Single-Position Robotic-Assisted Prone Lateral Fusion: Technical Description and Feasibility
Article information
Abstract
Single-position lateral interbody fusion surgery has gained traction over the years because of reduced surgical time and improved operating theater workflow. With the introduction of robotics in spine surgery, surgeons can place pedicle screws with a high degree of accuracy and efficiency; moreover, the robot allows us to localize the disk space and perform endplate preparation accurately with minimal radiation. In this study, we discuss the potential synergistic benefits of integrating robotic-assisted spine surgery and single-position prone lateral surgery. We share our technique and provide the operative nuances of using the Mazor X Stealth Edition system (Medtronic, Minneapolis, MN, USA). We highlighted the potential synergistic benefits of integrating both the prone lateral and robotic-assisted surgical techniques, including the challenges encountered. This approach is not meant to replace other techniques or be used in all patients. Instead, it adds to our arsenal for managing spine fusion.
Introduction
Because of reduced surgical time, cost efficiency, and restoration of spinal alignment, single-position lateral interbody fusion surgery has gained attention over the years [1,2]. The benefits of robotic-assisted navigation in spine surgery go beyond more accurate pedicle screw placement. Moreover, it reduces proximal facet joint violation, decreases intraoperative radiation, and has low complication rates [3–5].
In this paper, we aimed to describe the surgical technique and operative nuances of performing single-position robotic-assisted prone lateral lumbar fusion using Mazor X Stealth Edition (Medtronic, Minneapolis, MN, USA). We highlight how this platform can help improve accuracy not only for screw trajectory but also for lateral cage placement, reducing operative time and radiation to the surgical team.
Technical Note
Our patient is a 64-year-old female with a history of lumbar spinal stenosis for which she underwent left lumbar 4 (L4) to lumbar 5 (L5) minimally invasive tubular decompression 4 years before the writing of this report. The patient presented to our clinic with mild low back pain but severe right L5 radicular pain and numbness. She could only ambulate for less than 10 minutes before stopping to relieve her pain. On examination, motor power and sensation were preserved.
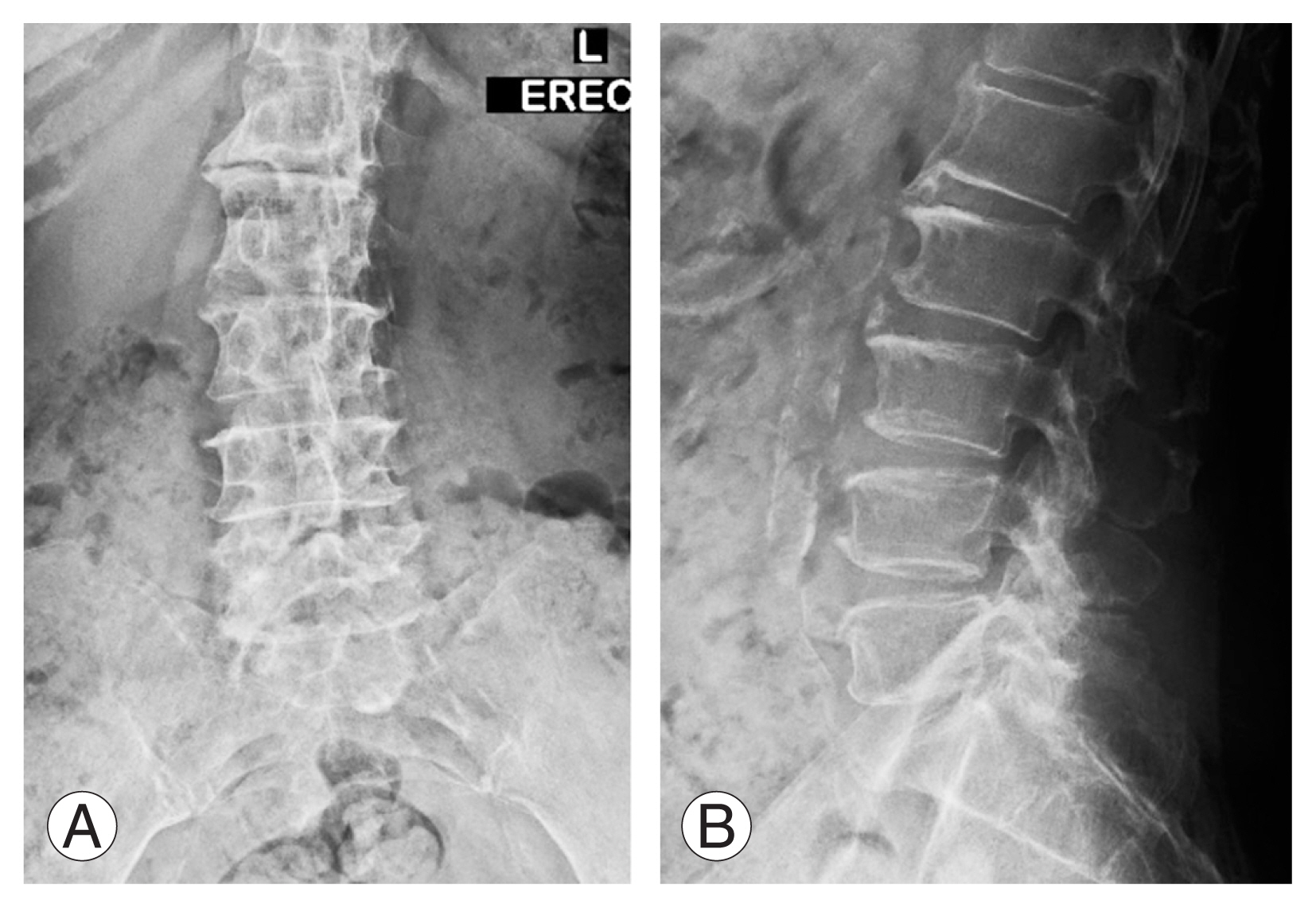
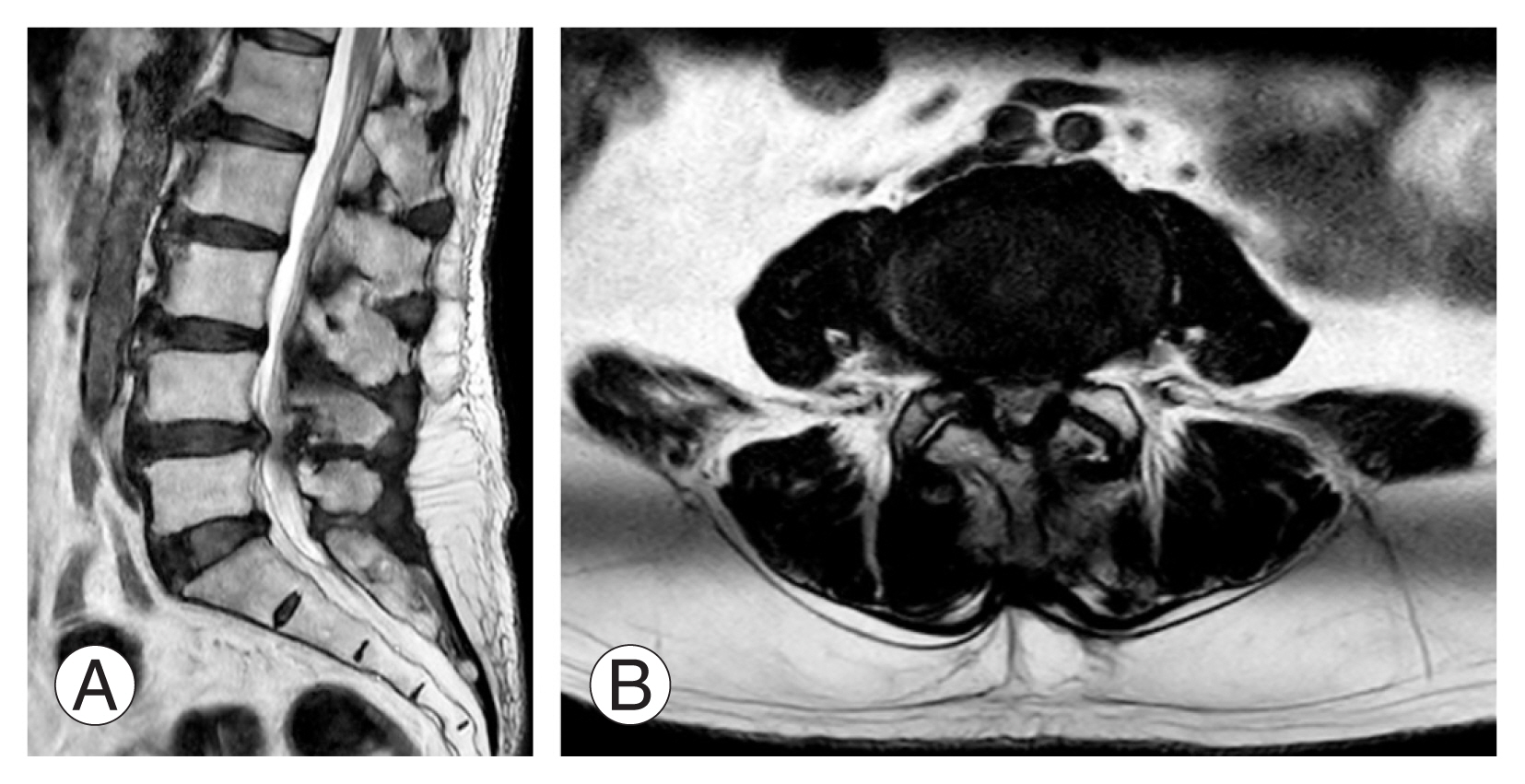
Lumbar spine X-ray showed lumbar spondylosis with mild degenerative scoliosis (Fig. 1). Flexion–extension views showed dynamic instability with grade 1 spondylolisthesis. Further evaluation using magnetic resonance imaging showed severe canal stenosis with compression of the cauda equina (Fig. 2). Furthermore, the case is suitable for lateral interbody fusion with a low iliac crest, psoas favorable, and good surgical corridor. The patient underwent single-position prone lateral interbody fusion of L4/5 and direct posterior decompression and instrumentation.
