Innovative Developments in Lumbar Interbody Cage Materials and Design: A Comprehensive Narrative Review
Article information
Abstract
This review comprehensively examines the evolution and current state of interbody cage technology for lumbar interbody fusion (LIF). This review highlights the biomechanical and clinical implications of the transition from traditional static cage designs to advanced expandable variants for spinal surgery. The review begins by exploring the early developments in cage materials, highlighting the roles of titanium and polyetheretherketone in the advancement of LIF techniques. This review also discusses the strengths and limitations of these materials, leading to innovations in surface modifications and the introduction of novel materials, such as tantalum, as alternative materials. Advancements in three-dimensional printing and surface modification technologies form a significant part of this review, emphasizing the role of these technologies in enhancing the biomechanical compatibility and osseointegration of interbody cages. In addition, this review explores the increase in biodegradable and composite materials such as polylactic acid and polycaprolactone, addressing their potential to mitigate long-term implant-related complications. A critical evaluation of static and expandable cages is presented, including their respective clinical and radiological outcomes. While static cages have been a mainstay of LIF, expandable cages are noted for their adaptability to the patient’s anatomy, reducing complications such as cage subsidence. However, this review highlights the ongoing debate and the lack of conclusive evidence regarding the superiority of either cage type in terms of clinical outcomes. Finally, this review proposes future directions for cage technology, focusing on the integration of bioactive substances and multifunctional coatings and the development of patient-specific implants. These advancements aim to further enhance the efficacy, safety, and personalized approach of spinal fusion surgeries. Moreover, this review offers a nuanced understanding of the evolving landscape of cage technology in LIF and provides insights into current practices and future possibilities in spinal surgery.
Introduction
Lumbar interbody fusion (LIF) has emerged as a pivotal technique for managing various spinal pathologies, from degenerative disk disease to spondylolisthesis and spinal instabilities [1]. The clinical relevance of LIF lies in its ability to restore spinal alignment, relieve neurological symptoms, and provide long-term stability, representing a significant advancement in spinal surgery. The evolution of this procedure reflects the persistent search for optimal patient outcomes by balancing surgical invasiveness with efficacy [2].
The choice of interbody cages, which have evolved based on the advancement of biomaterial science, has significantly influenced the efficacy of LIF. Various biomaterials are used in interbody cage development, from traditional materials such as titanium (Ti) and polyetheretherketone (PEEK) to newer materials such as tantalum. Three-dimensional (3D) printing technologies and surface modifications using plasma-spraying technology have taken interbody cage development to the next level [3]. These developments highlight the synergistic relationship between surgical techniques and biomaterials science, which is crucial for improving LIF outcomes. More recently, the introduction of biodegradable materials and the development of the expandable cage technique have further expanded the world of interbody cages.
Despite extensive research and clinical applications of various cages in LIF, gaps remain in understanding the comprehensive effect of cage design and material on patient outcomes. Previous studies have often focused on isolated aspects of cage performance, such as subsidence rates or fusion efficacy, without a holistic view of how these factors interact with overall spinal biomechanics and long-term outcomes [4]. In addition, no consensus has been reached regarding the optimal cage type for specific clinical scenarios, highlighting the need for a more nuanced understanding. This review aimed to bridge these gaps by providing a comprehensive overview of the evolution of cage designs and materials in LIF, critically evaluating their clinical implications, and identifying areas for future research and innovation.
Evolution of Cage Materials in Lumbar Interbody Fusion
1. Early developments and traditional materials
1) Titanium
The evolution of LIF cages began with the development of simple materials and techniques. Earlier cages, primarily composed of stainless steel and Ti, were designed to provide mechanical stability and facilitate bone grafting procedures [5]. Despite challenges such as stress shielding and radiopacity, these materials were chosen for their strength and biocompatibility. The use of cages in spinal procedures was pioneered in the early 1980s, marking a significant shift from traditional bone grafting methods [6,7].
Then, Ti and its alloys became the primary choice for cage fabrication because of their favorable properties, including biocompatibility and ability to promote bone ingrowth. Ti6Al4V is typically chosen for interbody cage production because of its strength, corrosion resistance, low density, biocompatibility, cost-effectiveness, and compatibility with magnetic resonance imaging [8–10]. To improve fusion rates and reduce complications, such as cage migration or subsidence, advancements in the design and application of Ti cages in the 1990s and early 2000s led to various configurations, such as cylindrical and box-shaped designs [7,11].
