Endoscopic Anterior Lumbar Interbody Fusion: Systematic Review and Meta-Analysis
Article information
Abstract
Laparoscopic anterior lumbar interbody fusion (L-ALIF), which employs laparoscopic cameras to facilitate a less invasive approach, originally gained traction during the 1990s but has subsequently fallen out of favor. As the envelope for endoscopic approaches continues to be pushed, a recurrence of interest in laparoscopic and/or endoscopic anterior approaches seems possible. Therefore, evaluating the current evidence base in regard to this approach is of much clinical relevance. To this end, a systematic literature search was performed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the following keywords: “(laparoscopic OR endoscopic) AND (anterior AND lumbar).” Out of the 441 articles retrieved, 22 were selected for quantitative analysis. The primary outcome of interest was the radiographic fusion rate. The secondary outcome was the incidence of perioperative complications. Meta-analysis was performed using RStudio’s “metafor” package. Of the 1,079 included patients (mean age, 41.8±2.9 years), 481 were males (44.6%). The most common indication for L-ALIF surgery was degenerative disk disease (reported by 18 studies, 81.8%). The mean follow-up duration was 18.8±11.2 months (range, 6–43 months). The pooled fusion rate was 78.9% (95% confidence interval [CI], 68.9–90.4). Complications occurred in 19.2% (95% CI, 13.4–27.4) of L-ALIF cases. Additionally, 7.2% (95% CI, 4.6–11.4) of patients required conversion from L-ALIF to open surgery. Although L-ALIF does not appear to be supported by studies available in the literature, it is important to consider the context from which these results have been obtained. Even if these results are taken at face value, the failure of endoscopy to have a role in the ALIF approach does not mean that it should not be incorporated in posterior approaches.
Introduction
Minimally invasive spine surgery (MISS) is increasingly recognized as an appealing alternative to open approaches. The typical advantages of MISS include faster postoperative recovery, decreased infection rates, reduced soft-tissue damage, and lesser postoperative pain [1–4]. The most widely-described MISS techniques (e.g., tubular microdiscectomy) are for posterior/posterolateral approaches. Anterior MISS approaches have also been described, including mini-open and laparoscopic/endoscopic approaches for anterior lumbar interbody fusion (ALIF), which is a trusted approach for the management of sagittal imbalance by restoring lordosis at the lumbosacral junction. In comparison to posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF), ALIF avoids the extensive dissection of paraspinal muscles. It also affords placement of larger interbodies with full apophyseal ring engagement, reducing subsidence risk and potentially offering superior restoration of lumbar lordosis and foraminal height relative to the PLIF and TLIF techniques [5,6]. At present, the majority of ALIF procedures are conducted via an open or mini-open laparotomy with the assistance of vascular or general access surgeons to minimize approach-related morbidity. However, laparoscopic ALIF (L-ALIF) is an alternative approach that employs laparoscopic cameras and ports, similar to those already employed in general surgery, to facilitate a less invasive approach to ALIF. This technique originally gained traction during the 1990s and early 2000, but has subsequently fallen out of favor due to the steep learning curve, the higher reported incidence of retrograde ejaculation, and the relatively high rate of conversion to open surgery [7]. The difficulty in placing a large ALIF device laparoscopically is another impediment to its wider use.
However, in the past decade, there has been a renewed interest in endoscopic approaches to degenerative disk disease (DDD) and other spinal pathologies. As the envelope for endoscopic approaches continues to be pushed, a recurrence of interest in laparoscopic anterior approaches, such as L-ALIF, seems possible. Therefore, evaluating the current evidence base to identify the pitfalls and potential points for improvement of this approach is of much clinical relevance. The objective of the present systematic review and meta-analysis was to evaluate the contemporary literature on laparoscopic/endoscopic ALIF.
Methods
1. Search strategy
A literature search was performed in PubMed, Scopus, and Web of Science databases in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The following Boolean and MeSH search terms were used: (((“laparoscopy”[MeSH Terms])) OR (endoscopic spine)) AND (anterior) AND (lumbar) OR (“anterior spine approach”[All Fields] OR “anterior spine decompression”[All Fields] OR “anterior spine exposure”[All Fields] OR “anterior spine exposures”[All Fields] OR “anterior spine fusion”[All Fields] OR “anterior spine fusions”[All Fields] OR “anterior spine instrumentation”[All Fields] OR “anterior spine surgery”[All Fields]).
2. Selection criteria
Duplicate publications were eliminated using the Rayyan-Intelligent Systematic Review web application (https://www.rayyan.ai/). The titles and abstracts of the remaining studies were screened. If the title and abstract of the study did not specify laparoscopic or endoscopic ALIF, the full-text was reviewed. Screening was independently performed by two reviewers. Disagreements in study selection, if any, were resolved by consensus with a third reviewer acting as arbitrator. The list of studies excluded during the full-text review was compiled and made available via the Figshare (Digital Science Inc., Holtzbrinck Publishing Group, Macmillan Publishers Ltd., London, UK) online open access repository. (All pertinent datasets are available and can be obtained from the authors upon request.)
3. Variables and inclusion/exclusion criteria
Studies qualifying the following criteria were chosen: (1) primary clinical studies reporting data on rates of pseudoarthrosis and/or perioperative complications following L-ALIF; (2) study design: randomized-controlled trial (RCT), case-control study, and retrospective cohort study; and (3) availability of full-text in the English language. Articles were excluded if: (1) they reported data from less than five patients; (2) mean follow-up duration was less than 6 months; (3) there was no specific mention of laparoscope or endoscope use; and (4) the study involved any non-anterior approaches to the lumbar spine (i.e., lateral or posterior).
4. Data extraction
Data from the selected articles were collected in a standardized proforma. Pertinent study variables including the first author’s surname and year of publication, institution and country of authors, study design, sample size, and relevant patient demographics were included. The primary outcome of interest was the radiographic fusion rate, and the secondary outcome of interest was the occurrence of one or more perioperative complications. Data were independently extracted by two reviewers with two separate reviewers ensuring the accuracy of the extracted data (each of whom is co-authors).