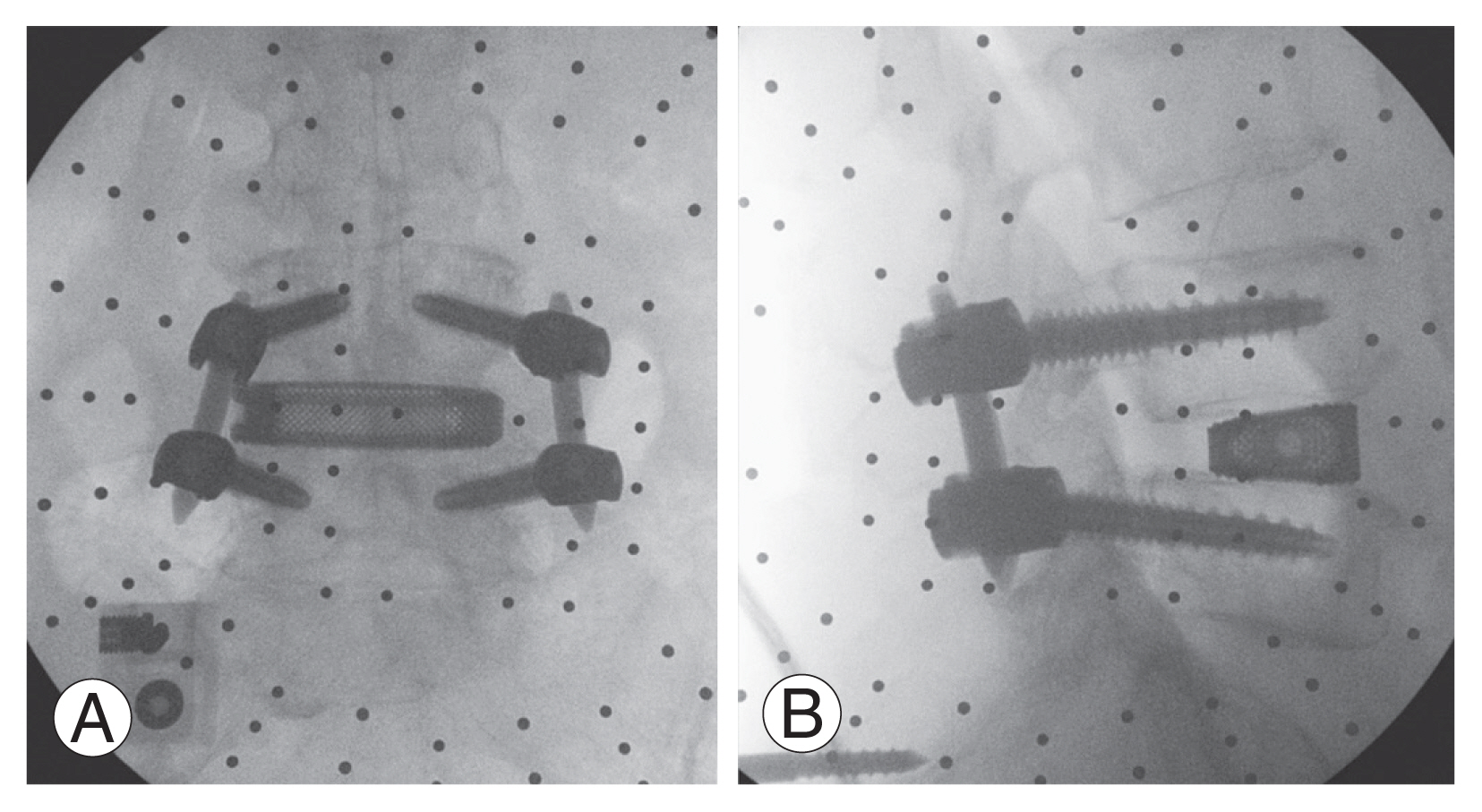
A computed tomography (CT) image was obtained before the operation and uploaded for planning. Preoperative templating of the sizes and trajectory of the screws and the oblique lateral interbody fusion cage was performed using the Mazor X Stealth Edition system. This preoperative templating could be performed at home or in the operating theater before the initiation of surgery. The Mazor X Stealth Edition system allows the segmentation of the spine into its spinal units, and thus, the screws can be accurately positioned even after cages are inserted (Fig. 3).