Despite its widespread use, Ti presents certain challenges. For example, the mismatch in the elastic modulus between Ti cages and native bone leads to concerns about stress shielding, potentially affecting long-term implant stability and integration. Seaman et al. [12] highlighted that the high elastic modulus of Ti6Al4V can lead to cage subsidence and loss of disk height restoration. In addition, the radiopaque nature of Ti hinders the precise assessment of fusion progression using imaging techniques, prompting the exploration of alternative materials [13]. Recent advancements in 3D printing and surface treatment technologies have enabled the creation of 3D-printed Ti interbody devices with elastic moduli comparable with those of the native bone [14–16].
2) Polyetheretherketone
The introduction of PEEK has significantly altered the use of cage materials for lumbar fusion surgery. PEEK is known for its biomechanical compatibility with bone, characterized by an elastic modulus that closely mirrors that of cortical bone and radiolucency, which facilitates postoperative imaging [17–19]. Clinical comparisons between PEEK and Ti cages have yielded inconclusive results regarding superiority, with each material exhibiting distinct advantages and disadvantages [12,20]. Compared with Ti alloys, PEEK reduces stress shielding and bone resorption, mitigating implant loosening risks [21,22]. In a meta-analysis, Seaman et al. [12] in 2017 revealed comparable fusion rates between Ti and PEEK interbody cages but highlighted a 3.59-fold higher subsidence likelihood with Ti. Consequently, from the perspective of subsidence and stress shielding, PEEK has advantages over Ti.
However, the hydrophobic characteristics and bioinertness of PEEK may impede its osteointegration [23]. Furthermore, biofilm formation on PEEK cage surfaces impedes binding to the host bone, thereby hindering solid fusion [24]. PEEK cages have also been associated with local inflammation, leading to complications such as bone nonunion and osteolysis [20,25,26]. Efforts to address these shortcomings have led to surface modification of PEEK cages to enhance bioactivity [27–29].
3) Tantalum
Tantalum is increasingly used in orthopedics because of its excellent histocompatibility and corrosion resistance and shows promise as an interbody fusion cage biomaterial [30–32]. Tantalum and its derivatives are superior to Ti and its alloys in terms of mechanical strength, corrosion resistance, and biocompatibility [33–37]. Tantalum exhibits superior osseointegration and antibacterial properties. Among its derivatives, porous tantalum has garnered considerable interest because of its elastic modulus and porous architecture, which closely resembles cancellous bone [38]. Currently, tantalum and its derivatives are effectively employed in artificial joint replacements [39], treatment of femoral head necrosis [40], and dental material applications [41], benefiting various patients. In spinal surgery, the application of tantalum extends to the treatment of infectious bone defects and anterior cervical discectomy and fusion (Fig. 1) [42–46].
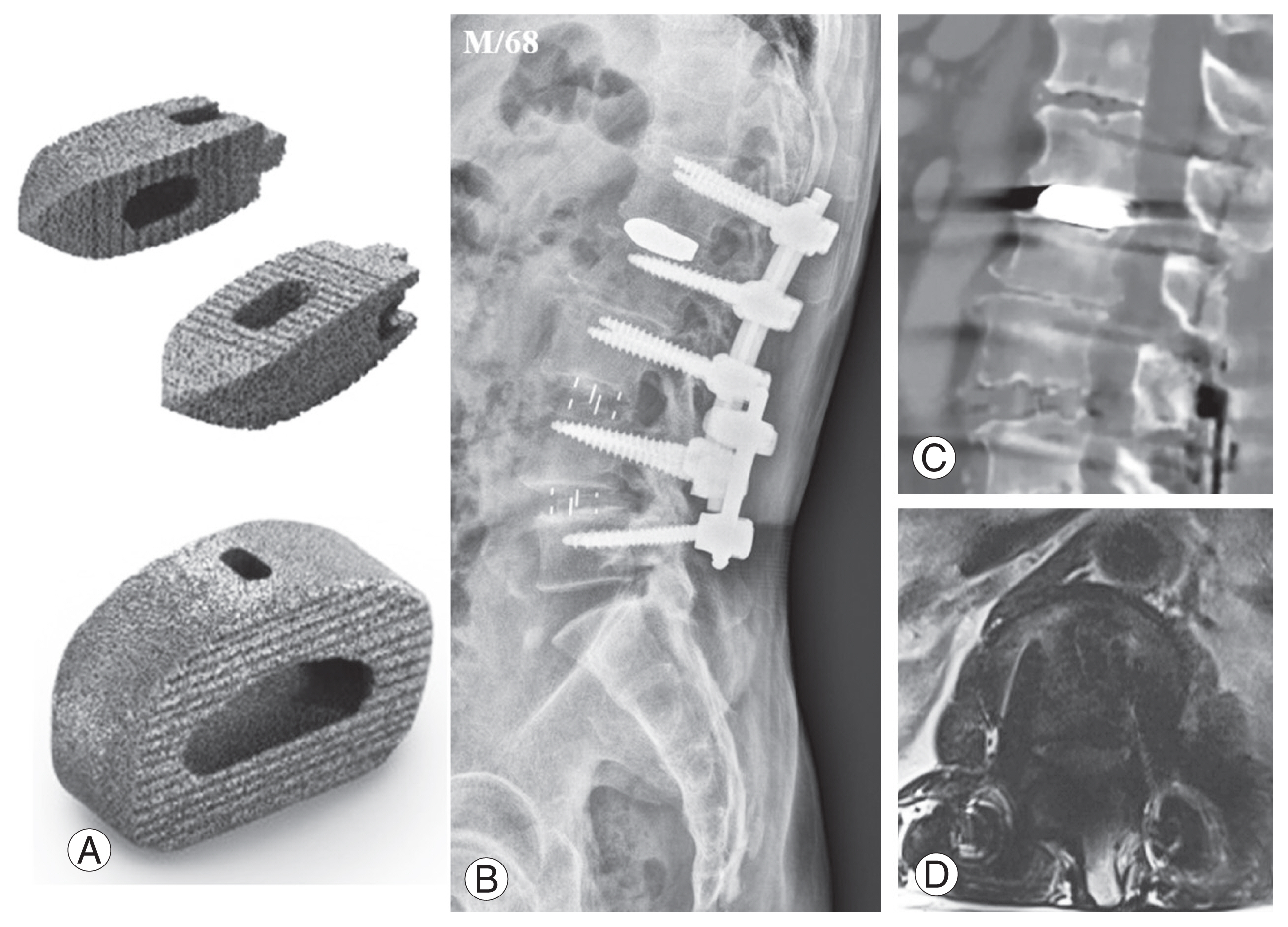
(A–D) Examples of tantalum cages. Representative cases illustrate the application of tantalum cages, such as in a 68-year-old male patient where a tantalum cage was placed in the L1–2 intervertebral space, resulting in artifact generation on postoperative computed tomography and magnetic resonance imaging.