5. Statistical analysis
Where applicable, descriptive statistics were generated using Microsoft Excel 2019 (Microsoft Corp., Redmond, WA, USA). Quantitative meta-analysis was performed using the R “metafor” package (R Core Team and the R Foundation for Statistical Computing, Vienna, Austria). Results were presented as forest plots; representing estimated weighted rates (and their corresponding 95% confidence intervals) for the following variables: (1) fusion rates; (2) complication rates; (3) rates of conversion to open surgery; and (4) rates of reoperation. Heterogeneity among the studies was evaluated using the chi-square, I2 and τ2 tests. When I2 ≥50%, indicating substantial heterogeneity, a random-effects model was used for meta-analysis. When I2 <50%, indicating relatively less heterogeneity, a fixed-effects model was used; p-values <0.05 were considered indicative of statistical significance for all analyses.
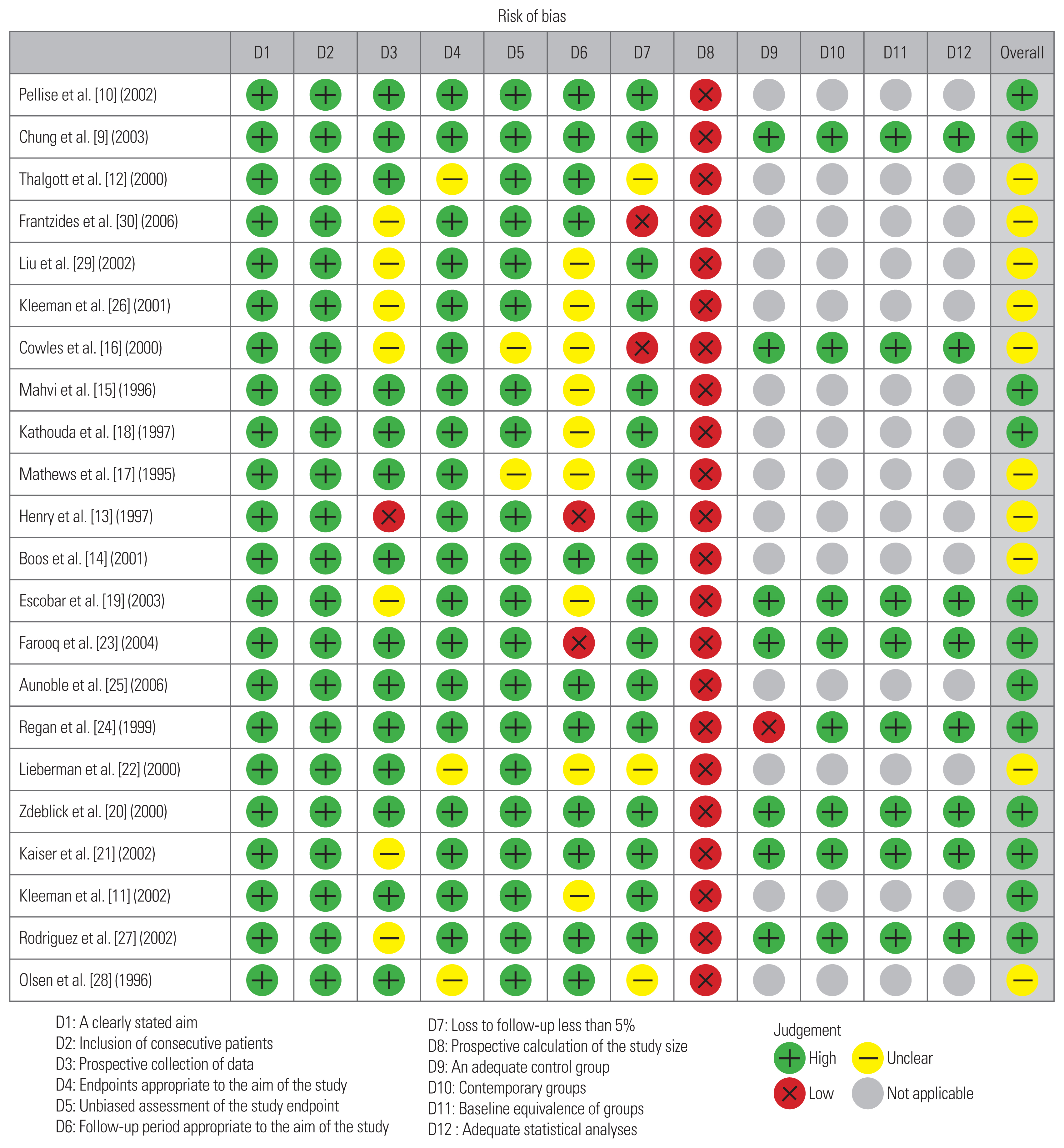
6. Study appraisal: assessment of quality and bias
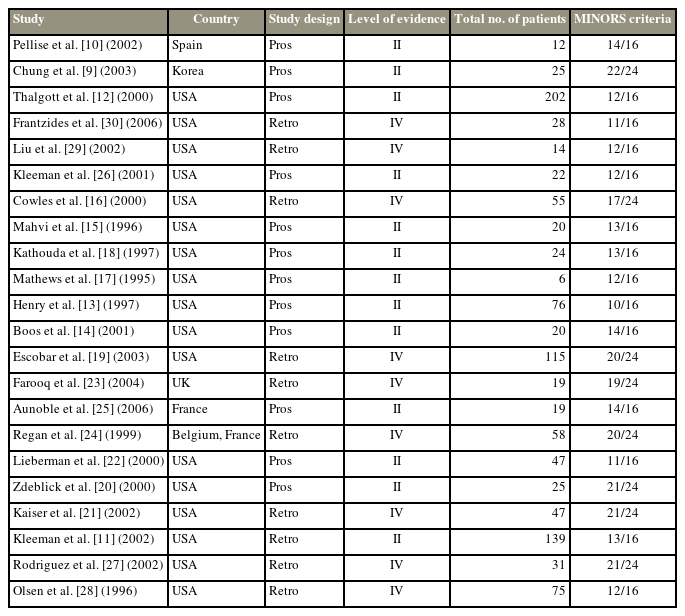
The methodology of the selected studies was assessed for sources of potential bias and a level of evidence was assigned according to a previously described method [8]. Selection bias was defined as bias resulting from patient loss to follow-up or occurrence of exposure and outcome before patient selection. Response bias was defined as that pertaining to surveys and patient-reported outcome measures. Recall bias was defined as bias occurring when treatment outcomes affected the patient’s memory of prior events. Finally, interviewer bias was identified when there was a lack of blinding or when there was some systematic difference in the way information was solicited or recorded. Assessment of study quality was reported on a scale of I (highest) to V (lowest) using the levels of evidence categorization system developed by the Oxford Center for Evidence-Based Medicine. To summarize, only high-quality RCTs receive the designation of level I evidence. Level II evidence generally