Mazor X Stealth preoperative planning of interbody cage size (A) and interbody cage trajectory (B, orange arrow).
The patient was placed in the prone position on a Jackson table with cushions over her chest, anterior superior iliac spine (ASIS), and thigh. The ASIS cushion should be placed at or slightly below the ASIS to ensure sufficient surgical field and better establishment of lumbar lordosis (Fig. 4). Fluoroscopic images were taken to confirm that the patient was placed in as true anteroposterior (AP) and lateral as possible. The Mazor robot was then mounted on the Jackson table.
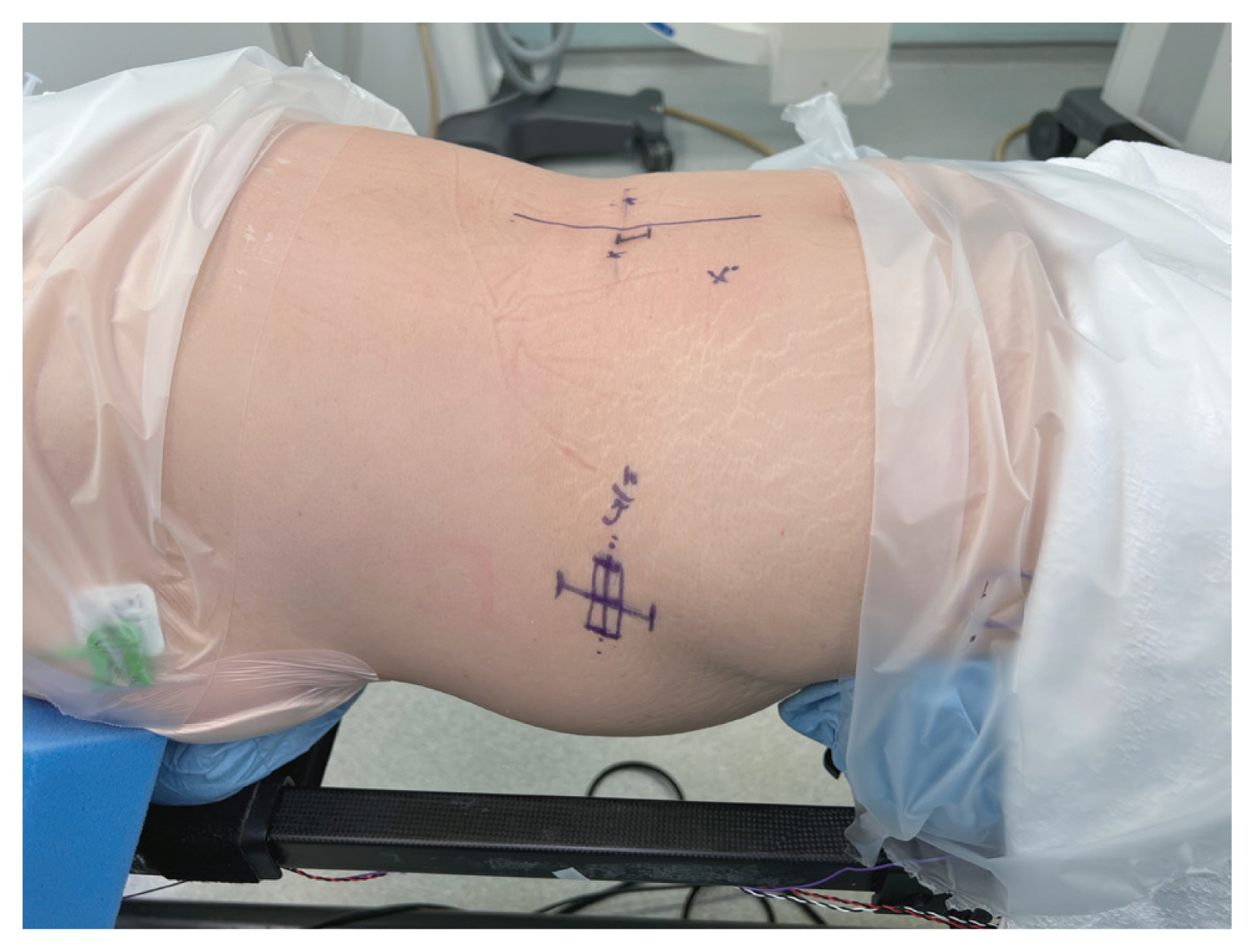
Hip pad at the level of anterior superior iliac spine and ensure sufficient surgical field. Preoperative neuro-localization of lumbar 4–5 disc space.
