|
 |
- Search
| Asian Spine J > Volume 18(3); 2024 > Article |
|
Abstract
Purpose
This study aimed to investigate survival and prognostic factors after surgery for a metastatic spinal tumor.
Methods
A retrospective multicenter study was conducted. The study participants included 345 patients who underwent surgery for spinal metastases from 2010 to 2020 at nine referral spine centers in Japan. Data for each patient were extracted from medical records. To identify the factors predicting survival prognosis after surgery, univariate analyses were performed using a Cox proportional hazards model.
Results
The mean age was 65.9 years. Common primary tumors were lung (n=72), prostate (n=61), and breast (n=39), and 67.8% (n=234) presented with osteolytic lesions. The epidural spinal cord compression scale score 2 or 3 was recognized in 79.0% (n=271). Frankel grade A paralysis accounted for 1.4% (n=5), and 73.3% (n=253) were categorized as intermediate or high risk according to the new Katagiri score. The overall survival rates were -71.0% at 6 months, 57.4% at 12, and 43.3% at 24. In the univariate analysis, Frankel grade A (hazard ratio [HR], 3.59; 95% confidence interval [CI], 1.23–10.50; p<0.05), intermediate risk (HR, 3.34; 95% CI, 2.10–5.32; p<0.01), and high risk (HR, 7.77; 95% CI, 4.72–12.8; p<0.01) in the new Katagiri score were significantly associated with poor survival. On the contrary, postoperative chemotherapy (HR, 0.23; 95% CI, 0.15–0.36; p<0.01), radiation therapy (HR, 0.43; 95% CI, 0.26–0.70; p<0.01), and both adjuvant therapy (HR, 0.21; 95% CI, 0.14–0.32; p<0.01) were suggested to improve survival.
The spine is the most frequent site of bone metastasis, leading to considerable morbidities, including spinal pain and neurological dysfunction [1]. The management of spinal metastases requires a multidisciplinary approach including pharmacotherapy, radiation therapy, and surgery [2-4]. Surgery plays a valuable role in the management of intolerable pain, acquisition of structural stability, and restoration or preservation of neurological function [5].
The current development of surgical techniques has given rise to an expanding indication for surgery [6]. Minimally invasive surgery can be applied to patients with a short life expectancy and poor condition [7,8]. Proper prediction of postoperative prognosis is important when considering surgical indications. Tomita et al. [9], Tokuhashi et al. [10], and Katagiri et al. [11] have examined prognostic factors for patients with spinal metastasis. However, it is uncertain whether these prognostic factors are still applicable in the current situation with improvements in treatment modalities. Moreover, few reports have discussed prognostic factors in surgical treatment for spinal metastases in the 2010s. In this study, we examined survival and prognostic factors after surgery for a metastatic spinal tumor from 2010 to 2020.
This study was conducted as a retrospective multicenter analysis at nine facilities in Japan. Patients who underwent surgical treatment for spinal metastases between January 2010 and December 2020 were selected from each hospital’s medical records. For each patient, the following indicators were extracted and analyzed: age, sex, primary tumor, metastatic spine level, bone lesions, epidural spinal cord compression (ESCC) scale, spinal instability neoplastic score (SINS), Frankel grade, new Katagiri score, bone-modifying agents, surgical procedure, perioperative complications, adjuvant therapy, length of hospital stay, final disposition, and survival period.
The ethical committee of Kyoto University Graduate School and Faculty of Medicine approved this study (aproval no., R2901). Informed consent was obtained from all individual participants included in the study.