Results from various clinical studies have demonstrated that porous tantalum cages (PTCs) are effective and safe for spinal surgery, offering several advantages. In anterior LIF (ALIF), PTCs significantly improve lumbar lordosis (LL), reduce back pain, and enhance patients’ quality of life without major complications [47]. Thoracolumbar burst fractures provide superior sagittal profile restoration compared with iliac crest bone grafts, with a lower tendency for correction loss over time [48]. Thus, PTCs could be a viable alternative to autologous bone grafting, potentially avoiding donor-site morbidity. Furthermore, in posterior LIF (PLIF), PTCs show promising results in early bone integration and stability, as indicated by computed tomography (CT) findings of trabecular bone remodeling and lower incidences of vertebral endplate cyst formation compared with Ti-coated PEEK cages [49]. Collectively, these studies have suggested that PTCs can achieve immediate stabilization, facilitate bone fusion, and improve long-term outcomes in spinal surgery.
2. Advancements in 3D printing and surface modification
1) 3D-printing technology
The introduction of 3D-printing technology in spinal cage production marks a pivotal development, allowing the creation of patient-specific implants with intricate, customizable porous structures [50]. It has opened new avenues for designing cages that promote bone ingrowth and vascularization, potentially optimizing the fusion process [51,52]. By using materials such as Ti, 3D-printed structures offer a harmonious blend of mechanical resilience and biological functionality, demonstrating the potential to enhance osseointegration and reduce the risk of non-device-related reoperation [53–55]. For example, 3D printing features an elastic modulus closely matching that of the native bone, whereas a conventional Ti alloy cage has an approximately 10-fold higher elastic modulus [14–16].
The biomechanical superiority of these 3D-printed cages has led to favorable results in previous clinical studies. Adl Amini et al. [56] showed that 3D-printed Ti cages exhibited a significantly lower early subsidence rate than PEEK cages in patients with standalone lateral LIF (LLIF). Corso et al. [54] analyzed 186 patients (50.5% male; mean age, 59.2±12.5 years) with a minimum follow-up of 6 months. Of these, 96 were treated with 3D-printed Ti implants and 90 with PEEK across 186 implant levels, of which 51.6% used 3D-printed Ti implants [54]. They concluded that in terms of non-device-related reoperation events, 3D-printed Ti cages demonstrated a minimal risk profile compared with traditional non-3D-printed cages. Yang et al. [57] reviewed 150 patients who underwent 1- to 2-level PLIF with a minimum follow-up of 2 years. Compared with PEEK cages, 3D-printed Ti cages achieved significantly higher fusion rates at both postoperative 1 (3D-printed Ti, 86.9%; PEEK, 67.7%; p=0.002) and 2 (3D-printed Ti, 92.9%; PEEK, 82.3%; p=0.037) years [57]. No significant difference was found in the subsidence rates between the two materials. These results suggest that 3D-printed Ti cages are a viable and safe option for PLIF because they provide a stable construct.