Neuro-localization of the L4/5 disk space was performed using a metal wire to mark the anterior, midportion, and posterior margins on the lateral view. The incision was centered over the midportion of the AP diameter of the disk space extending 1 cm proximal and 1 cm distal in an oblique fashion.
A posterior superior iliac spine pin was inserted, and the Mazor robot was connected to the patient. The robot performed registration and guided the marking of the screw entry points. We used a single longitudinal incision on the decompression side and stab incisions on the contralateral side. At the predetermined trajectory, the robotic arm guides the knife to perform stab incision down to the bone. This was followed by the Midas drill and tapping of the screw hole through the robotic sleeve using real-time robotic navigation. Guidewires were inserted into the screw holes, and the screws were inserted only after all screw holes were prepared. This reduces the risk of inaccuracy from the high torque generated during screw insertion.
A lateral incision was made, and the retroperitoneal space was entered. The retroperitoneal content was swept away using a gauze swab to expose the psoas muscle. The psoas muscle was exposed proximally and distally to avoid inadvertent entry into the peritoneal space. The equipment needed is shown sequentially in Fig. 5.
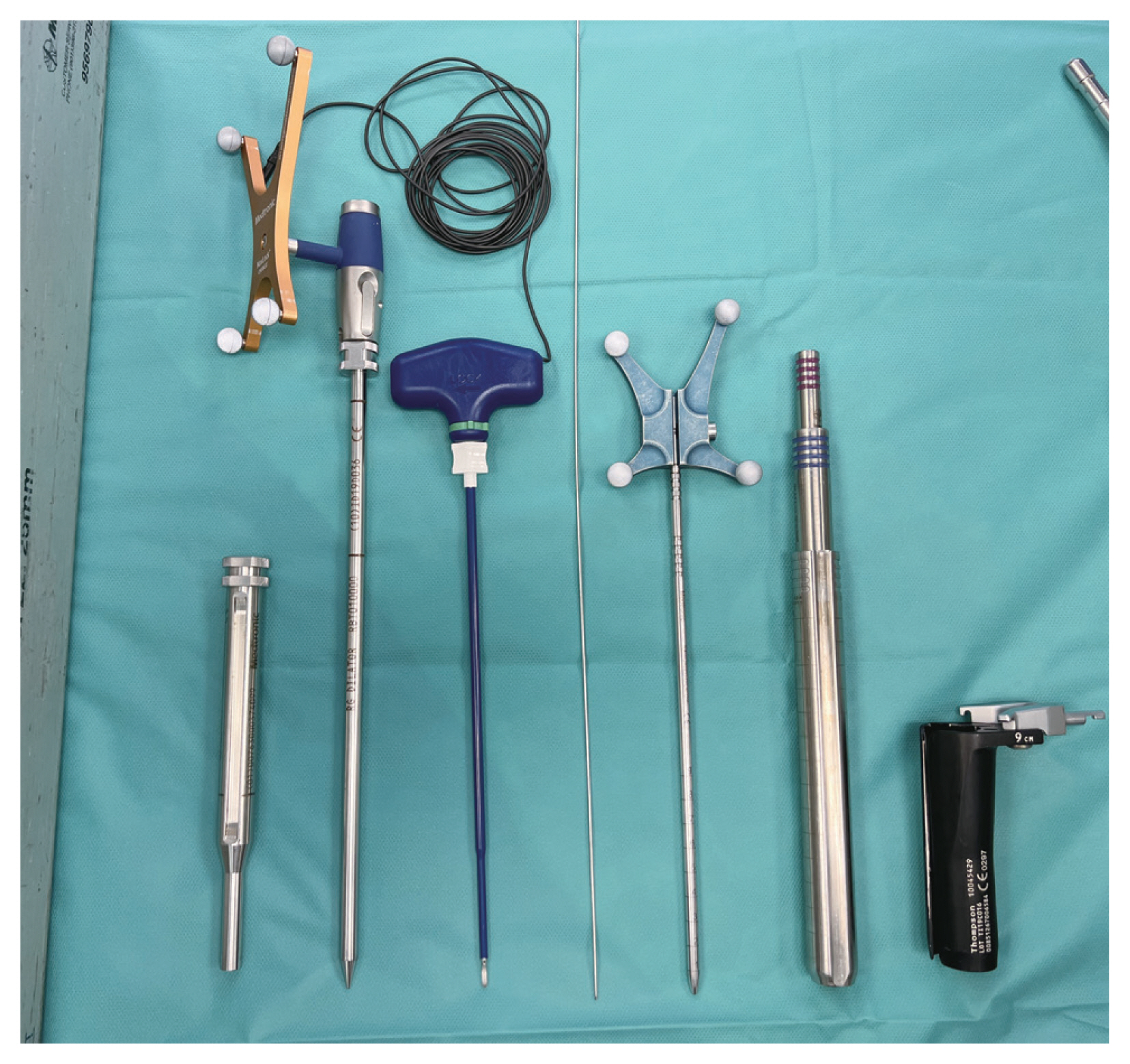
Equipment needed for prone lateral interbody fusion in sequential order from left to right: robotic arm guide cannula, navigated soft tissue dilator, NIM XPAK nerve stimulator, guidewire, navigated MAST dilator, sequential dilator sleeves, and round two-blade retractor.
