|
 |
- Search
| Asian Spine J > Volume 11(5); 2017 > Article |
|
Abstract
Spondylolysis from pars fracture is a common injury among young athletes, which can limit activity and cause chronic back pain. While current literature has examined the relative benefits of surgical and conservative management of these injuries, no study has yet compared outcomes between conventional direct repair of pars defects and modern minimally invasive procedures. The goals of surgery are pain resolution, return to play at previous levels of activity, and a shorter course of recovery. In this review, the authors have attempted to quantify any differences in outcome between patients treated with conventional or minimally invasive techniques. A literature search was performed of the PubMed database for relevant articles, excluding articles describing conservative management, traumatic injury, or high-grade spondylolisthesis. Articles included for review involved young athletes treated for symptomatic spondylolysis with either conventional or minimally invasive surgery. Two independent reviewers conducted the literature search and judged articles for inclusion. All studies were classified according to the North American Spine Society standards. Of the 116 results of our initial search, 16 articles were included with a total of 150 patients. Due to a paucity of operative details in older studies and inconsistencies in both clinical methods and reporting among most articles, little quantitative analysis was possible. However, patients in the minimally invasive group did have significantly higher rates of pain resolution (p<0.001). Short recovery times were also noted in this group. Both groups experienced low complication rates, and the majority of patients returned to previous levels of activity. Surgical repair of spondylolysis in young athletes is a safe and practical therapy. Current literature suggests that while conventional repair remains effective, minimally invasive procedures better clinical outcomes. We await further data to conduct a more thorough quantitative analysis of these techniques.
SpondylolysisŌĆöa fracture of the pars interarticularisŌĆöis a common injury in young patients, and is often associated with spondylolisthesis. Typically, the rostral vertebral body gradually translates over the inferior body with minimal resistance from the posterior column. In younger patients, especially athletes, it is seen at the L5 level and is typically bilateral [1].
Historically, the incidence of spondylolysis has been reported as 6% in the general population [2], although newer studies utilizing computed tomography (CT) technology have reported higher rates, as much as 11.5% [3] In young athletes, pars injuries occur with a much greater incidence, reported at anywhere from 23%ŌĆō63% [45]. Athletes at greatest risk engage in high-impact sports or activities that involve repetitive hyperextension, flexion, and rotation. There is some evidence for a hereditary predisposition to spondylolysis, such as a tendency for thin, more readily fractured vertebral bone, or an isolated weakness or thinning of the pars itself [67]. These fractures are also observed twice as frequently in men compared to women [258].
