Artificial neural networks for the detection of odontoid fractures using the Konstanz Information Miner Analytics Platform
Article information
Abstract
Study Design
An experimental study.
Purpose
This study aimed to investigate the potential use of artificial neural networks (ANNs) in the detection of odontoid fractures using the Konstanz Information Miner (KNIME) Analytics Platform that provides a technique for computer-assisted diagnosis using radiographic X-ray imaging.
Overview of Literature
In medical image processing, computer-assisted diagnosis with ANNs from radiographic X-ray imaging is becoming increasingly popular. Odontoid fractures are a common fracture of the axis and account for 10%–15% of all cervical fractures. However, a literature review of computer-assisted diagnosis with ANNs has not been made.
Methods
This study analyzed 432 open-mouth (odontoid) radiographic views of cervical spine X-ray images obtained from dataset repositories, which were used in developing ANN models based on the convolutional neural network theory. All the images contained diagnostic information, including 216 radiographic images of individuals with normal odontoid processes and 216 images of patients with acute odontoid fractures. The model classified each image as either showing an odontoid fracture or not. Specifically, 70% of the images were training datasets used for model training, and 30% were used for testing. KNIME’s graphic user interface-based programming enabled class label annotation, data preprocessing, model training, and performance evaluation.
Results
The graphic user interface program by KNIME was used to report all radiographic X-ray imaging features. The ANN model performed 50 epochs of training. The performance indices in detecting odontoid fractures included sensitivity, specificity, F-measure, and prediction error of 100%, 95.4%, 97.77%, and 2.3%, respectively. The model’s accuracy accounted for 97% of the area under the receiver operating characteristic curve for the diagnosis of odontoid fractures.
Conclusions
The ANN models with the KNIME Analytics Platform were successfully used in the computer-assisted diagnosis of odontoid fractures using radiographic X-ray images. This approach can help radiologists in the screening, detection, and diagnosis of acute odontoid fractures.
Introduction
Odontoid injuries can be fatal and require a thorough understanding of the radiographic projection of the craniocervical complex. These injuries often result from high-energy trauma and commonly occur in younger individuals or older adults [1,2]. The most common mechanism of injury is the hyperextension of the cervical spine, which pushes the head and C1 vertebrae backward. Odontoid fractures account for 20% of cervical spine fractures in adults and are the most common subtype among patients aged >65 years [3,4]. Specifically, spine surgeons are interested in this topic because these injuries have high morbidity and fatality rates [5,6]. This topic is also of particular interest to spine surgeons. The classification scheme proposed by Anderson and D’Alonzo [7] is the most frequently used for odontoid fractures. Three types of fractures can occur in the odontoid process, depending on the location and manner they occur, namely, type I, II, and III fractures. Cervical spine hyperextension is the most frequent mechanism of injury, in which the head and C1 vertebrae are pushed backward. Thus, this study reviews the presentation, assessment, and management of odontoid fractures, as well as the assessment, diagnosis, and treatment of C2 dens fractures [2,8].
Currently, lateral, anterior–posterior, and open-mouth X-ray views are utilized to assess cervical spine injuries; however, radiographs have lower sensitivity and specificity than computed tomography (CT) scans [2]. Trauma series have improved the sensitivity of plain films but have been reported to miss 61% of fractures, 36% of subluxations, and 23% of dislocations [9,10]. In recent years, neural networks and deep-learning (DL) techniques can be used to effectively detect spinal fractures using conventional spinal radiography [11]. Artificial intelligence (AI) and machine learning (ML) can also enhance predictive indicators in clinical spine surgery by extracting “radiomic” data from photographs that cannot be observed by visual inspection. AI tools can also enhance picture quality, patient centricity, imaging efficiency, and diagnostic accuracy, benefiting patients and clinicians. Moreover, AI can be used to remove image artifacts, harmonize images, enhance quality, reduce radiation, contrast exposure, and reduce imaging test duration [12–16].
With the above background, this study aimed to evaluate the potential of deep convolutional neural networks (CNNs) in detecting odontoid fractures. The technology could revolutionize medical care by aiding physicians in making more accurate diagnoses of odontoid fractures and reducing medical errors and personal bias. Moreover, it could serve as a model for other programs. By analyzing plain film data of undefined spinal disorders, the technology could reduce mortality rates, unnecessary examinations, and costs. This could be beneficial for physicians with limited experience in diagnosing odontoid fractures. The integration of medical imaging technology and AI has led to the development of computer-aided detection systems that improve lesion detection performance [17].