2) Surface modifications
The surface properties of interbody cages significantly affect osteointegration. Enhanced surface porosity promotes osteointegration by increasing the surface area and incorporating osteogenic and angiogenic factors such as bone morphogenetic protein-2 (BMP-2) [58]. Previous studies have demonstrated the clinical and radiological advantages of these surface-modified interbody cages. Guyer et al. [59] found that porous Ti exhibited a stronger implant–bone interface than conventional PEEK and allografts, indicating its superior potential for osseointegration and faster achievement of spinal fusion stability.
For porous PEEK cages, Torstrick et al. [60] examined the effects of porosity and pore size on cellular responses to PEEK using micro-CT analysis. They discovered that porous PEEK exhibited increased cell proliferation and cell-mediated mineralization compared with smooth PEEK and Ti [60]. Furthermore, to address PEEK’s inherent hydrophobicity and bioinertness, surface modifications incorporating materials such as hydroxyapatite (HA), calcium silicate (CS), and Ti have been explored to augment PEEK’s bioactivity [27–29,61]. Sun et al. [61] investigated the integration of soft tissues with HA/PEEK composite scaffolds. Although the overall bonding strength was influenced mainly by pore size rather than by HA content, HA helped enhance the firm adhesion of soft tissue to PEEK-based composites, a key factor in preventing postoperative effusion [61].
For CS/PEEK cages, Chu et al. [21] used in a goat cervical interbody fusion model and demonstrated that CS/PEEK cages outperformed pure PEEK cages in terms of fusion strength at 12 and 26 weeks in an X-ray analysis. Micro-CT revealed greater new bone ingrowth with CS/PEEK cages, achieving near-complete fusion at 26 weeks. Spine kinematics assays confirmed that these cages also exhibited superior mechanical stability and stiffness. Histological evaluations have highlighted rapid osseointegration and bone formation around CS/PEEK cages [21].
Zhu et al. [62] reported that PEEK cages with Ti and HA coatings, in contrast to uncoated PEEK cages, achieved a significantly higher fusion rates 3 months after single-level transforaminal LIF (TLIF). Two recent meta-analyses comparing Ti-coated PEEK cages with uncoated PEEK cages in lumbar fusion surgeries revealed comparable effects on bone fusion and cage subsidence across all follow-up periods, indicating no significant differences in patient-reported outcomes [27,28]. However, Ti-coated PEEK cages offer the combined benefits of Ti and PEEK: an elastic modulus akin to that of human cortical bone, enhanced osteoid cell growth, and increased cell adhesion space.
According to Torstrick et al. [63], the microstructure of surface-coated PEEK, including its pore morphology, can be precisely manipulated by varying the size of the sodium chloride crystals, with pores adopting the cubic shape of the porogen. Their findings suggested that introducing a porous surface layer to polymeric implants can enhance clinical outcomes while preserving a sufficient load-bearing capacity [63]. Concerns are raised regarding the durability and impaction resistance of the coatings mainly because of substantial impact forces encountered during cage insertion into the intervertebral space. Torstrick et al. [60] also showed that while porous PEEK devices sustained minimal damage during aggressive cervical impaction, Ti-coated PEEK devices experienced a significant loss in their initial Ti coverage [60].
3. Biodegradable and composite materials
Recent advancements have also led to the use of biodegradable materials, such as polylactic acid (PLA) and polycaprolactone (PCL), in the fabrication of spinal cages. These materials are designed to degrade over time and are ideally replaced by natural bone, thus mitigating long-term complications associated with permanent implants [64,65]. Although initial applications face challenges related to mechanical integrity and controlled degradation, recent iterations have shown promising results. This is evident when these materials are used in conjunction with osteoconductive or osteoinductive substances to enhance spinal fusion [66,67]. The evolution of biodegradable cages continues to be a central theme in spinal surgery research, with a focus on optimizing their composition and structure to improve clinical outcomes. Given their ability to reduce long-term complications associated with traditional implants, biodegradable materials such as PLA and PCL are at the forefront of this innovation [3,64,65,68].