refers to evidence from lower-quality RCTs that may not be appropriately blinded or randomized; alternatively, the loss of patients to follow-up can result in the designation of level II evidence. Prospective comparative studies are generally assigned as level II. Evidence from case-control studies and retrospective comparative studies is classified as level III, while that from non-comparative retrospective studies and smaller case series is classified as level IV. Expert opinions and case reports typically constitute level V evidence. Finally, we used the MINORS (methodological index for non-randomized studies) criteria for quality and risk of bias assessment (Appendix 1). Risk of bias (ROB) was displayed using a visual ROB tool (https://mcguinlu.shinyapps.io/robvis/).
Results
1. Literature search
Our query initially returned 257 results, with 57 studies from PubMed, 88 studies from Scopus, and 112 studies from Web of Science. Following title and abstract screening, 46 qualified the criteria for full-text review. Twenty-two studies involving 1,079 patients fulfilled the inclusion/exclusion criteria and were selected for quantitative analysis [9–30] (Fig. 1).
2. Study characteristics and patient demographics
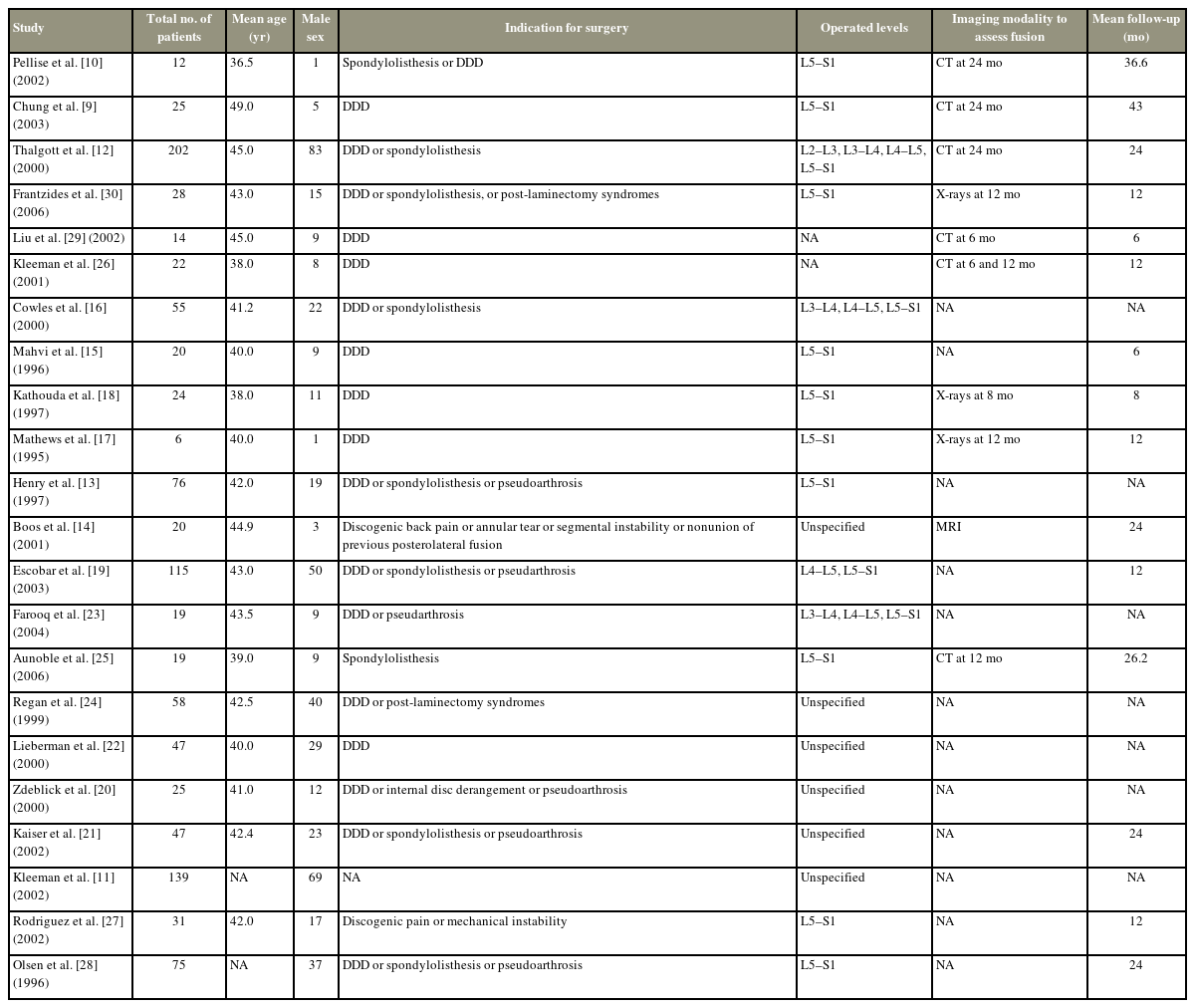
Table 1 summarizes the main characteristics of the 22 included studies. Twelve studies (54.5%) were conducted prospectively, while the other 10 (45.5%) were conducted retrospectively. The level of evidence ranged from II to IV. Selection bias (100%) was the most common bias among the included studies, along with interviewer bias (100%). Of the 1,079 included patients, 481 were males (44.6%). The mean age was 41.8±2.9 years (range, 36.5–49.0 years). The most common indications for L-ALIF surgery were DDD (reported by 18 studies, 81.8%) and spondylolisthesis (reported by nine studies, 40.9%). The most commonly operated levels were L5–S1, which was reported by 14 studies (63.6%). The mean follow-up duration in the pooled data was 18.8±11.2 months (range, 6–43 months) (Table 2).