Numerous studies have focused on using a DL model to detect or predict odontoid fractures; however, no study has reported on a DL model created without writing a single line of code using the Konstanz Information Miner (KNIME) Analytics Platform. Our results suggest that a single, thorough CNN can autonomously diagnose various odontoid abnormalities. The KNIME Analytics Platform, which addresses many of the shortcomings of existing tools, can also execute modern DL algorithms [18].
Materials and Methods
Study design and methodology
This study obtained 432 open-mouth (odontoid) radiographic views of cervical spine X-ray images from dataset repositories to construct an ANN model based on CNN theory. All images, including 216 normal radiographic images and 216 images of acute odontoid fractures, contained diagnostic information. The images were retrievevd from an online open-access dataset that anonymized patients [19].
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee and Institutional Review Board (IRB) of Faculty of Medicine, Chiang Mai University (IRB approval no., ORT-2566-09487). Informed consent was waived due to the retrospective nature of the study. All patients with symptomatic spinal conditions and cervical neck pain with plain film X-rays available from an online open-access odontoid fracture X-ray dataset [19] and open-mouth plain radiography images were included. Patients with a spine infection, congenital or other diseases, low-quality plain open-mouth images, and plain cervical spine films without open-mouth X-ray images were excluded.
The dataset consisting of 432 open-mouth plain X-ray images of adult patients with symptomatic cervical neck pain was categorized into normal (216 photos) and fracture (216 images) groups. Then, these images were divided into a training dataset, comprising 302 images (70%), and a test dataset, including 130 images (30%). The training dataset was subjected to cleaning, labeling, and annotation, which were carried out by a radiologist. The DL approach employed the deep CNN model for training and detecting odontoid fractures. The efficacy of the trained CNN model was assessed by subjecting the dataset to testing using an automated approach. Before assessing the accuracy of the model, the testing dataset was validated by three radiologists. The supervised learning approach was implemented by three researchers who performed manual annotations. The most optimal annotation imaging technique was chosen based on a consensus reached by the researchers through voting. The testing dataset was characterized by the absence of model training, indicating that the model had not been exposed to the testing image before evaluation.
Data augmentation
Owing to the small sample size, all images were randomly augmented using the Python package augmentor to prevent overfitting. The augmentor software package provided functions that were frequently used to create image data for ML, e.g., flipping, rotating, zooming, scaling, cropping, and translating. In this study, the following were applied to the conventional image augmentation set: horizontal flip, crop (zoom 0%–20%), rotation (between −15° and 15°), and shear (15° horizontal and 15° vertical). For training, segmented images were shuffled and randomized. The final data set included 302 training (70%) and 130 testing (30%) images.
Deep-learning training
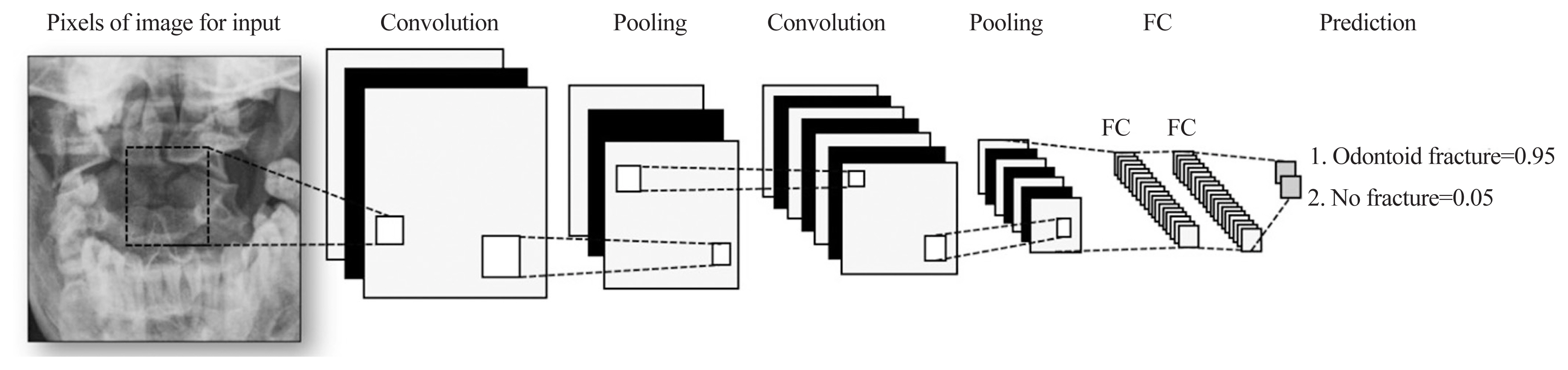
The DL method was used for training and detection of odontoid fractures using a deep CNN model (Fig. 1). The CNN training model was validated by evaluating the dataset’s classification using an automatic model. The KNIME Analytics Platform was employed for high-performance object detection. In this platform, an image is divided into a grid system in which each grid module detects objects within itself without the need for writing a single line of code. To evaluate the model, 100 epochs were executed; thereafter, the model’s accuracy reached 100%.