Surgical repair of symptomatic spondylolysis is classically indicated after 6 months of failed conservative management, with persistent pain and non-union at 9ŌĆō12 months [910]. Surgical decompression and fusion may also be indicated in symptomatic cases with neurologic deficit or radiculopathy.
While previous literature has compared the conservative and surgical management of spondylolysis [11], no study to date has analyzed differences in conventional direct repair (CDR) and minimally invasive surgery (MIS) techniques. This study will seek to compare outcomes between these approaches; in particular, the impact of MIS repair on return to play for athletes otherwise limited in their activity due to symptomatic fractures.
With strict adherence to preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, a literature search was conducted of the PubMed database for articles relating to surgical repair of fractures to the pars interarticularis in athletes. Keywords included ŌĆ£pars interarticularis fractureŌĆØ or ŌĆ£pars repairŌĆØ or ŌĆ£spondylolysis repair,ŌĆØ alone and in combination with phrases such as ŌĆ£athletes,ŌĆØ ŌĆ£minimally invasiveŌĆØ or ŌĆ£MIS,ŌĆØ and ŌĆ£direct repair.ŌĆØ
Results were limited to English language literature and human subjects. Case reports and case series detailing surgical intervention for symptomatic spondylosis in athletes were included. Studies describing conservative management, treatment of injuries due to trauma, and patients with high-grade spondylolisthesis (greater than Meyerding Grade I) were excluded. Two reviewers performed the database search and reviewed each article for inclusion/exclusion. The quality of evidence for each article was determined according to the standards of the North American Spine Society [12]. The last search was performed in April 2016.
Studies were first separated into MIS or CDR technique groups. Each study was analyzed for patient population, methods, outcomes, and complications. Various clinical metrics employed by different studies and other criteria were compared to assess differences in outcomes between MIS or CDR interventions.
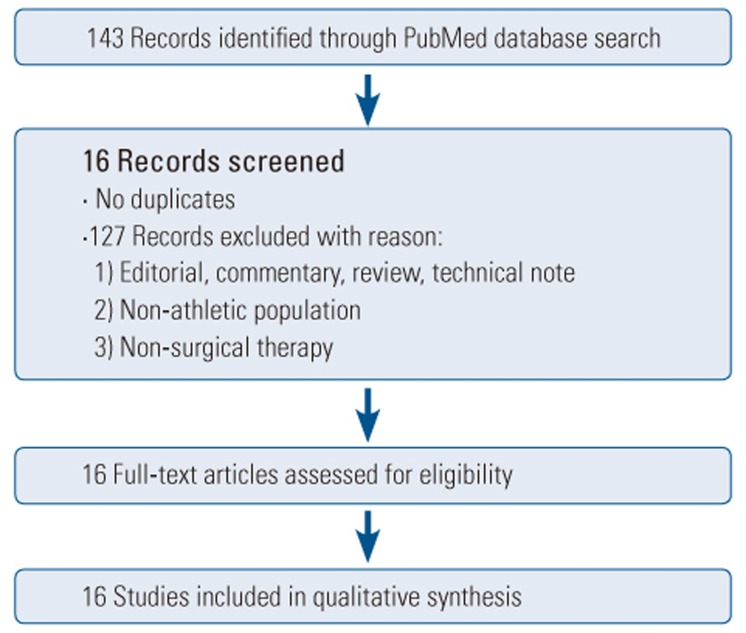
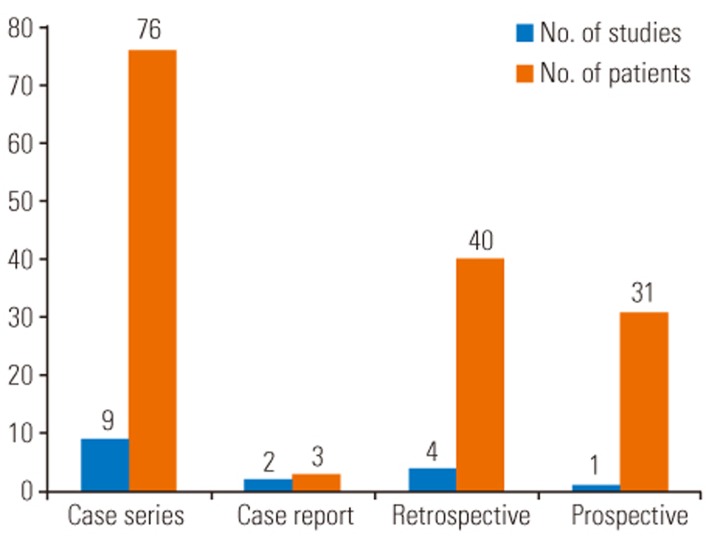
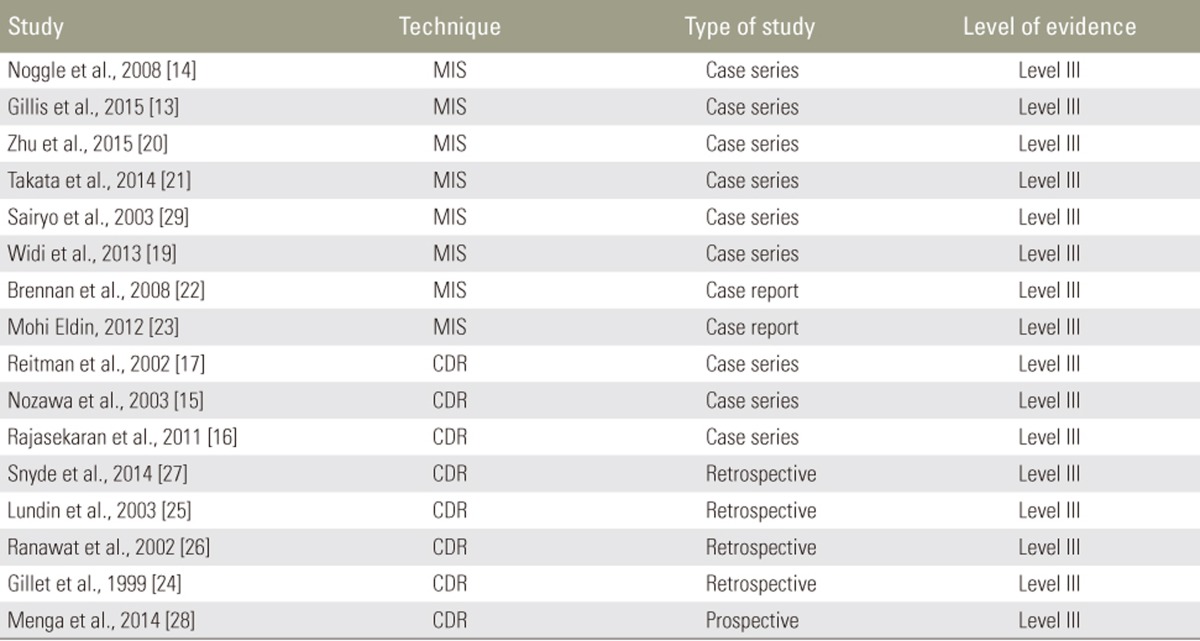
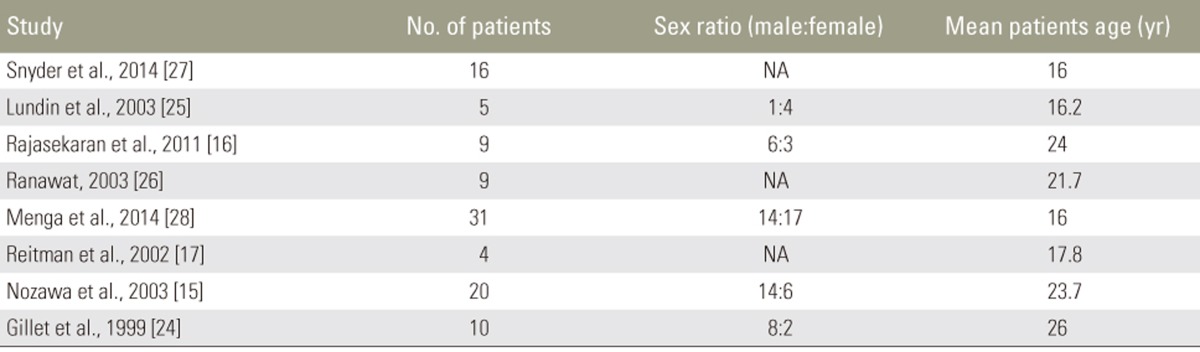
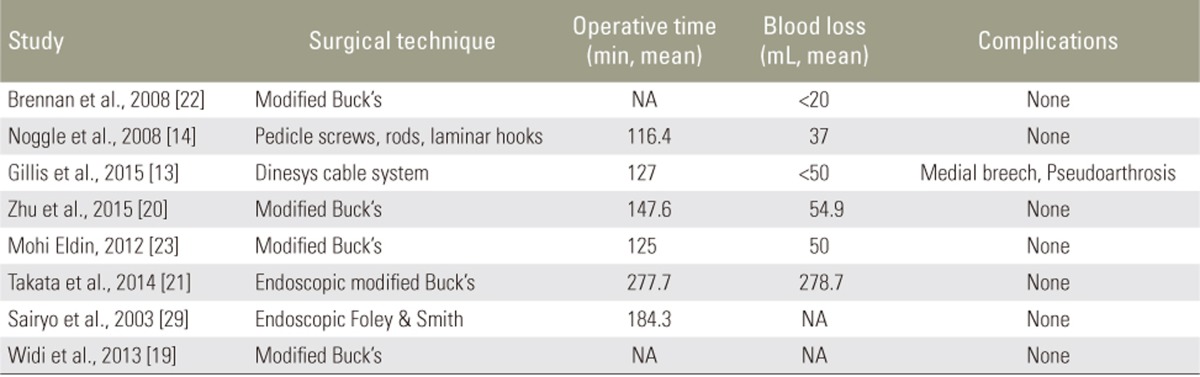
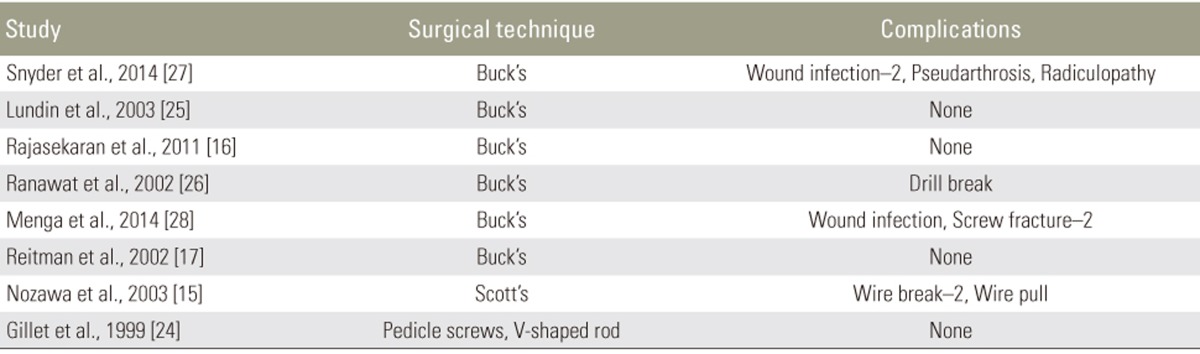
Our initial PubMed search yielded 143 results. After screening, 16 articles were included in this study, comprising 9 case series [131415161718192021], 2 case reports [2223], 4 retrospective studies [24252627], and 1 prospective study [28], with a total of 150 patients (Tables 1, 2). 8 of these articles describe MIS therapy in 46 patients [1314181920212223]. The remaining 8 articles describe CDR techniques in 104 patients [1516172425262728] (Figs. 1, 2).
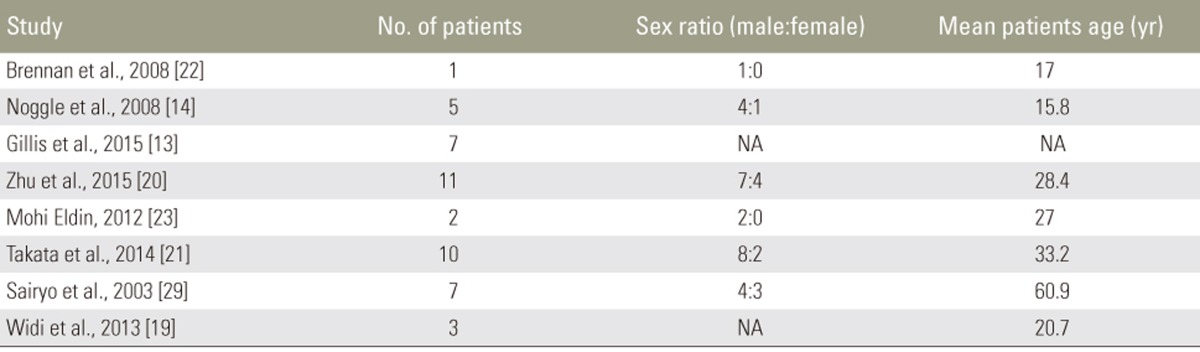
Of the 46 patients treated with MIS techniques, 72% were male (of 36 reported) and the average age was 32.9 years. Athletes in this group participated in professional and college football, professional hockey, and high school sports including track and volleyball (Table 3).