1) Polylactic acid
FDA-approved polyesters PLA and PCL were used as primary polymers. The formation of block copolymers such as poly L-lactic acid (PLLA), poly-D, L-lactic acid, and poly(lactic-co-glycolic acid) (PLGA) is achieved through the covalent bonding of different polymer units. Among these, aliphatic polyesters, particularly PLAs, are the most promising [69–71]. Previous studies have confirmed the biocompatibility of PLA with dural and neural tissues. More studies have indicated that PLA has no detrimental effects on neuronal cells or pH alterations during PLA implant degradation at the implantation site [72–75].
Despite their theoretical advantages, a systematic review focused on biodegradable implants, predominantly polylactides, and their comparison with conventional implants showed that the routine clinical application of absorbable cages lacks sufficient support primarily because of unfavorable long-term fusion rates [76]. The inferior clinical outcomes of biodegradable cages are hypothesized to arise from early degradation and loss of strength, leading to osteolysis and accelerated cage subsidence [77,78].
2) Polycaprolactone
Compared with PLA, which is a bulk-degrading polymer [79], PCL is bioerodible and maintains its initial elastic modulus and 95% mass for up to 12 months [80]. Owing to its superior rheological and viscoelastic properties to other aliphatic polyesters such as PLLA, poly-L-lactide-co-d, and L-lactide acid [81], PCL is a promising candidate for designing slow-degrading implants mainly because of its favorable melt extrusion properties. PCL is distinguished by its superior physicochemical properties, such as structural stability [82], flexibility [83], biocompatibility [84], and biodegradability [85]. In vivo, PCL demonstrates slow degradation, with virtually no molecular-weight changes observed after 6 months [86]. It exhibits greater resistance to degradation in biofluids than other polymers, and its low cost and accessibility add to its advantages [87,88]. PCL enhances cell viability and migration more effectively than rapidly degradable PLGA-3D scaffolds, as demonstrated in in vitro and in vivo studies [89]. Coinciding with advancements in additive biomanufacturing, PCL has gained prominence and become increasingly preferred for fabricating biodegradable cages for spinal fusion.
In large preclinical animal studies, a composite of PCL with ceramics, specifically calcium phosphate (CaP), has emerged as the optimal biomaterial for osseous healing in critical-size tibial defects [90,91]. This combination results in composite biomaterials with improved mechanical properties, controlled degradation rates, and enhanced bioactivity, making them well suited for bone tissue engineering applications [92,93]. Bioactive and bioresorbable scaffolds, made from medical-grade PCL incorporated with 20% β-tricalcium phosphate (TCP) and bioresorbable PCL scaffolds coated with a biomimetic CaP layer plus recombinant human BMP-2 (rhBMP-2), have been effectively used to achieve interbody spinal fusion in both lumbar porcine and thoracic ovine models [66,94]. According to Li et al. [95], autograft-free biodegradable PCL–TCP composite scaffolds facilitated bone tissue ingrowth and maintained mechanical load-bearing capacity after implantation, achieving a spinal fusion efficacy comparable to that of Ti cages with autografts in sheep anterior cervical discectomy and fusion surgeries. Similar to PLA, PCL faces the challenge of inferior mechanical properties compared with permanent materials such as Ti and PEEK. This performance gap becomes more evident as degradation occurs, potentially resulting in reduced stability over time.
3) Future of biodegradable materials
The final goal is to develop cages that offer the best strength and durability with eventual resorption and replacement by natural bone. The key focus areas include addressing issues such as premature degradation and ensuring adequate mechanical support during the critical bone healing and fusion period. Research is geared toward developing materials with optimized degradation rates, improved mechanical strength, and enhanced bioactivity to support the spine until complete osseointegration is achieved. Mechanically, improving the stiffness of PCL scaffolds can be achieved by increasing their mineral content, particularly with HA. According to Shor et al. [96], adding 25% HA to a composite resulted in a 40% increase in the compressive modulus. Furthermore, the stiffness of the PCL/HA mixture increased proportionally with the HA content [97].
Unmodified PCL surfaces exhibited limited cell adhesion, attachment, proliferation, and bioactivity. The use of nano-HA coatings, a type of CaP with a composition and crystal structure akin to human bone, may enhance cytocompatibility [98]. Yong et al. [99] indicated that a CaP-coated PCL-based scaffold with 0.54 μg of rhBMP-2 is as effective as an autograft from the rib head. This generated a conducive environment for thoracic interbody spinal fusion in a sheep thoracic spine model [99]. Recently, Duarte et al. [100] showcased a novel biopolymer of PCL doped with polydopamine and polymethacrylic acid, which, when foamed directly into a bone defect through a specialized high-pressure portable device, achieved immediate stabilization of osseous components. This technique yielded a 3D structure with morphological properties similar to those of the trabecular bone, showing significant potential for instrumentation-free interbody fusion.