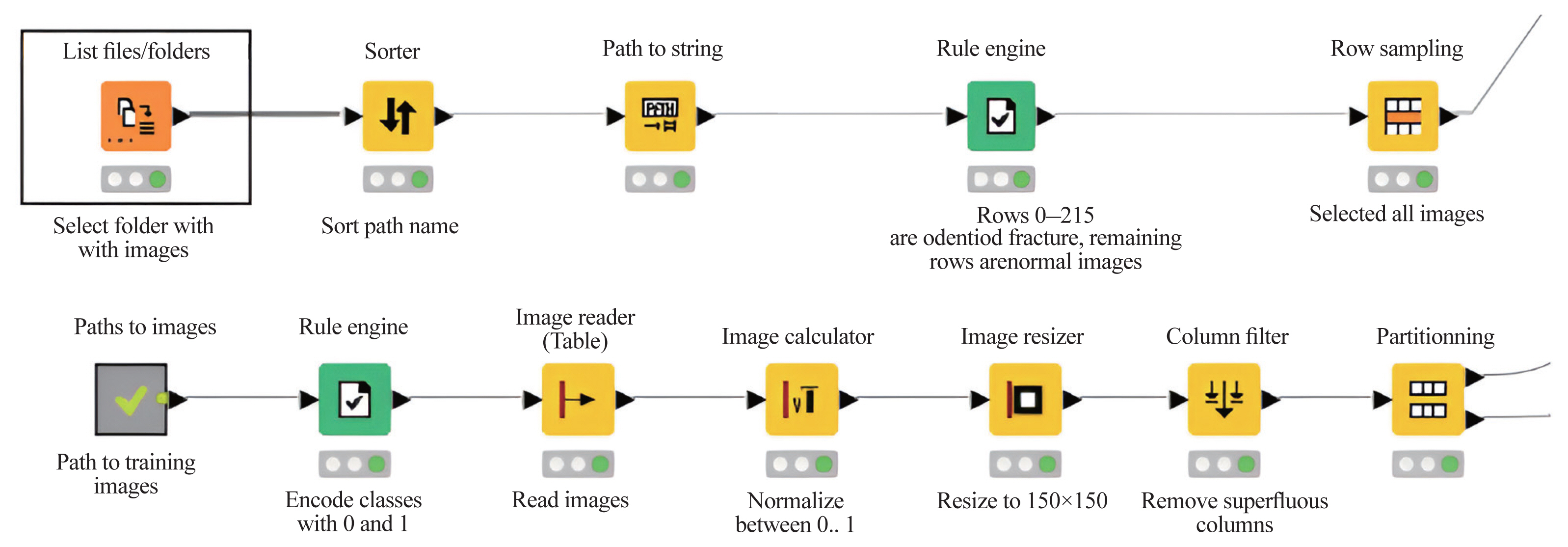
For ML analyses, the KNIME Analytics Platform (Fig. 2) was employed, which is a business intelligence or predictive analytical tool that has been used in biomedical research [20]. The KNIME Analytics Platform, an easy-to-use data integration, analysis, and exploration workflow system, can handle large amounts and different types of data in a platform-independent computing environment. It has successfully met complex end-to-end demands in several fields, such as cheminformatics and mass spectrometry [21]. In this study, this platform had two parts: network design (Fig. 3) and reading and preprocessing of pictures (Fig. 4). The model was first trained 50 times and then checked against the test set.