Of the 104 patients treated with CDR techniques, 57% were male (of 75 reported) and the average age was 23.1 years. Athletes in this group participated in college gymnastics, baseball, and soccer, professional cricket, and amateur running, swimming, and golf. This group also included manual laborers, such as butchers, bricklayers, and fishmongers (Table 4).
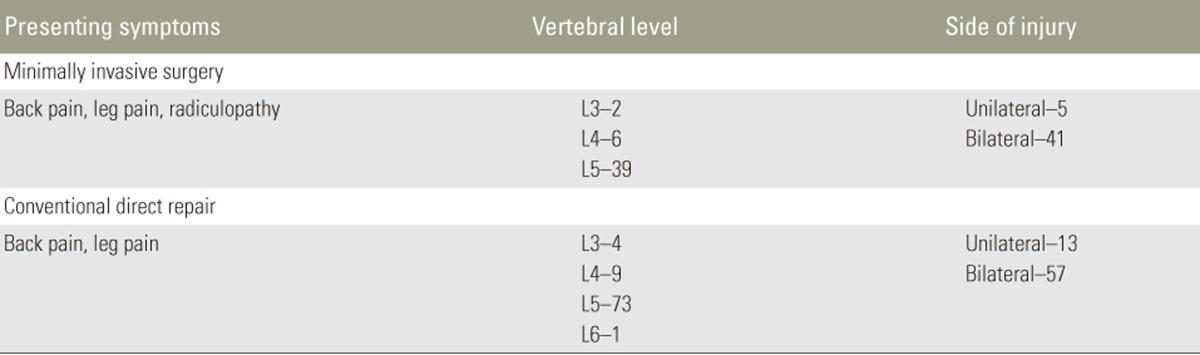
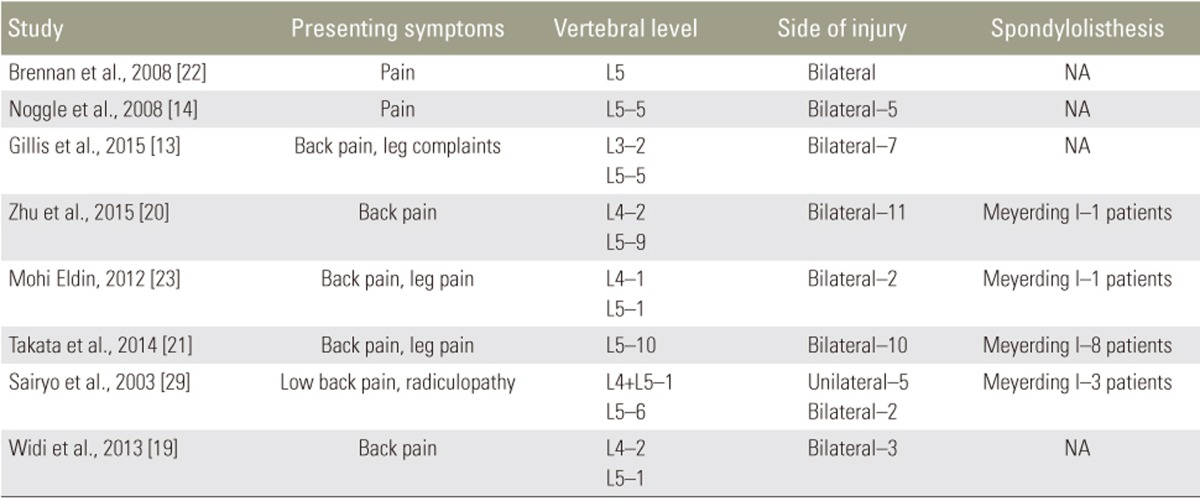
The majority of patients presented with back pain. Other symptoms included leg pain and radiculopathy. Most patients had no spondylolisthesis, and those who did were all Meyerding Grade I (n=13, 28%). Vertebral level was reported in 47 injuries. While the majority of fractures were at the L5 level (n=39, 83%), injuries were also treated at L4 (n=6, 13%) and L3 (n=2, 9%). Most injuries were bilateral (n=41, 89%) (Tables 5, 6).
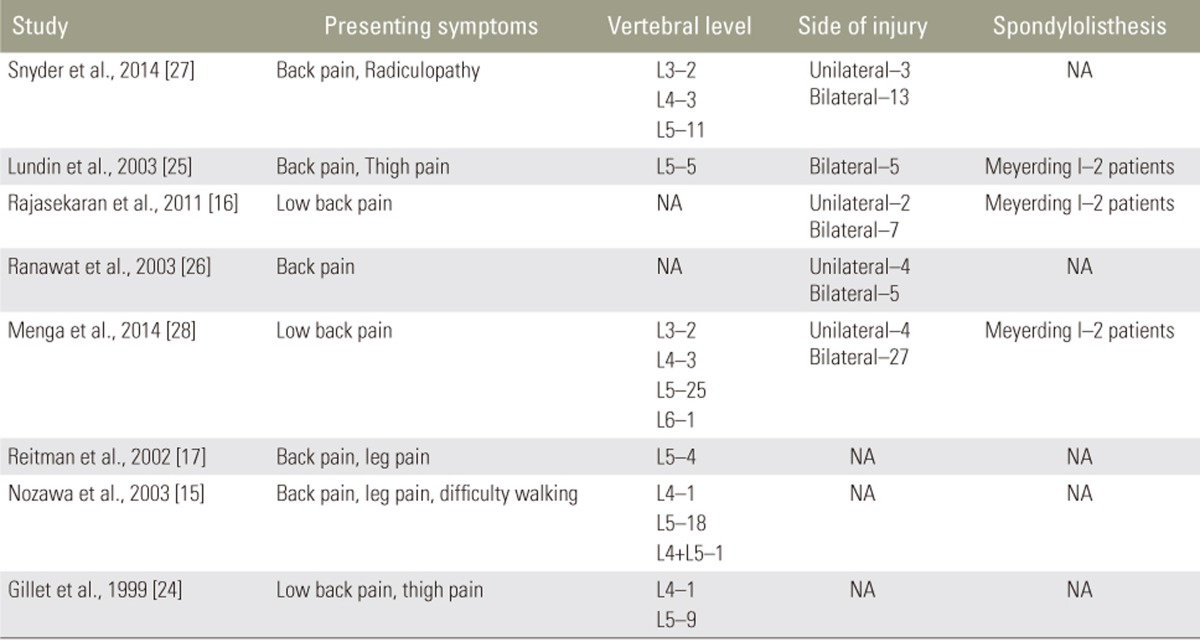
Most patients presented with back pain. Other symptoms included leg or thigh pain, radiculopathy, and difficulty walking. The vast majority of patients had no spondylolisthesis, and those who did were all Meyerding Grade I (n=6, 5.8%). Vertebral level was reported in 83 injuries. While the majority of fractures were at the L5 level (n=69, 83%), injuries were also treated at L4 (n=9, 11%), L3 (n=4, 5%), and L6 (n=1, 1.2%). Most injuries were bilateral (n=52, 80%) (Table 7).
The majority of patients in both groups were treated by Buck's procedure or MIS/endoscopic modifications of Buck's procedure. In the CDR group, approximately 71% of patients were treated with Buck's procedure. Other techniques were also listed in Tables 8 and 9.
Of the 8 studies, most employed a MIS modification of Buck's procedure (n=5, 62.5%). In a typical percutaneous adaptation of Buck's technique, the patient is positioned prone, and the target pars defect is localized by fluoroscopy. A needle is introduced to the adjacent lamina, and a guide-wire is next drilled through the injured pars with X-ray guidance. The trajectory is sequentially dilated, and the defect tapped and drilled before introduction of the pars screw. Two studies employed endoscopic procedures, including modifications of Buck's procedure with pedicle screws and the Foley and Smith approach. The mean reported operative time was 176.5 minutes (range, 116.4ŌĆō277.7 minutes). The mean reported blood loss was 112.4 mL (range, 20ŌĆō278.7 mL). The mean reported hospital stay was 1.67 days (Table 8).
The most common technique was Buck's procedure (n=6, 75%). Only Rajasekaran et al. reported operative time, with a mean of 58 minutes (range, 45ŌĆō75 minutes). 2 studies reported blood loss: Lundin et al. reported a mean loss of 146 mL (ragne, 100ŌĆō200 mL), and Rajasekaran et al. reported a mean loss of 98 mL (range, 50ŌĆō140 mL). Only Lundin et al. reported postoperative hospital stay, with a mean length of 3.2 days (Table 9).
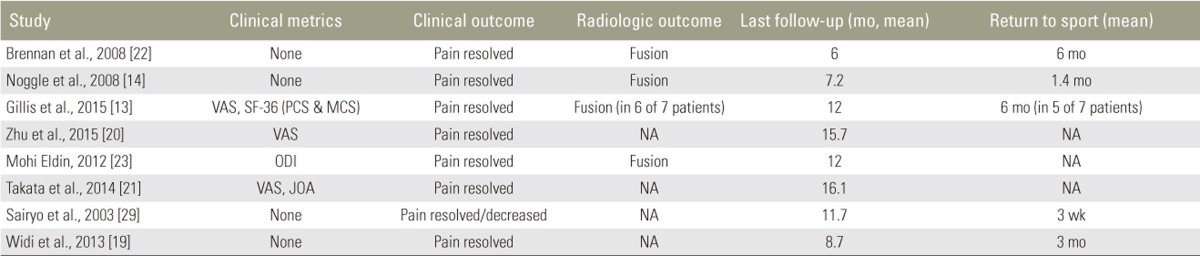