3. Arthrodesis
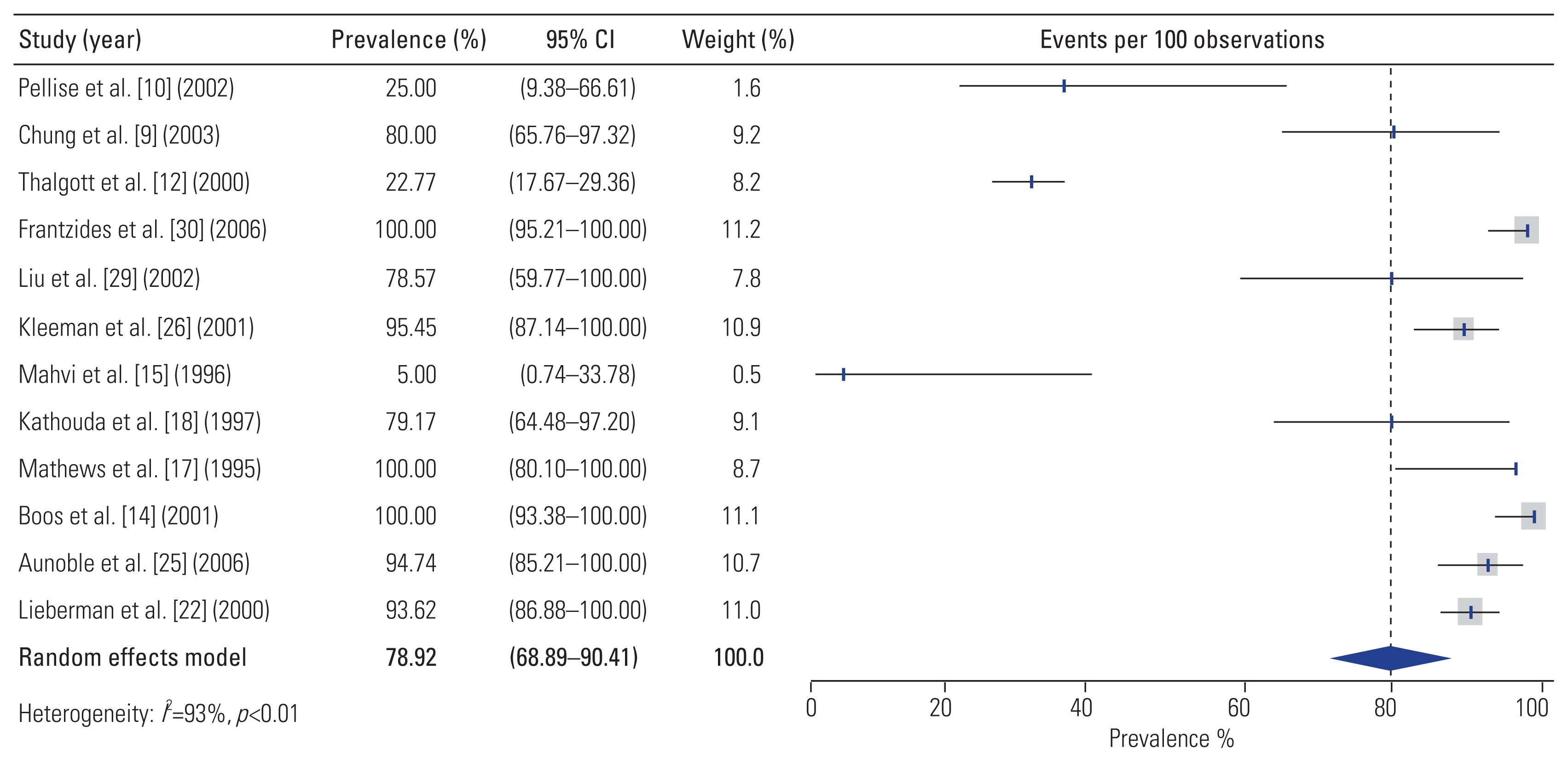
Twelve studies reported radiographic fusion rates following L-ALIF. The overall pooled rate of fusion was 78.9% (95% CI, 68.9–90.4) (Fig. 2); however, there was significant between-study heterogeneity associated with this estimate (I2=92.8%, p<0.001).