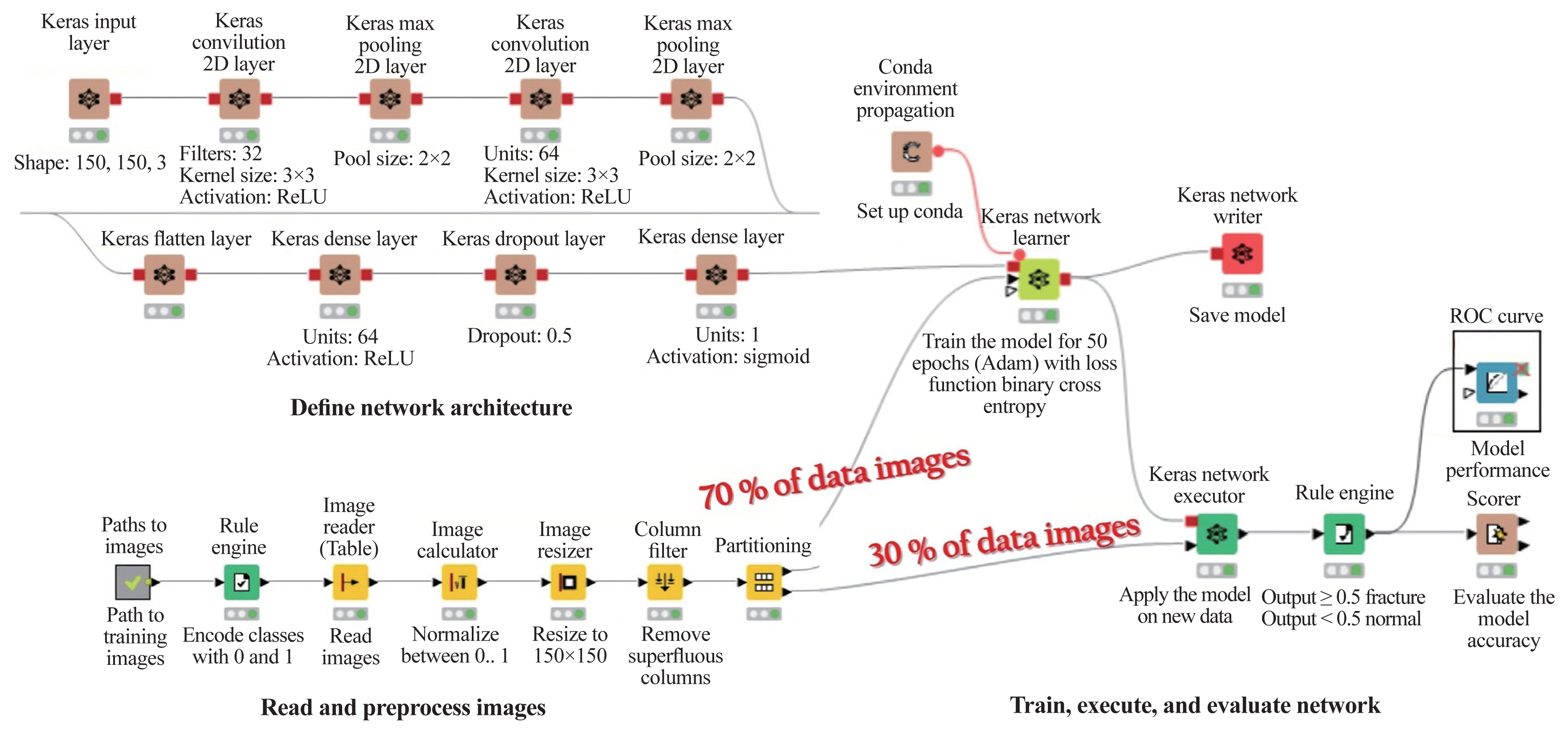
Konstanz Information Miner’s graphic user interface-based programming enabled class label annotation, data preprocessing, model training, and performance evaluation. 2D, two-dimensional; ROC, receiver operating characteristic.