4. Static vs. expandable cages in LIF
1) Static cages
Static cages, which are predominantly used in LIF, are pivotal in addressing degenerative spinal disorders [101,102]. The evolution of interbody fusion cages from the earliest threaded BAK designs to the current Ti or PEEK cages has led to shapes that more closely resemble intervertebral space. This design shift offers larger cancellous bone-filling spaces, increased fusion area, enhanced load-bearing capacity, and improved stability. These cages, characterized by their fixed shape and size, are designed for strength and ease of insertion, which are crucial elements in lumbar surgery. Their simple and robust design provides reliable support to the spinal segment, ensuring a consistent approach for various lumbar pathologies [20,103–105].
Recently, physicians and patients has placed a growing emphasis on minimally invasive surgical techniques for implanting the largest feasible intervertebral implant through the smallest possible incision with minimal surgical exposure. Compared with the posterior approach, the anterior approach facilitates the use of larger bone cages and grafts, demonstrating enhanced deformity correction capabilities and superior initial stability [106–108]. Significant advancements in surgical methods and instrumentation for ALIF and LLIF have been observed in the last 50 years. Critical factors such as cage dimensions, including width, length, height, and contact surface area, are pivotal in maximizing surface contact and ensuring ALIF and LLIF stability [102]. Radiologically, static cages have been instrumental in achieving the desired outcomes in spinal surgeries. Studies have indicated their efficacy in restoring and maintaining segmental lordosis (SL) and disk height, which are critical for preserving the natural curvature and biomechanics of the spine [109–111].
Recent developments in endoscopy-assisted spine fusion surgeries have demonstrated clinical and radiological outcomes comparable to those of conventional open surgery [112–115], emphasizing the need for specialized cage designs suitable for minimal incision techniques. Recently, Kim et al. [116] demonstrated the feasibility of using a larger cage originally designed for LLIF in biportal endoscopic TLIF to achieve a favorable fusion rate (Fig. 2). With the increasing adoption of minimally invasive techniques, technological advancements have led to the development of interbody devices designed to expand after placement.
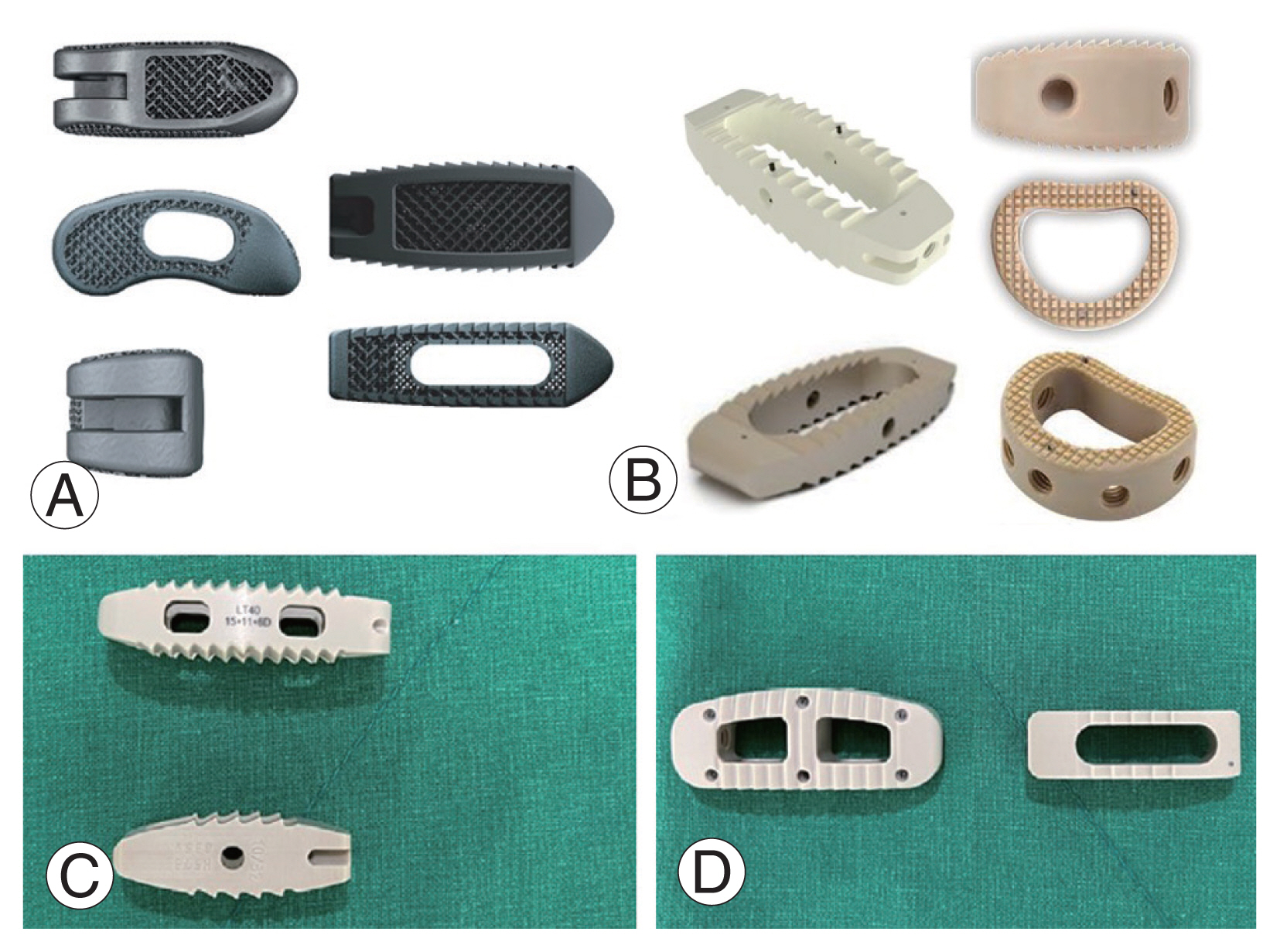
The lumbar interbody cages vary in design and size. (A) A titanium cage suitable for transforaminal lumbar interbody fusion (LIF) and posterior LIF. (B) A larger polyetheretherketone cage designed for oblique LIF, lateral LIF, or anterior LIF. Views (C, D) present lateral and axial perspectives of two distinct cages. The larger cage measures 15 mm in width and 40 mm in length, making it suitable for endoscopic transforaminal lumbar interbody fusion, while the smaller cage’s dimensions are 10 mm by 32 mm. From Kim JE, et al. World Neurosurg 2023;178:e666–72 [116], with permission from the authors.
2) Expandable cages
