rate of wear-related issues will continue as time passes. Prosthesis wear is usually accompanied with a poor biomechanical status such as subsidence, migration, and undersizing. Here, wear could adversely affect outcomes. In such patients, unknown complications can occur which spine surgeons have never experienced in fusion surgery. Regular long-term follow-up and spine surgeons' awareness of these potential complications are strongly warranted.
5. Multilevel TDR and hybrid surgery in multilevel cervical disc diseases
In multilevel cervical disc diseases, ACDF is the most widely accepted surgical procedure with a satisfactory pain and functional outcome and significantly high radiological fusion. However, clinical outcomes and radiological fusion rates deteriorate as the number of involved disc levels increases because longer fusions may cause greater stresses at adjacent levels than a single-level fusion [8586]. Unlike ACDF, TDR has benefits such as preservation of both spinal mobility and stability [4187], reduction of symptomatic ASD, and avoidance of fusion-related complications. Therefore, TDR appears to be a more effective procedure than ACDF for multilevel surgery if both have the same effects on neurological decompression. Because 2-level TDR was recently approved in the United States, little data is available for comparison between 2-level TDR and 2-level ACDF.
A recent post-hoc comparison clinical study with a 4-year follow-up between 1-level Mobi-C cervical artificial disc and 2-level TDR was published [88]. The patients, 179 with 1-level and 234 with 2-level, were concurrently enrolled in a multicenter US FDA IDE clinical trial. The authors found no difference between 1-level and 2-level TDR in all outcome measures, overall complications, and subsequent surgery rates. The authors concluded that their clinical trial demonstrated that 2-level TDR is as safe and effective as 1-level TDR for a few patients. Approximately 1 year before the publishing of this report, Davis et al. [39] reported their 4-year follow-up results on the same cohort of patients who underwent 2-level TDR as shown in the previous report, but with a smaller number of TDR patients. This comparison study between TDR and ACDF was conducted in 225 patients with TDR and 105 patients with ACDF at 24 centers in the United States. Patients with TDR, especially those undergoing treatment of two contiguous cervical levels, demonstrated significantly greater improvement than patients with ACDF for most of the outcome measures, including overall success compared to baseline. ACDF patients exhibited a higher rate of radiographic ASD. Previously, this group had reported 2-year follow-up results from the same patient cohort. They found that 2-level TDR produced statistically better outcomes for both pain and function [89]. Continuation of long-term follow-ups of this patient cohort should establish 2-level TDR as a superior alternative to ACDF. Very recently, Joaquim and Riew [90] performed a systematic review of the clinical studies evaluating patients who underwent multilevel CTDR (2 or more levels). In their systematic review of 14 clinical studies, most of the literature supports use of CTDR in multilevel cervical disc degeneration. The authors proposed further prospective, controlled, multicenter, and randomized studies to elucidate the superiority of CTDR over ACDF for treating cervical disc degeneration in selected cases.
As mentioned above, in surgical treatments of multilevel cervical degenerative diseases, multilevel fusion has a greater chance of causing ASD than single-level fusion. Hybrid surgery (HS), where ACDF and TDR are incorporated at different levels, can be a beneficial surgical treatment for multilevel cervical degenerative lesions to avoid the harmful effects of long-level fusions such as exacerbating ASD by combining the advantages of both ACDF and TDR techniques. However, not all the levels with cervical disease may be acceptable for TDR. In multilevel cervical degenerative diseases, there are often lesions that are acceptable and unacceptable for TDR. Surgeons can combine these techniques, using TDR at levels where it is effective and ACDF at levels that favor bony fusion. The major advantage of HS is that the technique allows for maintaining motion in multilevel diseases, which were previously fused and immobilized in long segments. HS should be performed in carefully selected patients and its feasibility as an alternative to TDR and ACDF for managing multilevel cervical degenerative disc diseases requires further elucidation.
Hey et al. [91] reported the results of a direct comparison study of three groups (ACDF, TDR, and HS) in multilevel cervical degenerative diseases with a minimum 2-year follow-up and concluded that HS could be a feasible and safe alternative to multilevel TDR or multilevel ACDF. In many aspects, the results of HS were a balance between TDR and ACDF. Alvin and Mroz [92] reported similar results in a comparison study of TDR and ACDF in 2-level cervical degenerative disc diseases in which they concluded that TDR group may be superior to ACDF group. Lee e al. [93] reported the results of 2- to 3-level HS in multilevel cervical spondylosis with 2-year follow-up, and demonstrated that HS is a safe and effective alternative to ACDF.
When compiling all data to date, multilevel cervical TDR and HS appear to be safe and effective alternatives to ACDF in selected patients. Considering the various pathologies concurrently observed in a single patient with degenerative cervical disease, TDR should be applied only in selected levels of multilevel-lesions. TDR should not be used in discs with ossification of posterior longitudinal ligament, discs with advanced degenerative disease, facetopathy, stenosis, or instability. Thus, a surgeon may have more chances to choose HS in patients with multilevel degenerative cervical disease. Multilevel TDR and HS in the cervical spine are associated with favorable outcome measures, ASD, complications, and second surgery rates. However, these surgical treatment modalities still require additional clinical data and long-term follow-ups.
Conclusion
CTDR is a new and innovative technology with limited indications for clinical application in spinal surgery. Most studies dealing with CTDR and its outcomes have reported favorable results in various outcome measures. However, some controversial issues such as outcomes, ASD, HO, wear debris, multilevel TDR, and HS remain unresolved. Based on the study results reported in the literature so far, favorable evidence related to outcomes, ASD, multilevel TDR, and HS exist. However, these issues still remain debatable. It will take considerable time to provide proper answers for these issues. We need to wait and examine results obtained from long-term follow-ups prior to forming conclusions about these controversial issues and the clinical relevance of CTDR.
Acknowledgments
The author is thankful to Miss So Ha Park for her sincere editorial assistance.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.