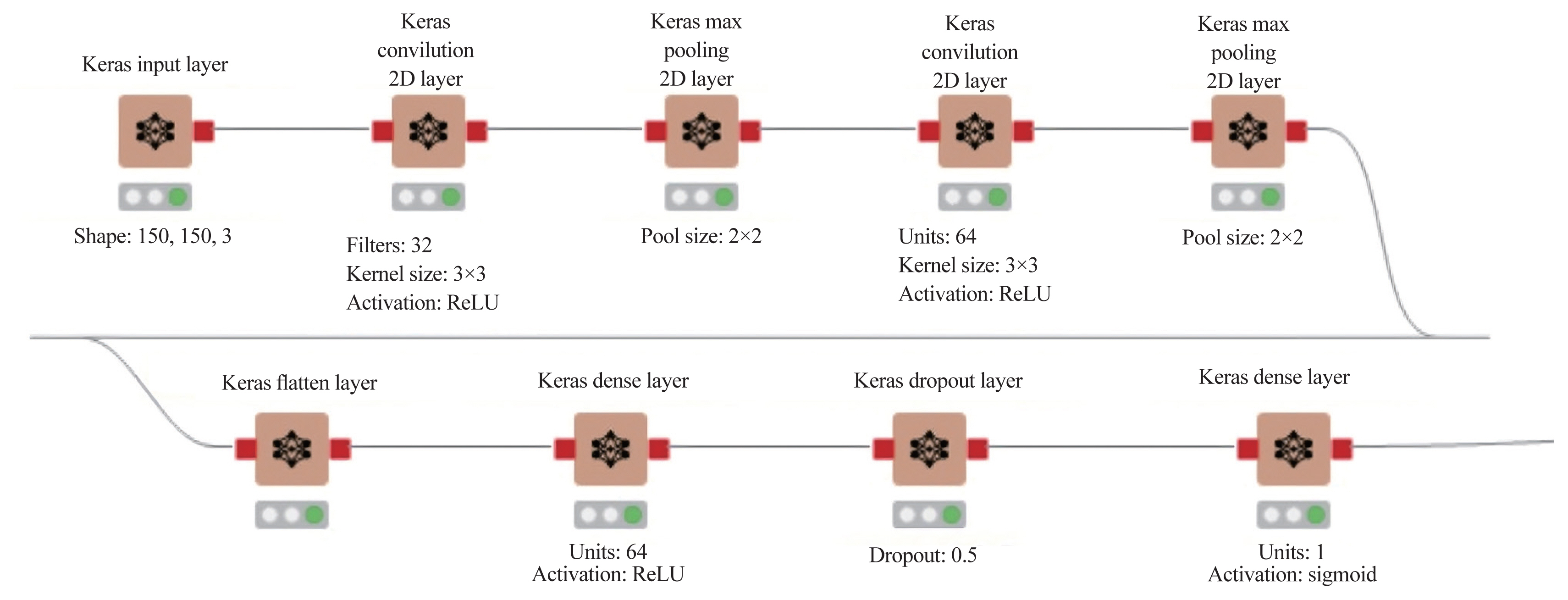
The Konstanz Information Miner analytics Platform defining network architecture. 2D, two-dimensional.
Results
After evaluating the model’s performance for detecting odontoid fractures, 130 X-ray images of both broken and normal odontoids were used, representing 20% of the test data. For 127 images, the model gave the right answer; however, for three other images, it gave the wrong answer based on normal photos. The accuracy of the forecast was 97.692%, with a test sample error rate of 2.308% (Table 1).
For fracture images, the true-positive, false-positive, true-negative, and false-negative values were 65, 3, and 62, respectively. However, for normal images, the values were 62, 0, 65, and 3, respectively. Table 2 shows the sensitivity (recall) and specificity (predictive value) F-measures for the performance of the AI model, which were 100%, 95.4%, and 97.77% for sensitivity (recall), and specificity (predictive value) F-measure and prediction error, respectively.