Next, the robotic arm was moved into the surgical field for the disk space trajectory while the surgeon maintained retraction using a handheld retractor. The location of the disk space was marked using a navigated soft tissue dilator through the robotic arm guide cannula (Fig. 6). The nerve stimulator was then used to ensure that the lumbar plexus was not within the surgical field. Robot-guided sequential dilation of the psoas muscle was accomplished using a navigated MAST dilator. The nerve stimulator was used once again before clearing the soft tissue over the disk space with gentle bipolar electrocautery.
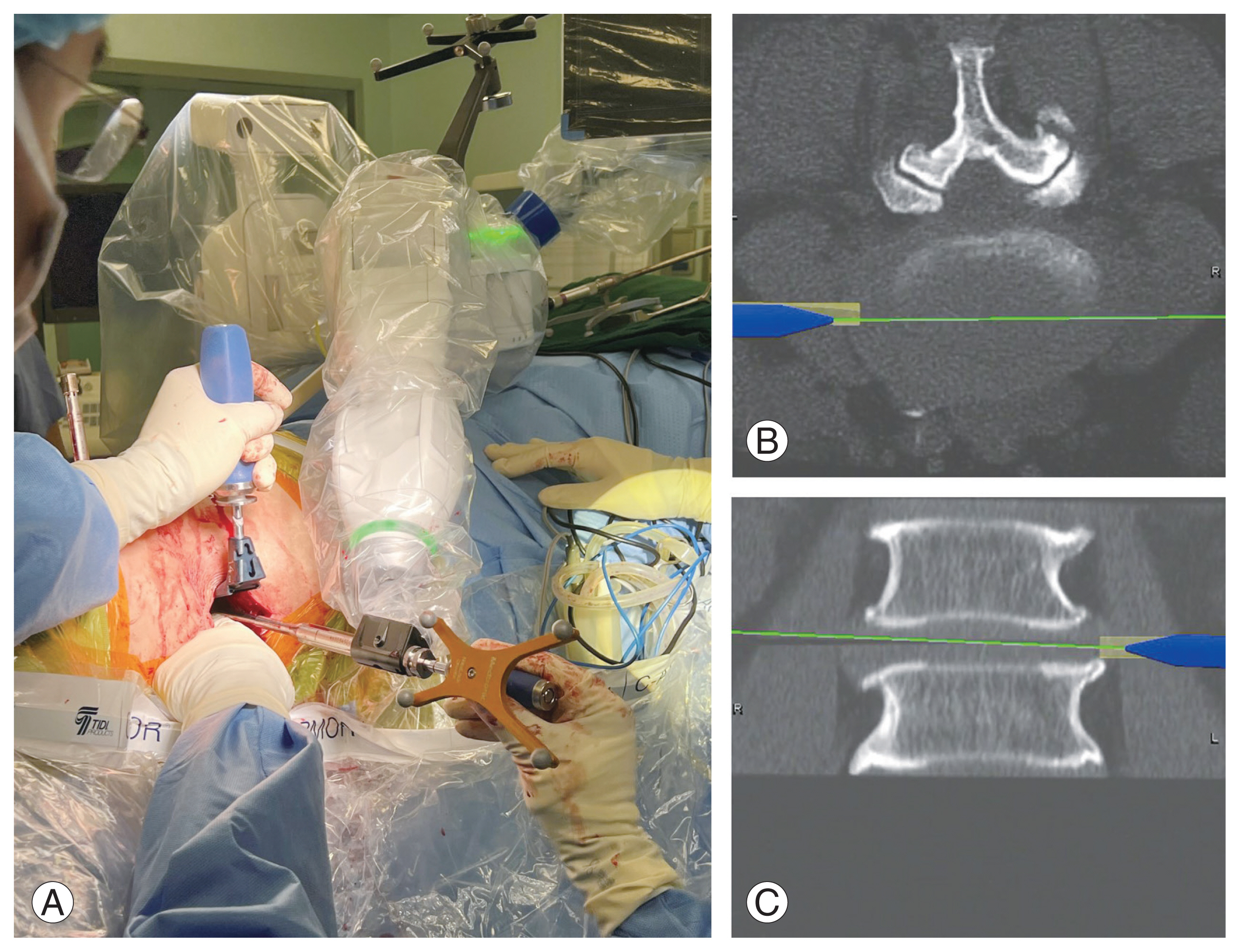
Neuro-localization of the disc space with navigator soft tissue dilator surgical field (A), robotic projection axial (B), and robotic projection coronal (C).
The stability of the flex arm can be improved by ensuring that the hinges are firmly supported by green towels on the patient’s body. A pin can be inserted through a retractor blade into the vertebral body to secure the blades of the retractor system because abdominal fat tends to push the dilators anteriorly. A flexible light source was attached to the blades to provide a direct illumination of the disk space.
With adequate soft tissue retraction and clear visualization of the disk space, standard annulotomy was performed. The Mazor system is compatible with Medtronic lateral access navigated instruments (Fig. 7). This allowed discectomy to be performed with minimal intraoperative fluoroscopy. Sequential trials were used to distract and open the disk space. The actual cage loaded with bone grafts was inserted in a press fit fashion under fluoroscopic guidance.