The primary tumor was defined as the original tumor of the spinal metastasis such as lung carcinoma, prostate carcinoma, and breast carcinoma. The level of spinal metastases that required surgery was summarized, ranging from one to nine vertebrae depending on the patient. Bone lesions were evaluated using computed tomography as “osteolytic,” “osteoblastic,” and “mixed.” Spinal cord compression was graded using axial T2-weighted magnetic resonance imaging based on the ESCC scale [12]. SINS was introduced to evaluate spinal instability by assessing the level of spinal metastases, pain, bone lesions, radiographic spinal alignment, vertebral body collapse, and posterior involvement of spinal elements [13]. The severity of paralysis was classified into five levels according to the Frankel grade [14]. The new Katagiri score is a prognostic indicator for patients with metastatic spinal disease based on the primary tumor, visceral metastases, laboratory data, performance status, preoperative chemotherapy, and multiple skeletal metastases [11]. Bisphosphonates and a human monoclonal antibody (HmAb) targeting the receptor activator of NF-κB ligand (RANKL) were also prescribed as bone-modifying agents to prevent skeletal-related events.
The surgical indication was the presence of progressive nerve palsy or intolerable back pain because of spinal cord compression or spinal instability caused by pathologic fractures. Surgery was also performed prophylactically in asymptomatic patients with high SINS or new Katagiri scores. Surgical procedures were mainly divided into palliative and definitive surgeries. Palliative surgery includes decompression and instrumented stabilization to improve paralysis and back pain. Neural tissue was decompressed using the posterior median approach, sometimes with the partial removal of the tumor to achieve sufficient neural decompression. Pedicle screws were inserted and connected using rods for stabilization. The fusion range was determined for each patient by considering the affected vertebrae, bone quality, and spinal alignment. Definitive surgery was performed using tumor excisional procedures when the long-term prognosis was expected; however, adjuvant therapy was not assumed to be effective. Biopsy and vertebroplasty were excluded from the surgical intervention.
During the postoperative hospital stay, perioperative complications were defined as medical problems and were categorized as “neurologic,” “respiratory,” “cardiac,” “gastrointestinal,” “urinary and renal,” “venous thromboembolism,” “wound,” “decubitus ulcer,” “infection,” or “others.” Final disposition was categorized as “home discharge,” “transfer to another hospital,” or “in-hospital death.” The length of hospital stay was defined as the duration from admission to discharge, transfer, or death.
Data are expressed as the mean±standard deviation. Kaplan-Meier survival curves are described. Univariate analysis to identify predictors of survival was performed for variables such as age, sex, ESCC scale, SINS, Frankel grade, new Katagiri score, surgical procedure, and postoperative adjuvant therapy using a Cox proportional hazards model. In the univariate analyses, a hazard ratio (HR) with a 95% confidence interval (95% CI) was estimated; HR indicates the probability of death in a particular group compared with that in a reference group. All p-values <5% were considered significant.