Compared with static devices, expandable cages have a minimal profile and can be expanded in situ to reduce iatrogenic endplate damage during cage insertion [117]. The cages were designed to adjust their size and shape to conform to the unique anatomical needs of the patient’s intervertebral space. Their ability to expand after insertion allows for a customized fit and enhanced spinal stabilization, significantly evolving from traditional static cage designs.
Expandable cages can be used for TLIF, ALIF, and LLIF [118]. Although expandable cages are initially implemented in TLIF [119], this procedure can be limited to cases with extensive scarring and high-grade spondylolisthesis [4]. Meanwhile, ALIF and LLIF allow the insertion of wide and large interbody cages, resulting in a greater endplate contact surface than TLIF cages [111]. However, the implantation of such large cages often requires strong impaction when static cages are used. By contrast, expandable LLIF cages obviate the need for the forceful impaction associated with static spacers, thereby potentially reducing the risk of cage subsidence [118].
Radiologically, the use of expandable cages in lumbar fusion has yielded promising results. Expandable cages have been reported to yield superior disk height increments and SL restorations in patients who had undergone lumbar fusion compared with static cages [120–124]. A study indicated that these cages effectively maintain or improve SL and disk height, which are critical factors in achieving optimal spinal alignment and biomechanics after surgery. Recent meta-analyses indicate that the design of expandable cages plays a key role in reducing the incidence of cage subsidence in lateral interbody fusion, a frequent complication in lateral lumbar surgeries, helping to maintain the structural integrity of the fused spinal segment (Fig. 3) [125].

(A) A 71-year-old female patient underwent L4–5 oblique lumbar interbody fusion (OLIF) with a static polyetheretherketone cage and exhibited cage subsidence in the 3-month postoperative follow-up X-ray. (B) A 75-year-old female patient received L4–5 OLIF with an expandable cage and has sustained proper alignment without any signs of cage subsidence for 3 months.
3) Comparative studies and current evidence
Whether expandable cages are associated with improved clinical outcomes in patients with lumbar fusion compared with static cages remains unclear [125–128]. Three recent meta-analyses assessing the clinical outcomes of expandable cages in TLIF revealed no significant differences in Visual Analog Scale scores for back and leg pain, Oswestry Disability Index (ODI), and fusion rates between static and expandable cages [126–128]. Another meta-analysis evaluated the clinical outcomes of expandable cages in both TLIF and PLIF and found no significant differences in ODI, fusion rates, LL, blood loss, and operation time when comparing static with expandable cages [125]. However, the aforementioned meta-analysis documented the role of expandable cages in reducing operative time and intraoperative blood loss, thereby contributing to faster patient recovery and reduced hospital stays [126]. These findings reveal the potential of expandable cages to enhance patient comfort and accelerate postsurgical rehabilitation and recovery.