Three studies employed the visual analogue scale (VAS) to assess pre- and postoperative symptoms: Gillis et al. reported a reduction of 3 and 1.5 points for back and leg pain respectively, Zhu et al. reported a decrease from 7.1┬▒2.3 to 1.8┬▒0.4 (p=0.01), and Takata et al. reported a mean decrease from 7.6 to 2.5 for back pain and 3.4 to 2.0 for leg pain. Takata et al. also utilized the Japanese Orthopedic Association (JOA) functional score, reporting a mean increase from 15.8 to 26.4. Gillis et al. used the short form survey (SF)-36 physical and mental components (PCS and MSC, respectively) to assess patients' subjective outcome, reporting improvements of 12.2 and 2.9 for the PCS and MCS, respectively. Mohi Eldin reported Oswestry disability index (ODI) reductions in both patients, by 53% and 55%, respectively.
All patients had good clinical outcomes with resolution of pain. Our pooled analysis of patients' postoperative pain resolution found a significantly higher rate of complete resolution among patients treated with MIS techniques (p=0.001). Unfortunately, inconsistency in clinical metrics and reporting between studies precludes further quantitative outcomes analysis.
Five studies reported length of hospital stay, with a mean length of 1.67 days (range, 1ŌĆō3 days). All athletes returned to their previous level of activity with the exception of two patients treated by Gillis et al. One patient underwent surgery for fusion at the same level as his pars repair 4 years postoperatively, at which time a broken Dynesys cable was noted. Another patient developed pseudoarthrosis with a recurrence of preoperative symptoms 18 months after surgery, after which CT scan demonstrated incomplete fusion across the pars. The average time for successful return to sport among athletes was 2.7 months (range, 0.75ŌĆō6.0 months).
Four studies reported radiologic assessment of fusion. Fusion was achieved in all patients but one treated by Gillis et al. (described above). On average, patients were followed postoperatively for 12.7 months (range, 6ŌĆō23 months) (Table 10).
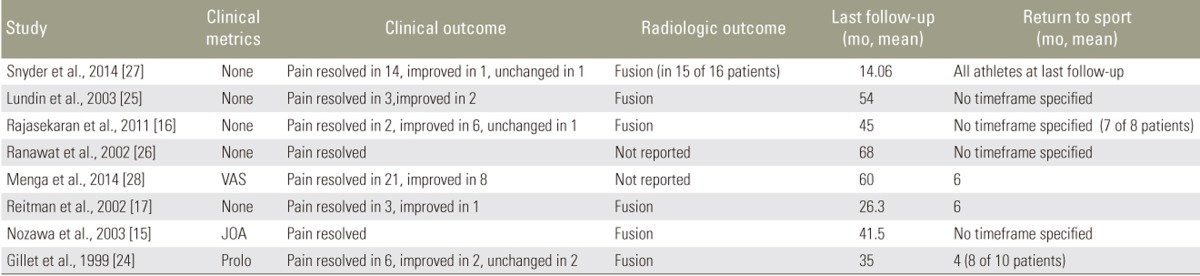
Menga et al. employed the VAS to assess pre- and postoperative symptoms, reporting a decrease from a mean score of 7 preoperatively (range, 1ŌĆō10) to 2 postoperative (range, 0ŌĆō10). The 25 athletes treated had a postoperative mean score of 1 (range, 0ŌĆō4). Nozawa et al. used the JOA functional score, reporting a mean preoperative score of 21.2┬▒3.9 (range, 13ŌĆō26) and a mean postoperative score of 27.7┬▒1.0 (range, 26ŌĆō29). Finally, Gillet et al. used the Prolo Economic Status score to assess postoperative outcomes. Postoperatively, patients had an average economic score of 4.0 (2ŌĆō5) and an average functional score of 4.0 (range, 2ŌĆō5), with a combined average of 8.0 (range, 4ŌĆō10). The majority of patients reported a resolution of pain (n=78, 75%). Of the remaining patients, over half had some improvement (n=20, 19%), while some had no postoperative change (n=4, 3.9%).