4. Complications
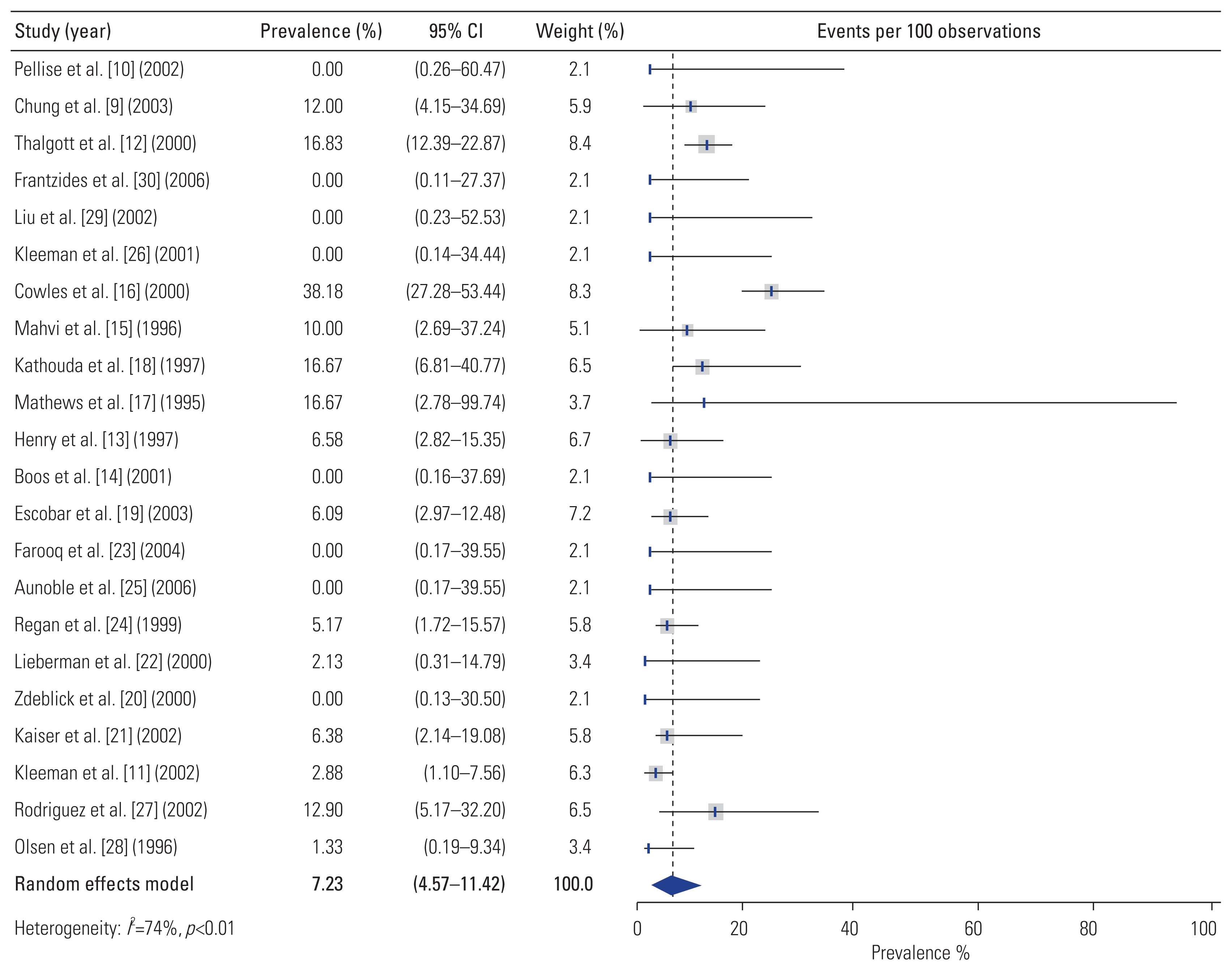
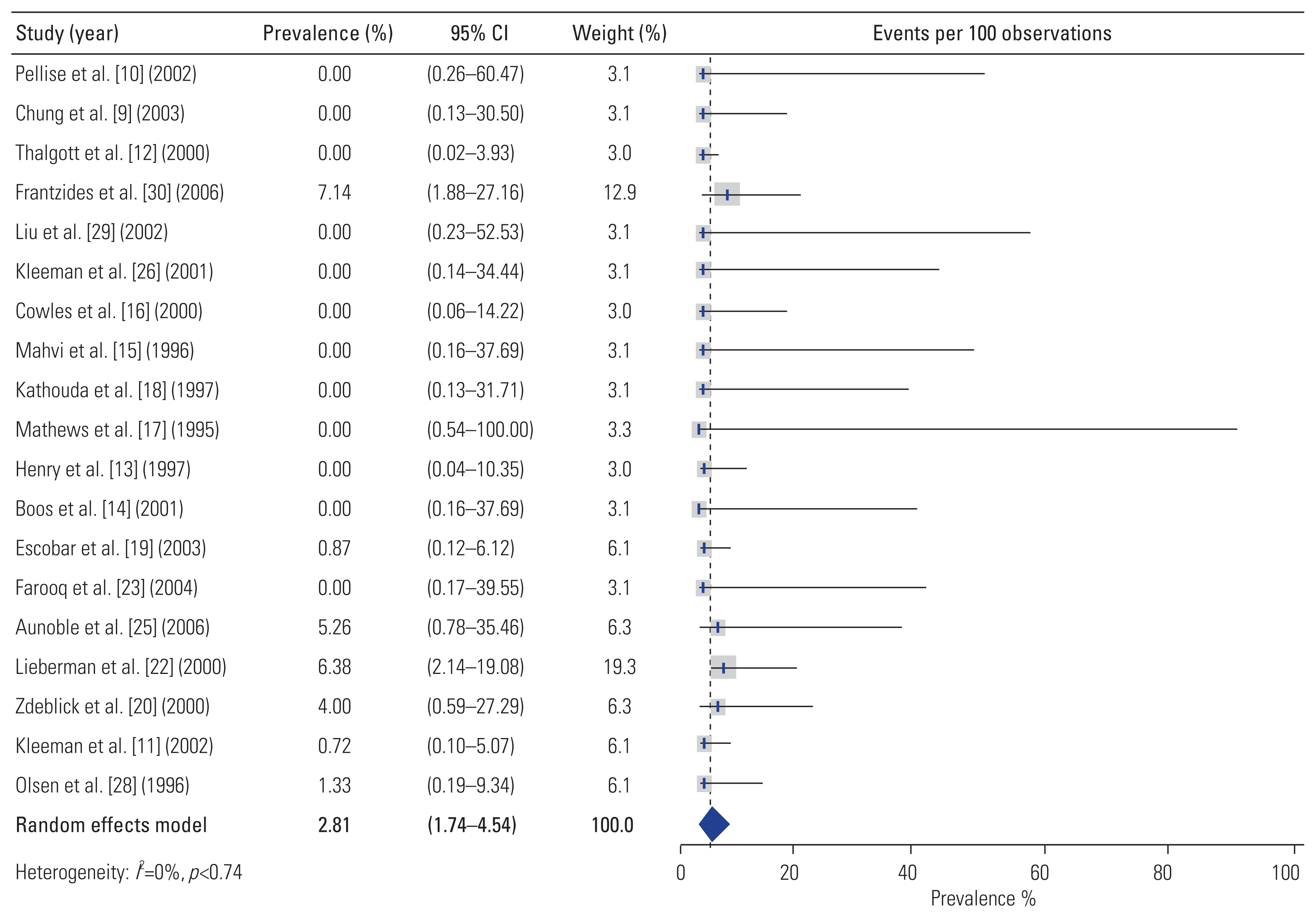
Overall, complications occurred in 19.2% (95% CI, 13.4–27.4) of patients who underwent L-ALIF (Fig. 3). Additionally, 7.2% (95% CI, 4.6–11.4) of patients required conversion from L-ALIF to open surgery (Fig. 4). Furthermore, 2.8% (95% CI, 1.7–4.5) of patients required reoperation following the primary surgery (Fig. 5). The rate and types of complications are summarized in Table 3. The results of the ROB assessment for each of the included studies are presented in Fig. 6. In general, there was a moderate risk for bias, though several studies were of high-quality and low ROB.

Estimated weighted rates of complications following laparoscopic anterior lumbar interbody fusion. CI, confidence interval.
Estimated weighted rates of reoperation following laparoscopic anterior lumbar interbody fusion. CI, confidence interval.
Discussion
ALIF is a dependable procedure for the management of degenerative spinal deformity involving the lumbar spine owing to its ability to offer significant physiological lordotic correction. Additionally, it offers the ability to place a large footprint interbody with end-to-end apophyseal ring engagement that theoretically reduces the risk of implant subsidence.
As previously mentioned, anterior approaches offer the ability to directly treat pathologic disks and to achieve physiologic sagittal plane correction. Additionally, anterior approaches avoid issues associated with posterior and lateral techniques, notably the risk of nerve root injury and extensive trauma to the paraspinal musculature [31]. Many argue that the large working distance and extensive manipulation of abdominal contents involved in the ALIF procedure represent a disproportionate risk relative to other approaches. The most notable of these complications include injury to the prevertebral great vessels (iliac vessels, inferior vena cava, aorta), thromboembolism secondary to venous manipulation, infection, and retrograde ejaculation [32]. The recruitment of access surgeons and the use of preoperative computed tomography angiography help minimize these risks. However, the risks are nevertheless non-zero and represent a target for potential improvement. One such avenue is the development of a minimally invasive ALIF approach. MISS techniques, including the L-ALIF, were popular in the mid-to-late 1990s. Such approaches are akin to laparoscopic abdominal procedures in that they minimize the structural compromise of the abdominal wall, reduce intraoperative blood loss and morbidity, and facilitate faster mobilization and recovery. However, a direct comparison of open ALIF and L-ALIF procedures suggested that the latter approach offers few (if any) benefits while being associated with higher complication rates and a steep learning curve [20]. These disadvantages may stem from a