Performance of model evaluation for detecting odontoid process fractures by the sensitivity (recall), specificity (predictive value) F-measure, and prediction error
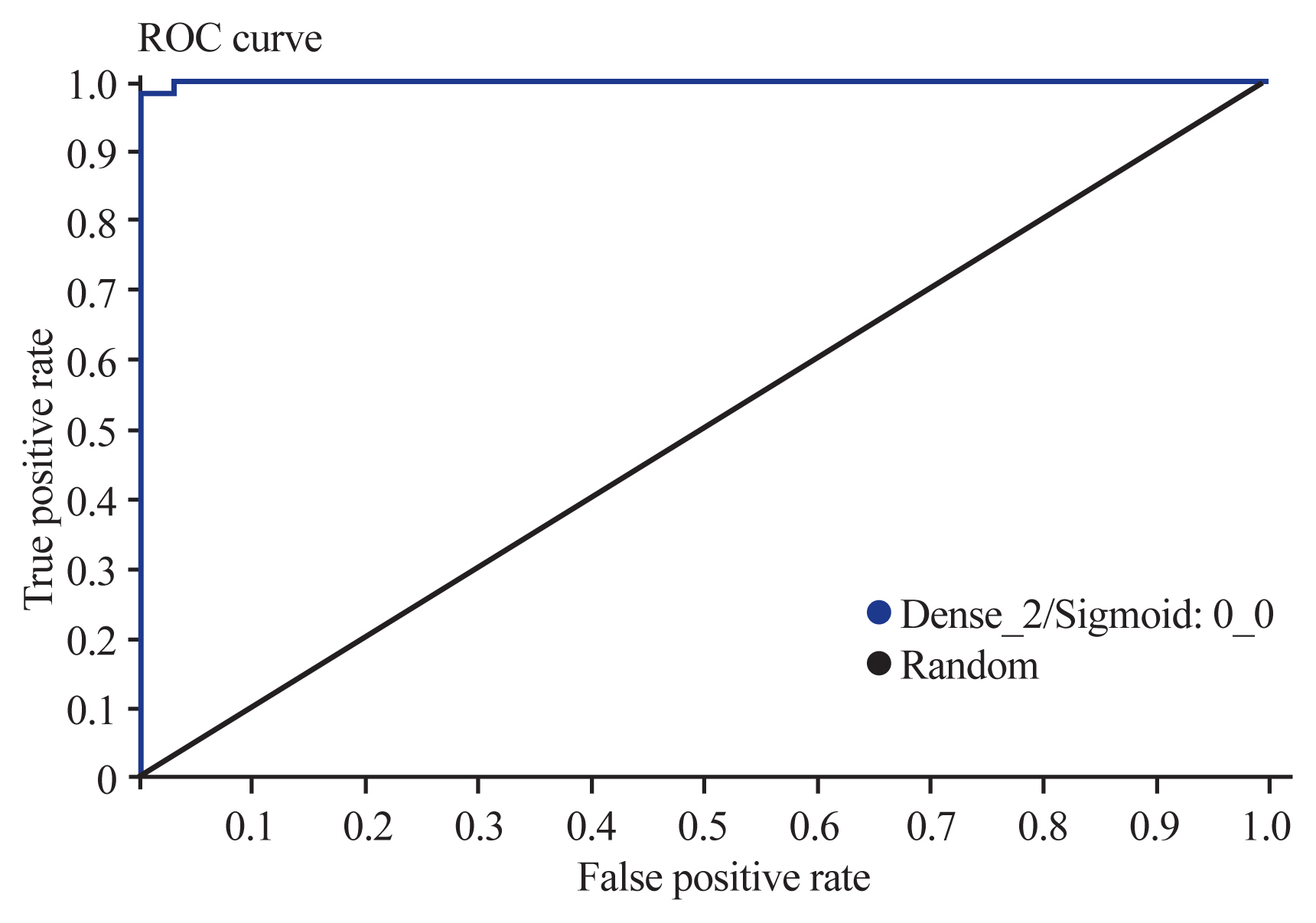
The accuracy of the model was analyzed and evaluated using the area under the receiver operating characteristic (ROC) curve. The overall accuracy was graded as follows: 0.9–1.0, very good to excellent; 0.8–0.9, good; 0.7–0.8, fair; 0.6–0.7, poor; 0.5–0.6, fail; <0.5, worthless, better to roll the dice. The model’s accuracy was equal to 97% of the area under the ROC curve for the diagnosis of odontoid fractures, which showed excellent accuracy (Fig. 5).