Only Lundin et al. reported postoperative length of hospital stay, with a mean stay of 3.2 days (range, 3ŌĆō4 days). All patients returned to their previous level of activity but three: Rajasekaran et al. reported one patient with persistent back pain in whom MRI suggested disc degeneration. Gillet et al. reported two patients whose poor surgical outcome prevented return to previous activity. Three studies reported the length of time before patients returned to sport, with a mean of 5.6 months (range, 4ŌĆō6 months).
Six studies reported postoperative radiographic outcomes. Of these, all patients had confirmed fusion except for one patient treated by Snyder et al. had two pars defects, one of which did not fuse successfully. On average, patients were followed postoperatively for 44.8 months (range, 9ŌĆō135 months) (Table 11).
Only two complications were reported, both in patients treated by Gillis et al. As described above, one patient developed pseudoarthrosis and experienced a recurrence of symptoms at 18 months postoperatively. Another patient experienced postoperative radiculopathy due to a medial breech, requiring screw revision (Table 8).
Eleven complications were reported. Snyder et al. reported two patients who developed postoperative superficial wound infections, which were treated with no effect on recovery. Two patients required revision surgery: one for pseudarthrosis and the other for radiculopathy, both of which developed postoperatively. Ranawat et al. reported one case of a perioperative drill break, which required removal during surgery. Menga et al. reported one patient with a postoperative superficial skin infection which was treated successfully without impact on recovery, and two patients with screw fractures: one was asymptomatic and found incidentally, the other was symptomatic (VAS=10). Finally, Nozawa et al. reported one patient in which a wire was displaced 2 weeks postoperatively and required repair with a pedicle screw, and two patients with asymptomatic postoperative wire breakage at 30 and 32 months respectively. As fusion had occurred by this point, neither patient required repair or revision (Table 9).
In 1970, Buck [30] described a method of spondylolysis correction by which screws are placed directly through the fractured pars and ipsilateral lamina. The goal of this procedure was to repair the pars defect so as to prevent the development of spondylolisthesis, the treatment of which had a relatively low success rate. His technique remains popular, and has formed the basis of CDR approaches to pars defects. Today, growing interest in MIS neurosurgery has led to the development of MIS modifications of Buck's procedure for the internal fixation of pars defects.
Direct repair is not, however, the only strategy available to treat these injuries. Transforaminal endoscopic microdiscectomy was performed in a 31-year-old patient with foraminal stenosis and symptomatic pain radiating to his legs. This patient had minimal spondylolisthesis and stable flexion/extension X-ray images [31]. Postoperatively, the patient experienced a complete resolution of his symptomatic pain. This techniqueŌĆöas with the MIS techniques reviewed hereŌĆömay be particularly applicable in patients who do not require fusion. Kakiuchi [32] has reported spondylolysis repair by fixation with pedicle screws and laminar hooks in 16 patients, 13 of whom had complete pain resolution, while the remaining 3 complain of only occasional postoperative back pain. These patients had an average age of 32 years (range, 12ŌĆō60 years).