From 2010 to 2020, a total of 345 patients were identified, and they were included in the study, and their backgrounds are summarized in Table 1. The mean age of patients admitted was 65.9 years, and the mean follow-up period was 13.7 months. The primary tumor was located in the lung in 72 patients (20.9%), prostate in 61 (17.7%), and breast in 39 (11.3%), accounting for half of the cases. The most common site of spinal involvement was the thoracic spine (69.2%), followed by the cervical (15.1%), lumbar (14.7%), and sacral (1.0%) spines. A total of 234 patients (67.8%) presented with osteolytic lesions. Spinal cord compression corresponding to ESCC scale 2 or 3 was recognized in 271 patients (79.0%). SINS representing spinal instability was “0–6” in 45 patients (13.0%), “7–12” in 215 (62.3%), and “13–18” in 82 (23.8%). The severity of paralysis was Frankel grade E in 60 patients (17.4%), D in 109 (31.6%), C in 147 (42.6%), B in 24 (7.0%), and A in 5 (1.4%). A total of 175 patients (50.7%) were categorized into the intermediate-risk group with a new Katagiri score of “4–6,” 91 (26.4%) into the low-risk group of “0–3,” and 78 (22.6%) into the high-risk group of “7–10.” Bone-modifying agents were prescribed to only 84 patients (24.3%), bisphosphonate to 37 (10.7%), and HmAb targeting RANKL to 47 (13.6%).
Data on surgical treatment, perioperative complications, and outcomes are presented in Table 2. Most patients (97.4%) underwent palliative surgery mainly by both decompression and stabilization, whereas only 5 (1.4%) underwent definitive surgery. The in-hospital complication rates were 4.3% for neurological, 3.5% for respiratory, 0.9% for cardiac, 4.3% for gastrointestinal, 5.5% for urinary and renal, 2.0% for venous thromboembolism, 5.5% for wound, 0.6% for decubitus ulcer, 4.6% for infection, 7.8% for others, and 39.1% for overall complications. In addition, 12 deaths (3.5%) occurred within 30 days of surgery, ranging from 13 days.
As shown in Table 3, 318 patients (92.2%) received adjuvant therapies, including chemotherapy or radiation therapy, before or after surgery. Postoperative chemotherapy was administered to 106 patients (30.7%), postoperative radiation therapy to 56 (16.2%), and both postoperative chemotherapy and radiation therapy to 137 (39.7%). Table 2 also shows that the mean length of hospital stay was 46.1 days; 52.8% of patients were discharged home, 35.9% were transferred to another hospital, and 8.7% died in the hospital.
Neurological status after surgery at the final followup is shown in Table 4. Frankel grade A was noted in 6 patients (1.7%), B in 19 (5.5%), C in 94 (27.3%), D in 126 (36.6%), and E in 99 (28.8%). The number of ambulatory patients with Frankel grade D or E increased from 168 (48.8%) to 225 (65.4%) after surgery. The neurological status improved by 1 grade in 99 patients, 2 in 21 patients, and 3 in four patients. Approximately half (n=177) of the patients had maintained preoperative neurological status.
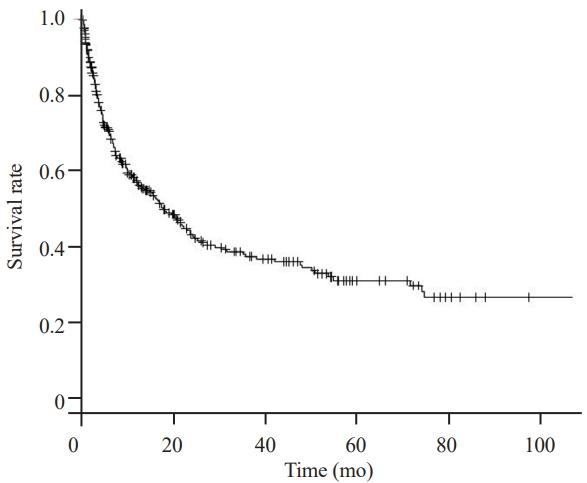
A Kaplan-Meier curve for all patients is shown in Fig. 1. The 6-, 12-, and 24-month overall survival rates were 71.0%, 57.4%, and 43.3%, respectively. Fig. 2 indicates that based on the primary tumor type, survival for lung and colorectal cancer was the poorest, followed by kidney, prostate, and breast cancer. In the univariate analysis in Table 5, Frankel grade A, intermediate risk, and high risk in the new Katagiri score were significantly associated with poor survival, with HRs of 3.59 (95% CI, 1.23–10.50; p<0.05), 3.34 (95% CI, 2.10–5.32; p<0.01), and 7.77 (95% CI, 4.72–12.8; p<0.01) compared with Frankel grade E and low-risk groups, respectively. Postoperative chemotherapy (HR, 0.23; 95% CI, 0.15–0.36; p<0.01), radiation therapy (HR, 0.43; 95% CI, 0.26–0.70; p<0.01), and both types of adjuvant therapy (HR, 0.21; 95% CI, 0.14–0.32; p<0.01) were significantly associated with improved survival. Kaplan-Meier survival curves for Frankel grades and new Katagiri scores are shown in Figs. 3 and 4, respectively.
Spinal metastasis is critical for patients, leading to substantial morbidities, including spinal pain and neurological dysfunction [1]. Surgery plays a valuable role in treatment to reduce pain, acquire structural stability, and restore or preserve neurological function [5]. Prediction of life expectancy in patients with spinal metastasis is important when determining the treatment option, particularly when surgery is considered. The Tomita score, Tokuhashi score, and new Katagiri score have been proposed and used over the years as prognostic factors for spinal metastasis [9-11].