Regarding radiological outcomes, expandable cages can achieve superior disk height increments and SL restoration in patients who had undergone lumbar fusion compared with static cages [120–124,126,127]. However, a meta-analysis on the radiological outcomes of TLIF revealed no statistically significant differences in spinal sagittal alignment (SL and LL) or pelvic parameters [127]. Concurrently, expandable cages have been linked to a reduced incidence of subsidence [121–123,129,130]. This reduction may be attributed to their capacity to attain a tailored fit within the intervertebral space. However, two recent meta-analyses focusing on expandable TLIF cages did not demonstrate any significant difference in cage subsidence between static and expandable cages [126,127]. Frisch et al. [117] and Li et al. [131,132] reported that expandable LLIF cages resulted in an expandable group with a significantly lower subsidence rate. They also reported increased postoperative disk space measurements compared with preoperative levels, noting a statistically more significant change in static than in expandable cages [117,131,132]. This difference may be due to the overdistraction required for static cage insertion. Consequently, an expandable LLIF cage that avoids forceful insertion may help prevent subsidence. More studies are needed to determine whether expandable cages exhibit variability in their subsidence prevention efficacy based on the surgical technique employed and understand the underlying reasons for such differences.
The association between expandable cages and improved clinical outcomes compared with fixed cages in patients who had undergone lumbar fusion remains uncertain. Expandable cages have several advantages in certain aspects. Therefore, choosing between static and expandable cages should be based on patient-specific factors and surgical objectives. Surgeons must weigh these findings against individual patient needs, surgical goals, and specific pathology being addressed to choose the most appropriate interbody device.
4) Future directions in cage technology for LIF
As technology continues to evolve, future studies should explore the integration of bioactive substances into 3D-printed cages. Embedding growth factors or osteoinductive materials within the scaffold structure may further promote bone growth and fusion [133,134]. In addition, ongoing advancements in materials science may introduce new biocompatible materials that enhance the functionality of 3D-printed cages. The combination of a customizable design and improved material properties and integration of bioactive agents are poised to significantly advance the efficacy and safety of LIF procedures, paving the way for more personalized and effective spinal treatments.
Research has increasingly focused on multifunctional coatings that combine osteoinductive properties with antibacterial capabilities. The development of dual-function coatings could revolutionize LIF procedures by enhancing bone growth and reducing risks [135]. Future studies must explore the incorporation of novel materials and bioactive agents into these coatings, potentially leading to even greater improvements in clinical outcomes. As this field evolves, the focus will likely shift toward customizing coatings based on specific patient needs and surgical context, further personalizing LIF treatments and improving patient-specific outcomes.
Continuous innovations in material science and technology are likely to shape the future of LIF. A study focused on developing materials that directly deliver targeted therapeutic agents, such as growth factors or antibiotics, to the fusion site [20]. In addition, the exploration of personalized implants tailored to each patient’s specific anatomical and pathological conditions represents a significant advancement in patient-specific care. These emerging materials and technologies can significantly improve the efficacy, safety, and patient outcomes of spinal fusion surgeries, thus marking a new era for treating spinal disorders.
Conclusions
The dynamic evolution of cage technology in LIF represents a significant advancement in the management of spinal disorders, offering spine surgeons diverse tools tailored to optimize patient outcomes. The transition from the use of traditional materials to the utilization of innovative synthetic, biodegradable, and composite materials reflects a deeper understanding of biomechanics and materials science. Advancements in 3D printing and customizable solutions have ushered in an era of patient-specific implants, ensuring a closer match between anatomical and pathological conditions. Investigations on surface modifications, bioactive coatings, and emerging materials such as smart biomaterials signifies a paradigm shift toward implants that support structural integrity and actively participate in the biological healing process. Moreover, the development of static and expandable cages, each with distinct clinical and radiological outcomes, highlights the importance of personalized treatment strategies for spinal surgery. These technological advancements integrated with clinical expertise can significantly enhance the efficacy, safety, and overall success of spinal fusion procedures, marking a pivotal step forward in orthopedic surgery.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: SYC, DHK; data curation: SYC, DHK; formal analysis: SYC, DHK; methodology: SYC, DHK; project administration: SYC, DHK; visualization: SYC, DHK; writing–original draft: SYC, SC, DHK; writing–review & editing: SYC, SC, DHK; and final approval of the manuscript: all authors.
