Correlation among Inflammatory Cytokine Expression Levels, Degree of Disk Degeneration, and Predominant Clinical Symptoms in Patients with Degenerated Intervertebral Discs
Article information
Abstract
Study Design
Observational study.
Purpose
To assess the correlation among inflammatory cytokine expression levels, degree of intervertebral disk (IVD) degeneration, and predominant clinical symptoms observed in degenerative disk disease (DDD).
Overview of Literature
Low back pain (LBP) is associated with inflammatory cytokine expression levels, including those of tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), and nerve growth factor (NGF). However, the association between cytokine expression levels and the physiological mechanisms of disk degeneration and clinical pain remain controversial.
Methods
Using the enzyme-linked immunosorbent assay, TNF-α, IL-6, and NGF expression levels were analyzed in 58 IVD samples that were harvested from patients with lumbar DDD. Patient samples were grouped according to the degree of IVD degeneration using the Pfirrmann grading system and magnetic resonance imaging, and the correlations between the disease groups and each cytokine expression level were assessed. In addition, on the basis of their predominant preoperative symptoms, the patients were assigned to either an LBP or leg pain group to determine the correlation among these disease manifestations and individual cytokine expression levels.
Results
A gradual increase in TNF-α (R=0.391) and IL-6 (R=0.388) expression levels correlated with the degree of IVD degeneration, whereas NGF (R=0.164) expression levels exhibited a minimal decrease with disease progression. Regarding the predominant clinical manifestation, only the LBP group exhibited a significant increase in TNF-α expression levels (p=0.002).
Conclusions
These results suggested that TNF-α and IL-6 play an important role in the pathophysiology of IVD degeneration at any stage, whereas NGF plays an important role during the early disease stages. Moreover, because TNF-α expression levels were significantly high in the LBP group, we propose that they are involved in LBP onset or progression.
Introduction
Intervertebral disk (IVD) degeneration leads to degenerative disk disease (DDD), characterized by lumbar disk herniation (LDH), lumbar spinal stenosis (LSS), and diskogenic low back pain (LBP) [12]. These diseases are often accompanied by acute or chronic intractable LBP and radicular pain. The most common cause of disk degeneration is compositional changes in IVD, compromising its biomechanical properties and impairing its function. Our understanding of the pathophysiological basis of radicular pain has recently evolved from the assumption of a pure mechanical nerve root compression to a more complex mechanism that involves both mechanical and biochemical mechanisms [345], thereby uncovering the potential of treating DDD patients with currently approved pharmaceuticals instead of surgery.
LBP is reportedly associated with inflammatory cytokine expression levels, including those of tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), and nerve growth factor (NGF), the latter of which is increased in IVDs of DDD patients [678]. Previous studies identified an association between the clinical symptoms of lumbar disease and intradiscal cytokine expression; however, the association between cytokine expression levels and the mechanisms of disk degeneration and clinical pain remain controversial [291011]. Thus, this study aimed to better understand the association between local intradiscal TNF-α, IL-6, and NGF expression levels and the degree of IVD degeneration, as well as its prominent symptoms.
Materials and Methods
1. Subjects
The study procedures were approved by the ethics committee of Chiba University, and informed consent was obtained from each patient. In this study, 58 IVD samples were harvested from 55 patients undergoing surgery for various lumbar diseases, including LDH, LSS, diskogenic LBP, and lumbar spondylolisthesis. The mean patient age was 59.6±17.1 (standard deviation) years, 31 were men, and 24 were women; three women provided two disk samples each (Table 1). Whole IVD samples that included both the annulus fibrosus (AF) and nucleus pulposus (NP) were harvested and immediately frozen to avoid the degradation of the clotting activity. Clinical DDD was confirmed by spinal magnetic resonance imaging (MRI) in all cases. Furthermore, cases with a complaint of both back and leg pain were enrolled from numerical rating scale score (NRS) and were assigned to the significantly strong symptom group in this study. Reoperation cases and those using influential drugs for IVD degeneration (e.g., steroids) were excluded.
2. Cytokine quantification
The inflammatory cytokine expression levels in each sample were determined using the enzyme-linked immunosorbent assay. Frozen IVD samples were pulverized, homogenized, and digested in a tissue lysis reagent. TNF-α, IL-6, NGF, and total protein assays were performed according to the manufacturers' protocols and quantified by measuring the reaction absorbance at 450 nm using a microplate reader (TNF-α/IL-6, R&D Systems, Minneapolis, MN, USA; NGF, Promega, Madison, WI, USA; total protein, Bio-Rad, Hercules, CA, USA). Cytokine expression levels were then normalized to the total protein levels for further analysis.