Of the patients included in this review, those undergoing MIS repair experienced significantly greater resolution of symptomatic pain. While disparities in reporting and metrics between various studies precludes a more rigorous statistical analysis, the MIS patient population in this study is more than 10 years older on average than the CDR population. This may reflect the general benefits of MIS surgery: less surgical stress (e.g., tissue destruction, blood loss), which allows expansion of the candidate population to older patients. We therefore argue for the application of MIS techniques in pars repair, as this approach offers greater treatment of symptoms in a wider range of patients.
The ultimate goal of pars repairŌĆöbeyond resolution of painŌĆöis the return to play of an injured athlete at preoperative levels. Current literature suggests a postoperative delay of anywhere from 6ŌĆō12 months before a patient who has undergone pars repair can return to play [101133]. The studies included in this review did not consistently report return to play. Many of those that did failed to specify the timeframe involved. However, 3 MIS studies did report return to play times of 1.4 months [14], 3 months [19], and even 3 weeks [18]. These outcomes may suggest that the trauma of surgery itself delays an athlete's return to play after pars repair, and that MIS techniques therefore afford the patient a more rapid return to previous activity levels.
This review does face limitations. The small number of patients involved prevents broad generalization of our conclusions. As mentioned, the studies included were rarely consistent in clinical metrics used or reported, preventing a thorough quantitative statistical analysis. These studies were predominately case series, and therefore the patients involved were not randomized, but selected for their various treatments with bias. In CDR, there were many complications that may not related to magnitude of surgery but related to hardware failure. This could reflect an artifact of the literature, as our report includes approximately twice as many CDR patients as MIS. There may also be some characteristic of the techniques or hardware employed by CDR/MIS techniques influencing hardware failure, but due to the disparity of variables reported by these studies we cannot conduct meaningful analysis.
The direct repair of spondylolysis is an effective therapy for athletes in whom conservative management has failed. As new MIS techniques are developed and reported, a growing body of evidence suggests that these approaches may offer patients higher resolution of symptomatic pain and a swifter recovery with return to play when compared to traditional CDR techniques. More numerous and thorough data are required to conduct a quantitative analysis and draw firm conclusions.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
1. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol 2011 40:683ŌĆō700. PMID: 20440613.


2. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am 1984 66:699ŌĆō707. PMID: 6373773.


3. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine (Phila Pa 1976) 2009 34:199ŌĆō205. PMID: 19139672.



4. Cavalier R, Herman MJ, Cheung EV, Pizzutillo PD. Spondylolysis and spondylolisthesis in children and adolescents: I. Diagnosis, natural history, and nonsurgical management. J Am Acad Orthop Surg 2006 14:417ŌĆō424. PMID: 16822889.


5. Foreman P, Griessenauer CJ, Watanabe K, et al. L5 spondylolysis/spondylolisthesis: a comprehensive review with an anatomic focus. Childs Nerv Syst 2013 29:209ŌĆō216. PMID: 23089935.


6. Albanese M, Pizzutillo PD. Family study of spondylolysis and spondylolisthesis. J Pediatr Orthop 1982 2:496ŌĆō499. PMID: 6761366.


7. Wynne-Davies R, Scott JH. Inheritance and spondylolisthesis: a radiographic family survey. J Bone Joint Surg Br 1979 61:301ŌĆō305. PMID: 383720.


8. Belfi LM, Ortiz AO, Katz DS. Computed tomography evaluation of spondylolysis and spondylolisthesis in asymptomatic patients. Spine (Phila Pa 1976) 2006 31:E907ŌĆōE910. PMID: 17108819.


9. d'Hemecourt PA, Zurakowski D, Kriemler S, Micheli LJ. Spondylolysis: returning the athlete to sports participation with brace treatment. Orthopedics 2002 25:653ŌĆō657. PMID: 12083575.


10. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep 2009 8:35ŌĆō40. PMID: 19142078.