combination of reduced operative visualization and the use of instrumentation (endoscope/laparoscope) with which the average neurosurgeon is unfamiliar [24]. Due to these disadvantages, the L-ALIF and other endoscopic anterior approaches have failed to take hold within the spine surgery community, and in fact, the number of reports of endoscopic ALIF has declined since the turn of the millennium, with the last report identified in the present review having been published in 2006.
Although its laparoscopic iterations did not appear to be as effective as modern open and MISS approaches, ALIF offers several distinct advantages over other approaches [33]. First, ALIF spares the traumatization of posterior muscles that would occur during a PLIF and/or open laminectomy. Thus, ALIF enables indirect neural decompression, less tissue injury, and is favorable for anterior releases in severe deformity. Approaching the disk space from the anterior direction affords the surgeon a large surface area to initiate fusion graft placement (one of the drawbacks of laparoscopic or endoscopic ALIF is the size limitations imposed upon the interbody grafts that can be used). Overall, the net effect of this surgery is that it creates an environment conducive to fusion, all while conferring the flexibility of performing a complete or subtotal discectomy [33]. Excellent footprint for implants and strong fusion rates for ALIF (in general terms) aside [34], the results of this study indicate that L-ALIF affords much lower overall rates of fusion (pooled rate: 78.9%) compared to the other ALIF techniques (typical fusion rates: 85%–95%) [35]. Again, L-ALIF is historically not the most successful technique, but with improved technology in the form of modern endoscopy and robotic guidance and neuronavigation, endoscopy may gain further traction for ALIF down the line [36].
1. L-ALIF: limitations, drawbacks, complications, and poor rates of arthrodesis
Though described here as a single unit, L-ALIF approaches can technically be classified into anterior retroperitoneal (RP) and transperitoneal (TP) approaches [13,15]. The anterior RP approach, first described by Harmon [37] in 1963, was developed for enabling multilevel spinal access while sparing the peritoneum and obviating the need for excess bowel retraction [38]. The TP approach entails the penetration of the peritoneum and extensive bowel retraction, resulting in a higher risk of vascular injury and other complications [38]. Division of the peritoneum in the TP approach inevitably places traction on the hypogastric plexus and surrounding vasculature (including the common iliac vein, which is at risk for ligation during this procedure). Damage to these neurovascular structures can cause retrograde ejaculation, which is a primary concern in TP L-ALIF approaches, but which is also seen following >2% of conventional ALIF procedures [39,40].