3. Correlation analysis of cytokine expression levels and degree of IVD degeneration
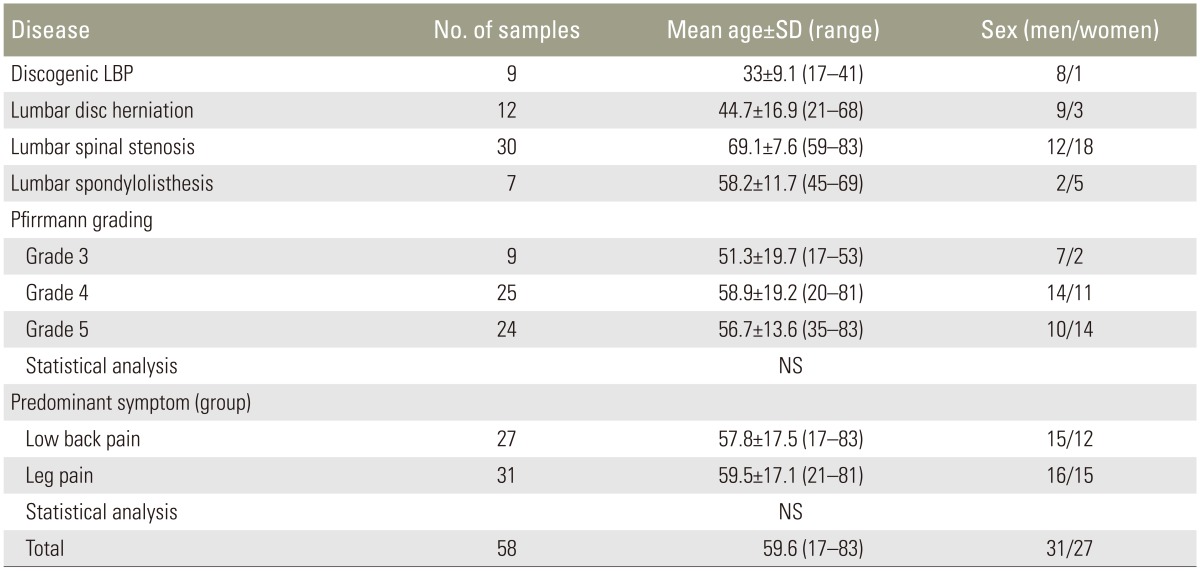
All patients underwent MRI for preoperative diagnosis and were stratified with respect to the disease severity using the Pfirrmann grading system (Table 1) [12]. The average relative expression levels of each cytokine were then used to assess its association with IVD degeneration.
4. Correlation analysis of cytokine expression levels and predominant clinical symptom
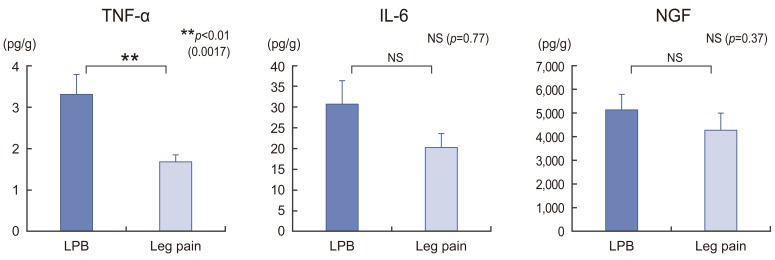
The 58 samples were divided into the following two groups on the basis of the patients' predominant perioperative symptom: LBP group (n=27) and leg pain group (n=31) (Table 1). Associations between the average relative expression levels of each cytokine and the predominant symptom were then determined.
5. Statistical analysis
Kruskal-Wallis and Mann-Whitney U tests were used to compare inflammatory cytokine expression levels with disease severity and predominant clinical manifestation. A p-values of <0.05 were considered to be statistically significant.
Results
1. Association between cytokine expression levels and degree of IVD degeneration
Gradual increases in TNF-α and IL-6 expression levels were observed with DDD progression (Pearson correlation, R=0.391 and 0.388, respectively). TNF-α expression levels were significantly higher in the grade 5 samples than in the grade 3 ones (p<0.05); however, no significant differences were found between the grade 3 and 4 samples and grade 4 and 5 samples. IL-6 expression levels were significantly higher in the grade 5 samples than in grade 3 ones (p<0.05); however, the levels were also higher in the grade 4 samples than in the grade 3 ones (p<0.05). No significant difference in IL-6 expression levels was found between the grade 4 and 5 samples.
Furthermore, NGF expression levels showed a minimal decrease in more advanced DDD (R=−0.164) and was significantly lower in the grade 5 samples than in the grade 3 ones (p<0.05). However, no significant differences were observed between the grade 3 and 4 samples and grade 4 and 5 samples (Fig. 1).

Correlation between cytokine expression levels and the degree of intervertebral disk (IVD) degeneration according to the Pfirrmann grading system and magnetic resonance imaging. A gradual increase in the tumor necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6) expression levels was observed with the progression of IVD degeneration. The nerve growth factor (NGF) exhibited a minimal decrease. NS, not significant. *p <0.05.