11. Bouras T, Korovessis P. Management of spondylolysis and low-grade spondylolisthesis in fine athletes: a comprehensive review. Eur J Orthop Surg Traumatol 2015 25(Suppl 1): S167ŌĆōS175. PMID: 25394940.


12. North American Spine Society. Levels of Evidence for Primary Research Question [Internet]. Washington, DC: North American Spine Society; cited 2017 Aug 20. Available from: https://www.spine.org/DocumentsResearchClinicalCare/LevelsOfEvidence.pdf
13. Gillis CC, Eichholz K, Thoman WJ, Fessler RG. A minimally invasive approach to defects of the pars interarticularis: restoring function in competitive athletes. Clin Neurol Neurosurg 2015 139:29ŌĆō34. PMID: 26363364.


14. Noggle JC, Sciubba DM, Samdani AF, Anderson DG, Betz RR, Asghar J. Minimally invasive direct repair of lumbar spondylolysis with a pedicle screw and hook construct. Neurosurg Focus 2008 25:E15.

15. Nozawa S, Shimizu K, Miyamoto K, Tanaka M. Repair of pars interarticularis defect by segmental wire fixation in young athletes with spondylolysis. Am J Sports Med 2003 31:359ŌĆō364. PMID: 12750127.


16. Rajasekaran S, Subbiah M, Shetty AP. Direct repair of lumbar spondylolysis by Buck's technique. Indian J Orthop 2011 45:136ŌĆō140. PMID: 21430868.



17. Reitman CA, Esses SI. Direct repair of spondylolytic defects in young competitive athletes. Spine J 2002 2:142ŌĆō144. PMID: 14588273.


18. Sairyo K, Sakai T, Yasui N. Minimally invasive technique for direct repair of pars interarticularis defects in adults using a percutaneous pedicle screw and hook-rod system. J Neurosurg Spine 2009 10:492ŌĆō495. PMID: 19442013.


19. Widi GA, Williams SK, Levi AD. Minimally invasive direct repair of bilateral lumbar spine pars defects in athletes. Case Rep Med 2013 2013:659078PMID: 23737800.



20. Zhu X, Wang J, Zhou Y, Zhang Z, Li C, Zheng W. Minimally invasive surgery for direct repair of lumbar spondylolysis by utilizing intraoperative navigation and microendoscopic techniques. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2015 29:1244ŌĆō1248. PMID: 26749732.

21. Takata Y, Sakai T, Tezuka F, Goda Y, Higashino K, Sairyo K. Clinical outcome of minimally invasive repair of pars defect using percutaneous pedicle screws and hook-rod system in adults with lumbar spondylolysis. Ann Orthop Rhematol 2014 2:1013.
22. Brennan RP, Smucker PY, Horn EM. Minimally invasive image-guided direct repair of bilateral L-5 pars interarticularis defects. Neurosurg Focus 2008 25:E13.

23. Mohi Eldin M. Minimal access direct spondylolysis repair using a pedicle screw-rod system: a case series. J Med Case Rep 2012 6:396PMID: 23176068.



24. Gillet P, Petit M. Direct repair of spondylolysis without spondylolisthesis, using a rod-screw construct and bone grafting of the pars defect. Spine (Phila Pa 1976) 1999 24:1252ŌĆō1256. PMID: 10382254.


25. Lundin DA, Wiseman D, Ellenbogen RG, Shaffrey CI. Direct repair of the pars interarticularis for spondylolysis and spondylolisthesis. Pediatr Neurosurg 2003 39:195ŌĆō200. PMID: 12944700.


26. Ranawat VS, Dowell JK, Heywood-Waddington MB. Stress fractures of the lumbar pars interarticularis in athletes: a review based on long-term results of 18 professional cricketers. Injury 2003 34:915ŌĆō919. PMID: 14636734.


27. Snyder LA, Shufflebarger H, O'Brien MF, Thind H, Theodore N, Kakarla UK. Spondylolysis outcomes in adolescents after direct screw repair of the pars interarticularis. J Neurosurg Spine 2014 21:329ŌĆō333. PMID: 24949906.


28. Menga EN, Kebaish KM, Jain A, Carrino JA, Spon-seller PD. Clinical results and functional outcomes after direct intralaminar screw repair of spondylolysis. Spine (Phila Pa 1976) 2014 39:104ŌĆō110. PMID: 24108299.


29. Sairyo K, Katoh S, Sakamaki T, Komatsubara S, Yasui N. A new endoscopic technique to decompress lumbar nerve roots affected by spondylolysis: technical note. J Neurosurg 2003 98(3 Suppl): 290ŌĆō293. PMID: 12691388.


30. Buck JE. Direct repair of the defect in spondylolisthesis. Preliminary report. J Bone Joint Surg Br 1970 52:432ŌĆō437. PMID: 4916960.

31. Madhavan K, Chieng LO, Hofstetter CP, Wang MY. Transforaminal endoscopic discectomy to relieve sciatica and delay fusion in a 31-year-old man with pars defects and low-grade spondylolisthesis. Neurosurg Focus 2016 40:E4.

32. Kakiuchi M. Repair of the defect in spondylolysis. Durable fixation with pedicle screws and laminar hooks. J Bone Joint Surg Am 1997 79:818ŌĆō825. PMID: 9199377.