From a safety and efficiency standpoint, it is understandable why many groups have entirely left behind L-ALIF in favor of the “mini-open” ALIF approach. This approach uses muscle-splitting techniques to minimize soft-tissue trauma (as compared to open ALIF) and provides improved visualization of the operative corridor through wider exposure (as compared to L-ALIF). Improved visualization and access help avoid damage to posterior neural elements. Mini-open approaches have been cited as technically more feasible than laparoscopic approaches and have therefore become very popular because (relative to conventional open approaches) they decrease muscular injury and operative time, accelerate recovery time, and reduce costs associated with surgery [19,20,41–44]. With the advent of single-position surgery, mini-open approaches have also been performed in the lateral decubitus position, enabling circumferential access which can potentially increase the efficiency of placing posterior instrumentation at the same time [45,46].
The limited body of evidence supporting the use of L-ALIF and other endoscopic spinal procedures—as well as concerns over several key complications—has also rendered the surgical community wary of incurring high initial capital investments for incorporating technology that has not been validated by the medical literature [32]. Here we systematically reviewed the literature on L-ALIF to assess whether the reported arthrodesis and perioperative complication rates would justify the adoption of this technique. The pooled fusion rate following L-ALIF was 78.9% (95% CI, 68.9–90.4), which is lesser than that reported after open ALIF (radiographic arthrodesis achieved in over 90% of cases) [35]. It is possible that the higher fusion rate after open ALIF reflects recent improvements in ALIF technology, including the US Food and Drug Administration (FDA) approval of recombinant human bone morphogenetic protein-2 and the availability of three-dimensional–printed titanium interbodies with improved osteoinductive properties [34]. However, there is insufficient evidence to support this claim.
Importantly, the incidence of perioperative complications with L-ALIF was 19.2% (95% CI, 13.42–27.38) with 7.2% (95% CI, 4.57–11.42) of patients requiring conversion from L-ALIF to open surgery. This complication rate is nearly quadruple that reported in a recent meta-analysis by Phan et al. [47], who found that perioperative complications occurred in only 5.95% of cases during open ALIFs assisted by an access surgeon. Therefore, there does not appear to be a compensatory reduction in perioperative complications in lieu of the lower observed fusion rates. It must be noted that many of the L-ALIF series included in our study reported results pertaining to the learning curve period, contributing to a higher rate of complications than would be expected in experienced hands. In line with this, some series, such as that of Olsen et al. [28] reported complication rates close to the pooled rate for open ALIFs reported by Phan et al. [47]. However, achieving such rates requires (1) strict patient selection; (2) use of a multidisciplinary surgical team including both a general surgeon and a spine surgeon, each with significant laparoscopic experience; and (3) the presence of both the general and spine surgeon throughout the surgical operation [28,47].
Another detraction from L-ALIF procedures is that they offer minimal improvement in visualization relative to the mini-open ALIF. The latter does not require the use of an endoscope to achieve adequate visualization and is, therefore, less equipment-intensive; it also has a much more gradual learning curve for access surgeons as it is similar to the approach used for the open treatment of abdominal aortic aneurysms, which is an Accreditation Council for Graduate Medical Education-mandated category minimum for those in vascular surgery training programs. Another limitation of the endoscopic approach that is often overlooked is the difficulty in placing interbodies at the large lumbar segments. Anterior interbody approaches offer the benefit of enabling the placement of implants with large cross-sectional areas to reduce implant subsidence, maximize correction, and improve fusion rates across the anterior column. Endoscopic approaches may not be conducive to the placement of the larger, modern interbodies due to the limited access window. At best, endoscopic visualization may help improve visualization via anterior and oblique/pre-psoas approaches or they may prove useful for minimally invasive resection of presacral neoplasms [32,48,49]. However, their use does not appear imperative and it is unclear that they have a defined role at present in the management of degenerative lumbar pathology.
2. Endoscopic learning curve: boom and then bust cycle
As mentioned earlier, endoscopic spinal fusion surgery became increasingly popular during the 1990s, peaking around the turn of the century. However, in the first decade of the 21st century, endoscopic surgery largely disappeared from the spine surgeon’s armamentarium. This occurred despite great advances in endoscopy, such as the development of pressurized irrigation systems and improved optical performance. Doubtlessly, several factors limited the penetration of endoscopy in the North American spine market; however, one of note was likely the limited exposure of most spine surgeons to endoscopic techniques during their training. Therefore, in the absence of obvious market pressure to adopt these skills, there was little incentive to face the steep learning curve associated with their adoption when the costs of this skill acquisition would likely be borne by patients who would otherwise still realize satisfactory outcomes using conventional approaches.
Increased penetration of arthroscopy into orthopedic training programs and of endoscopic skull base surgery into neurosurgery training programs has likely increased the familiarity of trainees with endoscopy equipment. However, the skills developed during these procedures are not directly translatable to spinal endoscopy [32]. To this end, a recent single-surgeon series found that a learning curve of 15 cases was required to achieve clinical outcomes similar to those seen using conventional techniques [50]. In this same study, a head-to-head comparison of outcomes following traditional and endoscopic decompression showed slightly worse outcomes among patients treated endoscopically, suggesting that the learning curve may even exceed the estimate reported by the authors. The steep learning curve in endoscopic spinal surgery likely stems from multiple factors, the foremost of which is the relative novelty of performing surgery in a small-scale working space under indirect visualization. Furthermore, the two-dimensional representations captured by conventional endoscopes remove the stereoscopic information that humans employ as one of the primary visual cues for depth perception. Acclimation to this alternative means of visualization could account for the initial increase in operative time and complications (e.g., activation of the high-speed endoscopic burr without realizing it is in contact with the dura). Finally, inadequate visualization offered by low-resolution endoscopes can lead surgeons to miss incidental dural tears requiring repair or compressive elements requiring removal to alleviate the symptoms. This highlights the importance of employing high-quality equipment as well as becoming facile with these instruments.