However, recent studies have cast doubt on the validity of these scores, reporting the possibility that they do not reflect the prognosis with the improvement of treatment modalities [15]. Moreover, a study reported that primary tumor etiology and time elapsed since surgery should be considered when determining survival probability [16]. Conversely, the new Katagiri score was demonstrated to be a useful prognostic factor for patients undergoing surgery for spinal metastasis, even in recent cases [17]. The new Katagiri score is notable because it includes all bone and spinal metastases and takes into account the influence of the primary tumor and the efficacy of targeted and selective chemotherapy [11]. For these advantages, the new Katagiri score was selected for this study. In this study, the mean of the new Katagiri scores was 4.9, which was classified into intermediate-risk group, with overall survival rates of 71.0% at 6 months, 57.4% at 12, and 43.3% at 24. These results were not far from a previous report of survival rates of 74.0% at 6 months, 49.3% at 12, and 27.6% at 24 for patients with a prognostic score of 4–6 [11]. Moreover, the new Katagiri score was demonstrated to be a significant prognostic factor with HRs of 3.34 (95% CI, 2.10–5.32; p<0.01) for the intermediate-risk group and 7.77 (95% CI, 4.72–12.8; p<0.01) for the high-risk group, compared with the low-risk group. The new Katagiri score is still considered to greatly influence survival after surgical treatment of spinal metastases.
Frankel grade A was also associated with poor survival after surgery for spinal metastasis. The Frankel grade represents the degree of paralysis and is not a prognostic predictor. However, several studies have suggested a correlation between Frankel grade and survival period. According to Candido et al. [18], cases that did not improve from Frankel grade A, B, or C demonstrated statistically shorter survival than those who recovered to Frankel grade D or E: 7.89 months versus 19.13 months. Vanek et al. [19] reported that preoperative neurological status significantly influenced survival time; the median survival was 5.1 months in Frankel grades A–C and 28.2 months in Frankel grades D and E (p<0.001). Moreover, patients with Frankel grade A had a poor improvement rate in function after surgery [20]. Patients with Frankel grade A and severe motor deficits are less likely to achieve improvement in function, which may lead to medical complications and poor survival rates.
Although the ESCC scale and SINS were originally not used for indicating survival prognosis, relationships with survival have been proposed. Quraishi et al. [21] concluded that spinal surgery tended to result in more complications and worse survival in patients with higher grades of spinal cord compression. Whether SINS affects survival prognosis is controversial. Masuda et al. [22] reported that the median survival of the SINS unstable group (SINS ≥13) was statistically shorter than that of the unstable group, whereas other studies demonstrated no prognostic value of SINS for postoperative survival [23,24]. While older age is also expected to be associated with short survival after surgery, previous reports mentioned that compared with age <80 years, age >80 years was a risk factor only for poor improvement in neurological status and quality outcome but not for survival rates [20]. In this study, The ESCC scale, SINS, or age was not found to be a predictor for survival, and patients aged >80 years tended to survive longer than those aged <60 years with HR of 0.67 (95% CI, 0.33–1.34; p=0.26), although it was not significant. Patients with a good background, such as performance status and general condition, were limitedly extracted as participants because this study only focused on patients undergoing surgery.