2. Correlation between cytokine expression levels and predominant clinical symptom
The analysis of cytokine expression levels with respect to the primary clinical manifestation revealed that TNF-α expression levels were significantly higher in the LBP group than in the leg pain group (p=0.002). However, IL-6 and NGF expression levels tended to be higher in the LBP group than in the leg pain group (p=0.77 and 0.37, respectively) but were not statistically significant (Fig. 2).
Discussion
In this study, we report that the trend of increased TNF-α and IL-6 expression levels coincided with advanced IVD degeneration. Several studies have demonstrated the role of inflammatory cytokines and growth factors in the pathophysiology of IVD degeneration [613141516]. For instance, a strong correlation exists between TNF-α expression levels and histological signs of IVD degeneration [11]. Lee et al. [9] also reported regarding the trend of increased TNF-α expression levels with disease progression; however, no significant correlation between IL-6 expression levels and the degree of IVD degeneration was observed.
Inflammatory cytokines have known roles in the pathophysiology of other osteoarthritis (OA) forms. Abe et al. [17] showed that the synovial joint fluid from patients with terminal hip OA contained significantly higher IL-1, IL-6, IL-8, and TNF-α levels than that from patients with early OA. Similarly, Orita et al. [18] studied the correlation between the synovial joint fluid cytokine levels and disease severity, measured by both clinical symptoms and Kellgren-Lawrence (KL) radiographic grade, in patients with knee OA and reported that while IL-6 had a significant negative correlation with the KL grade, TNF-α correlated with the clinical symptom severity. Thus, they concluded that TNF-α and IL-6 were associated with pain and joint function, respectively. On the basis of these previous reports, we expected that the regional expression levels of the inflammatory cytokines would increase along with IVD degeneration. Altogether, these results suggest that both TNF-α and IL-6 play important roles in DDD progression at any degenerative stage.
In contrast, the expression levels of neurotrophic NGF had only a minimal decrease that corresponded to IVD degenerative progression. Growth factors are polypeptides that bind cell membranes via specific receptors. In IVD, tissue fluids can deliver growth factors through the end plate via an endocrine mechanism [19]. Nerve fibers that innervate painful IVDs are accompanied by microvascular blood vessels that secrete NGF, and the pattern of tissue innervation is regulated by NGF-driven axonal growth and maturation [20]. Yamauchi et al. [21] reported that NGF extracted from degenerative NP might facilitate pain transmission by promoting the axonal growth in sensory nerve fibers and the production of substance-P, which is a pain-related neuropeptide. In our study, NGF expression levels were high during early IVD degeneration; thus, we speculated that NGF expression levels play an important role during early disk degeneration, which is characterized by discogenic LBP and radicular pain.
Moreover, our analysis revealed that TNF-α expression levels were significantly higher in the LBP group than in the leg pain group. In 2002, Burke et al. [2] reported that the increased IL-6 and IL-8 levels in disk tissues from patients with LBP were comparable with those in patients with sciatica; however, they did not any significant difference in TNF-α expression levels between the groups. Weiler et al. [11] reported that TNF-α expression levels were substantially higher in the disks of symptomatic patients than in those of older individuals, which were obtained at autopsy. Thus, they suggested that while TNF-α was not involved in initiating disk degeneration, it propagates degenerative disarrangement and pain induction in adults. Moreover, Lee et al. [9] demonstrated that TNF-α expression levels were significantly higher in DDD patients with LBP than in herniated NP patients with leg pain; however, no statistical differences were observed with respect to IL-6, IL-1, and IL-12 expression levels. Furthermore, several studies have identified TNF-α as a primary molecular constituent of herniated disk tissues, at least in tissue culture, providing convincing evidence regarding the cytokine's involvement generating and promoting disk-induced pain [61322].
This study had some limitations. First, we had no control group. Second, we quantified cytokine expression levels in whole disk tissues that included both AF and NP; however, Schroeder et al. [5] reported that cytokine expression levels were higher in AF than in NP of DDD patients, suggesting that AF initiates the inflammatory response in IVDs. Therefore, we might have obtained different results if we had separately evaluated AF and NP in our analysis. Third, we included various DDDs types, such as LDH, LSS, discogenic LBP, and lumbar spondylolisthesis in our sample population; however, whether these diseases have a similar pathogenesis with respect to IVD degeneration remains unclear; this should be considered in future studies. Finally, because the degree of mechanical compression by IVD bulging may exacerbate the leg pain experienced by patients, excluding cases where degenerated disks mechanically compress the spinal nerve may be necessary.
Conclusions
Inflammatory cytokines are involved in pain pathogenesis and degenerative disk progression in DDD patients. TNF-α has a greater involvement in LBP than other inflammatory cytokines and may be a viable therapeutic target for discogenic LBP.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.