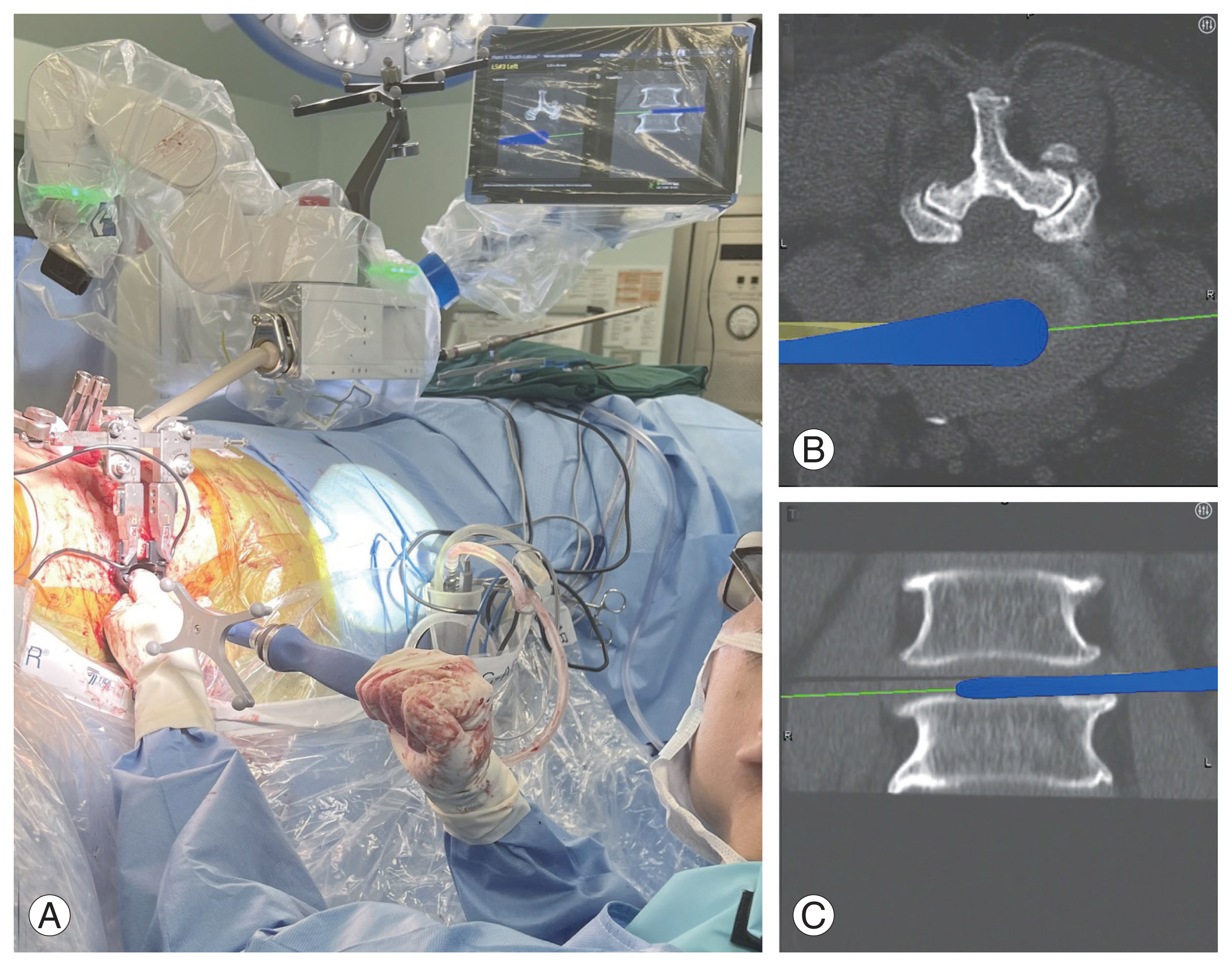
Disc space preparation using navigated cobbs’ operative field (A), robotic projection axial (B), and robotic projection coronal (C).
The assistant surgeon performed layered closure of the abdominal muscles. Concurrently, the main surgeon proceeded to decompress the spinal canal using the tubular retractor system, under navigation using the robotic stealth system (Fig. 8). This also reduced the amount of intraoperative radiation. After decompression, screws and rods were inserted in the usual fashion (Fig. 9).
Institutional Review Board (IRB) assessed, and IRB approval was not necessary for this study at our institution.
Discussion
Individually, robotic-assisted surgery and single-position prone lateral surgery have gained popularity because of their numerous advantages. However, the literature where both techniques are combined is limited. The potential benefits of integrating these technologies may provide synergistic benefits for minimally invasive spinal fusion surgery, as shown in the patient in this study.
The benefits of navigation and robotic surgery are as follows: greater precision, less soft tissue trauma, better screw positioning, and the ability to preplan interbody cage trajectory due to its ability to perform vertebral body segmentation [6]. The use of robots also significantly reduces radiation exposure because robotic arms can help us determine the ideal discectomy location, which was preplanned on CT. Furthermore, the robot navigation system allowed for radiation-free endplate preparation. Intraoperative fluoroscopy was mainly used to check cage size and positioning.
The benefits of single-position prone surgery are as follows: good restoration of lumbar lordosis, easy screw placement, and direct neural decompression [7]. Meanwhile, in single-position lateral surgery, the risk of lateral screw breaches is greater because of unfamiliarity and difficulty in direct neural decompression [8]. However, the drawbacks of single-position prone surgery are soft tissue retraction and disk space visualization during the lateral interbody procedure. Because truncal fat is being compressed and pushed into the surgical field, we recommend using longer retractors for access, pining the retractor to the vertebral body, and ensuring that the flex arm hinge is firmly rested on the green towels draped over the patient to stabilize the retractor. The use of a flexible light source attached directly to the retractor also improves visualization.
In conclusion, both robotic-assisted spine surgery and prone lateral surgery have gained interest over the past few years because of their numerous benefits in spinal fusion cases. We highlight the potential synergistic benefits of integrating both techniques, including the challenges encountered. This approach is not meant to replace other techniques or be used in all patients. Instead, it adds to our arsenal for managing spine fusion.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conception and design: JYL Oh. Writing–original draft: QY Yeo. Writing–review and editing: all authors. Final approval of the manuscript: all authors.