In this study, ESCC scale 2 or 3 was noted in up to 79.0% (n=271) of patients, and intermediate or high risk of new Katagiri score in 73.5% (n=253), which could explain the increased incidence of complications. Complication rates after palliative spinal surgery remain high, ranging from 14% to 34% [25,26]. Systemic cancer treatment makes patients immunocompromised, and radiotherapy increases the risk of wound problems and infections. Patients also have a high risk of venous thromboembolism. In this study, the total complication rate was 39.1%, which was slightly higher than that in previous reports.
This study has some limitations. First, only patients who underwent surgery were enrolled. Second, owing to the retrospective analysis of data from multicenters, some clinical information was lacking, and surgical indications and procedures were not standardized among facilities. Third, nine facilities enrolled in this study were affiliated hospitals, resulting in limited indications or procedures of surgery that might not reflect current trends in Japan. Fourth, some patients had less than 1 month of follow-up by an orthopedic doctor, which may affect perioperative outcomes, including complications and survival rates. Finally, patient satisfaction and quality of life were not assessed. Patient satisfaction has been reported to be affected by neurological improvement [27], and further studies including these aspects are necessary to validate the efficacy of surgical treatment for spinal metastases.
Frankel grade A or intermediate or high risk in the new Katagiri score were significant prognostic factors associated with poor survival after surgery in patients with spinal metastases. Taking these factors into account is of great importance when considering treatment options for patients with spinal metastases. For better survival, chemotherapy or radiation therapy should be considered after surgery.
Fig. 2.
The Kaplan-Meier survival curve for each tumor. The 12-month survival rate was 91.0% for breast; 87.8% for kidney; 71.7% for prostate; 37.9% for lung; and 16.1% for colorectal cancer.
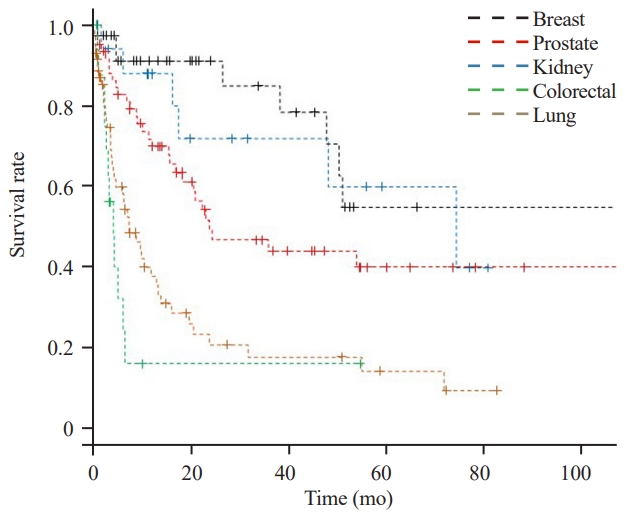
Fig. 3.
The Kaplan-Meier survival curve for Frankel grade. The 12-month survival rate was 62.0% for grade E; 65.0% for grade D; 51.7% for grade C; 44.1% for grade B; and 30.0% for grade A.
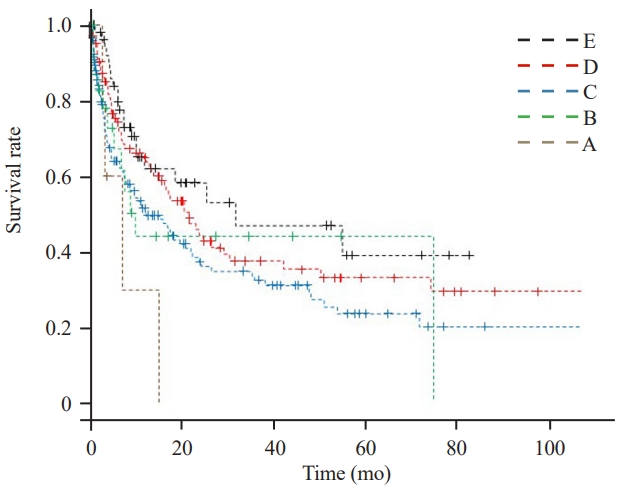
Fig. 4.
The Kaplan-Meier survival curve for new Katagiri score. The 12-month survival rate was 91.0% for 0–3; 55.0% for 4–6; and 19.6% for 7–10.
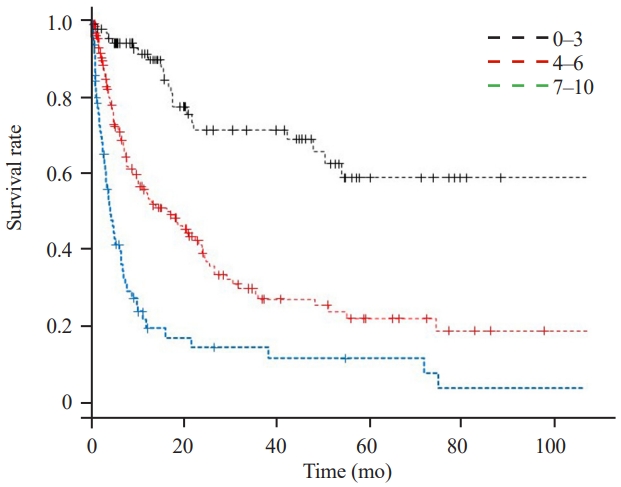
Table 1.
Demographic data for overall patients in the study (n=345)
Table 2.
Surgical procedures and perioperative outcomes (n=345)
Table 3.
Adjuvant therapies before or after surgery
| Chemotherapy |
Radiation therapy |
||||
|---|---|---|---|---|---|
| Neither | Before | After | Both | Total | |
| Neither | 27 | 4 | 34 | 2 | 67 |
| Before | 10 | 5 | 15 | 5 | 35 |
| After | 40 | 7 | 72 | 6 | 125 |
| Both | 37 | 22 | 40 | 19 | 118 |
| Total | 114 | 38 | 161 | 32 | 345 |
Table 4.
Comparison of Frankel grade between before and after surgery
| Preoperative Frankel |
Postoperative Frankel |
|||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | Total | |
| A | 1 | 3 | 1 | 5 | ||
| B | 1 | 7 | 11 | 2 | 3 | 24 |
| C | 3 | 9 | 59 | 60 | 16 | 147 |
| D | 2 | 20 | 58 | 28 | 108 | |
| E | 1 | 1 | 1 | 5 | 52 | 60 |
| Total | 6 | 19 | 94 | 126 | 99 | 344 |
Table 5.
Univariate analysis for predictors of survival
References
1. Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease: a review. J Neurosurg Spine 2010;13:94–108.