Discussion
Although the terms AI, ML, and DL are commonly used interchangeably, they are significantly different. The word “AI” refers to any strategy for teaching computers to behave intelligently like humans. ML works similarly to the human brain, whereas DL is the thinking process or human algorithm [22]. ML algorithms analyze data, learn from it, and make intelligent decisions based on what they have learned. DL, a subset of ML, uses algorithms to construct an artificial neural network (ANN) that can learn and make intelligent judgments about itself. AI is anticipated to significantly affect spinal imaging analysis. International medical institutions produce numerous images every day; however, manual annotation by a radiologist or orthopedist is a time-consuming and labor-intensive process. Although current graphical interface tools enable annotation, managing the ever-increasing volume of data remains difficult [22].
The KNIME Analytics Platform is an innovative data science software application. Being a free and open-source platform, it assures that users will always be on the cutting edge of data science. It has over 300 data source connectors and integrates all prominent ML libraries. With the advent of big data, DL researchers have developed deep CNNs with multiple hidden layers and complex structures that have powerful feature extraction and feature expression capabilities, allowing DL-related algorithms to make remarkable strides in the field of computer vision, particularly in image recognition, classification, and semantic segmentation [22]. This study describes the overall DL process of applying a simple CNN model via KNIME Analytics Platform. The platform does not require users to code because it can follow procedures with clicks, drags, and drops, thanks to the graphical user interface (GUI). In this study, a simple CNN model was trained with data obtained from 432 open-mouth (odontoid) radiographic views of cervical spine X-ray images. Our analysis revealed that the model achieved 97% accuracy. This system is expected to have the potential for use in odontoid fracture detection in acute trauma situations.
Previous studies using X-ray images of fractures to train CNN models have reported comparable results. An et al. [23] used the KNIME Analytics Platform and a simple CNN model to detect coronavirus disease 2019 with 93% accuracy without writing a single line of code. This will help reduce the entry barrier for AI research [23]. Another study that used the KNIME Analytics Platform measured the length of hospital stay of patients with femoral fractures and reported that all the algorithms surpassed 80% accuracy [24]. A prime example of the significant advances that AI has already achieved in spine surgery is the classification of degenerative discs by feature extraction from magnetic resonance images. This AI-based algorithm could achieve a 70.1% concordance with human observations by extracting important disc features such as shape and intensity using CNNs, which are naturally skilled at processing visual data. This is remarkably comparable to the documented rate of agreement between individual expert radiologists (70.4%) [25]. Lateral X-ray images are essential for the classification of odontoid fractures and guide treatment. However, this study aimed to prove the effectiveness and performance of the DL model (CNN) for the detection of odontoid fractures and non-fractures. This application is employed as a screening tool for fast detection for radiology technicians, general doctors, or non-spine specialty doctors. This tool is still a screening tool for a patient suspected of odontoid fractures in the emergency department of the author’s hospital. The lateral X-ray view showed it was normally used to detect odontoid fractures of C2 in patients with acute trauma because of their inability to open their mouth; however, according to the Advanced Trauma Life Support guideline, patients who could not open their mouth were subjected to CT [26].