Endoscopy is not unique among neurosurgical techniques in its steep learning curve. Nevertheless, its adoption is sparse, which is likely attributable to the paucity of training programs that incorporate endoscopic spinal surgery into their curricula [32]. The incorporation of such training into residency offloads the steepest part of the learning curve from the trainee’s independent practice. This can help remove the anxiety associated with performing such complicated cases during the early post-residency period when trainees seeking board certification are compiling cases for review. Nevertheless, a review of the 2021–2022 North American Spine Society fellowship directory could identify only five institutions advertising training in spinal endoscopy [51]. It is therefore unlikely that most graduates will receive this training. Consequently, unless there is interest or adequate incentive for recent graduates to attend the independent weekend courses where most endoscopic spine surgery is taught, these surgeons are unlikely to incorporate spinal endoscopy into their armamentarium [52]. Therefore, the relatively low penetration of spinal endoscopy within the broader market likely stems from a combination of a dearth of trained faculty in academic centers and a relatively high barrier to entry in the postgraduate period as freshly-minted attendings must bear the costs of the steep learning curve during a period where their complications are most closely scrutinized. Nevertheless, this low penetrance may be addressed with the apparent increased interest in spinal endoscopy as reflected by a quadrupling in the number of publications on this topic between 2015 and 2020 [53].
3. Economic factors and regional variations
Though the adoption of spinal endoscopy in the United States is low at present, this is not true worldwide. Several East Asian countries, notably China and South Korea, have seen much more widespread adoption of endoscopic spine surgery [52]. This may, in part, be due to the historic dominance of orthopedic surgeons within these markets. The extensive experience with arthroscopic procedures in orthopedic surgery residency may confer skills that are more directly transferable to endoscopic spine surgery, thus facilitating its adoption into their practice [32].
As a counterpoint though, it is plausible that economic factors in the United States healthcare system have helped stymie the popularization of endoscopic spine surgery. At present, reimbursement rates for discectomies and simple decompression surgeries are relatively low. Until 2017, there was no unique CPT code (Current Procedural Terminology code) for endoscopic decompression, at which point 62380 was adopted, which covers endoscopic decompression procedures such as laminotomy, foraminotomy, discectomy, and partial facetectomy [31]. However, the relative value units assigned to this code are insurance carrier-dependent. Therefore, it is unclear whether surgeons will be compensated for the increased training required to acquire and employ these endoscopic skills [54].
4. Endoscopic robotic spinal surgery: considerations regarding future role
Though the results presented here do not overall seem to support L-ALIF as a viable alternative to open ALIF, they are derived from series that are all nearly 2 decades old; as such, they do not necessarily inform the potential of ALIF using modern endoscopy. Significant strides in spinal endoscopy and other surgical technologies have been made which may allow modern versions of the L-ALIF approach to yield results similar or superior to those offered by the ALIF. Spinal robotics is one such surgical technology and, in fact, one of the currently marketed platforms, the ExcelsiusGPS (Globus Medical, Audubon, PA, USA), was derived from a thoracoscopy robot. The degree to which these technologies can be harmoniously integrated to improve patient outcomes is still under ongoing investigation [55,56].
5. Study limitations
Some limitations of the present study should be acknowledged. First, it is challenging to assess the effects of heterogeneity resulting from the diverse array of indications for endoscopic ALIF, including DDD, adult spinal deformity, disk herniation, and spondylolisthesis. Furthermore, there was significant heterogeneity among studies resulting from inconsistent measurement and reporting of clinical outcomes and patient complications.
Second, the studies included in this review were retrospective in nature, rendering them susceptible to bias. Furthermore, errors could have resulted from any inaccuracy in data retrieval or the pooling of certain measures. Although all retrieval decisions and analysis calculations were double-checked, the possibility of human error cannot be excluded. Furthermore, the series analyzed in this study employed old technologies and the field of spine surgery has evolved significantly in the last two decades. For example, only four studies were published following the FDA approval of bone morphogenic protein, the use of which has significantly improved ALIF arthrodesis rates. Additionally, cages used today perform better than the cages used during the time most of the L-ALIF studies included in this review were performed. To summarize, our meta-analysis cannot account for the effects of technological improvements.
Due to these limitations, the results of this study are not intended to be inherently definitive but may help inform the design of future studies and analyses that can better delineate the benefits and drawbacks of endoscopic ALIF surgery as the popularity of spinal endoscopy continues to grow.
Conclusions
Although L-ALIF does not appear to be supported by published studies, it is important to consider the context from which these results have been obtained. Owing to the paucity of recent studies, our findings do not account for recent technological advances. It is possible that technological advances may reposition L-ALIF as a valuable technique within modern spine surgery. The purpose of this study was to identify via a literature search whether any groups have investigated L-ALIF using modern endoscopes, and it appears that no such investigations have been conducted. Moreover, the limited training opportunities and lack of academic centers teaching endoscopic spine surgery in the United States represent a significant barrier that has likely prevented many spine surgeons from achieving optimal proficiency in endoscopic techniques. If more training centers incorporate endoscopic spine surgery into their curricula, endoscopic surgical training may potentially become a standard component of spine surgery training programs, after which the potential merits of endoscopic approaches such as L-ALIF can be more accurately assessed.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: MYO; data curation: CCK, EKT, CY, BP, SS; formal analysis: CCK; funding acquisition: not applicable; methodology: NJB; project administration: NJB, SA, MHP, MYO; visualization: ZP, MHP, MYO; writing–original draft: NJB, ZP, AML; writing–review & editing: all authors; and final approval of the manuscript: all authors.