2. Tsukamoto S, Kido A, Tanaka Y, et al. Current overview of treatment for metastatic bone disease. Curr Oncol 2021;28:3347–72.



3. Cho JH, Ha JK, Hwang CJ, Lee DH, Lee CS. Patterns of treatment for metastatic pathological fractures of the spine: the efficacy of each treatment modality. Clin Orthop Surg 2015;7:476–82.



4. Pennington Z, Porras JL, Larry Lo SF, Sciubba DM. International variability in spinal metastasis treatment: a survey of the AO Spine Community. Global Spine J 2023;13:1622–34.




5. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643–8.


6. Kumar N, Malhotra R, Zaw AS, et al. Evolution in treatment strategy for metastatic spine disease: presently evolving modalities. Eur J Surg Oncol 2017;43:1784–801.


7. Mezei T, Horvath A, Pollner P, Czigleczki G, Banczerowski P. Research on the predicting power of the revised Tokuhashi system: how much time can surgery give to patients with short life expectancy? Int J Clin Oncol 2020;25:755–64.




8. Dea N, Versteeg AL, Sahgal A, et al. Metastatic spine disease: should patients with short life expectancy be denied surgical care?: an international retrospective cohort study. Neurosurgery 2020;87:303–11.

9. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298–306.


10. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186–91.


11. Katagiri H, Okada R, Takagi T, et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med 2014;3:1359–67.




12. Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine 2010;13:324–8.


13. Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;35:E1221–9.

14. Frankel HL. Ascending cord lesion in the early stages following spinal injury. Paraplegia 1969;7:111–8.



15. Tabouret E, Cauvin C, Fuentes S, et al. Reassessment of scoring systems and prognostic factors for metastatic spinal cord compression. Spine J 2015;15:944–50.


16. Ahmed AK, Goodwin CR, Heravi A, et al. Predicting survival for metastatic spine disease: a comparison of nine scoring systems. Spine J 2018;18:1804–14.


17. Kobayashi K, Ando K, Nakashima H, et al. Prognostic factors in the new Katagiri scoring system after palliative surgery for spinal metastasis. Spine (Phila Pa 1976) 2020;45:E813–9.


18. Candido PB, Peria FM, Pinheiro RP, Costa HR, Defino HL. Outcomes and survival of spinal metastasis with epidural compression. J Craniovertebr Junction Spine 2021;12:287–93.



19. Vanek P, Bradac O, Trebicky F, Saur K, de Lacy P, Benes V. Influence of the preoperative neurological status on survival after the surgical treatment of symptomatic spinal metastases with spinal cord compression. Spine (Phila Pa 1976) 2015;40:1824–30.

20. Amelot A, Balabaud L, Choi D, et al. Surgery for metastatic spine tumors in the elderly: advanced age is not a contraindication to surgery! Spine J 2017;17:759–67.


21. Quraishi NA, Arealis G, Salem KM, Purushothamdas S, Edwards KL, Boszczyk BM. The surgical management of metastatic spinal tumors based on an Epidural Spinal Cord Compression (ESCC) scale. Spine J 2015;15:1738–43.


22. Masuda K, Ebata K, Yasuhara Y, Enomoto A, Saito T. Outcomes and prognosis of neurological decompression and stabilization for spinal metastasis: is assessment with the spinal instability neoplastic score useful for predicting surgical results? Asian Spine J 2018;12:846–53.




23. Zadnik PL, Goodwin CR, Karami KJ, et al. Outcomes following surgical intervention for impending and gross instability caused by multiple myeloma in the spinal column. J Neurosurg Spine 2015;22:301–9.


24. Versteeg AL, Verlaan JJ, Sahgal A, et al. The spinal instability neoplastic score: impact on oncologic decision-making. Spine (Phila Pa 1976) 2016;41 Suppl 20:S231–7.

25. Wise JJ, Fischgrund JS, Herkowitz HN, Montgomery D, Kurz LT. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine (Phila Pa 1976) 1999;24:1943–51.


-
METRICS

-
- 0 Crossref
- Scopus
- 522 View
- 58 Download
- Related articles in ASJ
-
Incidence and Risk Factors for Implant Failure in Spinal Metastasis Surgery2020 December;14(6)
Prognostic Factors of Neurological Complications in Spinal Surgeries2018 August;12(4)