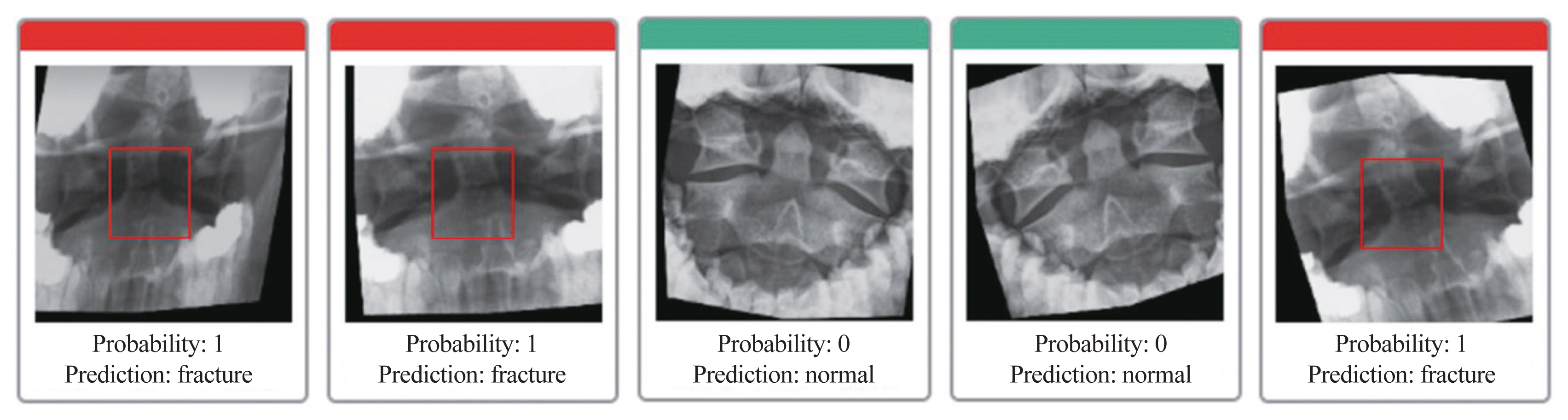
Odontoid injuries due to car accidents are the most common type of misdiagnosis during medical examinations, and that was one of the main incentives for conducting this study. A plain radiographic study via an open mouth or an odontoid view could be used for screening research. In clinical practice, internists and general practitioners frequently misdiagnose odontoid problems because of inexperience, resulting in further harm from excessive motility and allowing early movements. With AI-assisted diagnosis, CT could become the gold standard for determining the ultimate diagnosis of odontoid fractures. AI-assisted diagnosis can be used as a screening tool and has the potential to reduce human errors. An example of using a web-based application is shown in Fig. 6.
As study limitations, we used an open-assessment dataset of odontoid fractures that was imbalanced and had varied data distribution [27] because type II was the most common type based on the classification by Anderson and D’Alonzo [7]. As this was an open-assessment dataset of X-rays, the data were anonymized and did not include the identification of the participants, relevant dates, recruitment period, eligibility criteria, sources, selection methods, follow-up, and data collection [19]. This study presents a novel way for applying ML to identify and discriminate distinct forms of spinal cord diseases. ML was used to determine the local image gradient orientation attributes of each structure; however, this model cannot classify an os odontoideum. Because clinical X-ray studies in the dataset were anonymized, they could not be extended to all patients and thus could not provide a comprehensive description of the dataset. A larger and multicenter dataset is preferred for future training, validation, and testing of advanced DL. Future studies can categorize CT scans or magnetic resonance images according to the classification by Anderson and D’Alonzo [7] or the classification by Grauer et al. [8]. Positively, because this was a normal public dataset and the study was conducted entirely by computers, there was no opportunity for human prejudice or other sources of bias.
Conclusions
ANN models using the KNIME Analytics Platform can be successfully utilized for computer-assisted diagnosis of odontoid fractures using radiographic X-ray images. AI-computer-assisted diagnosis and radiologists can decrease both human and computer errors. The KNIME Analytics Platform can automatically identify versions of Python, CUDA, and Keras. It rapidly and easily makes a workflow by dragging the nodes installed in extensions. Since the models do not have builtin example codes, for some parts, it is necessary to search for and use published nodes directly from the KNIME Hub website. Furthermore, the data collection process is not time-consuming, allowing the inclusion of larger amounts of data covering broader periods in the future [28].
Codeless DL using the KNIME Analytics Platform can reduce the time required and lower the threshold for the application of DL research in healthcare because the KNIME Analytics Platform provides a user-friendly GUI and good compatibility. ANN models with the KNIME Analytics Platform have been successfully utilized for computer-assisted diagnosis of odontoid fractures using radiographic X-ray images. However, to decrease both human and computer errors, the AI-computer-assisted diagnosis is best used with radiologists.
Acknowledgments
The authors would like to express their sincere thanks to G. Lamar Robert and Chongchit Sripun Robert for editing the English manuscript. The authors would also like to thank Pawee Chalidapong for support.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: WL, PS; methodology: WL; software: WL; validation: AP, STC, PS, WL; formal analysis: PS, WL; investigation: WL, VK, KJ, AP, STC, PS; resources: WL, VK, KJ; data curation: WL, PS; writing–original draft preparation: WL, PS; writing–review and editing: WL, PS; visualization: STC, AP, WL; supervision: WL; project administration: WL; funding acquisition: WL. All authors have read and agreed to the published version of the manuscript.