The Impact of Preoperative Antithrombotic Therapy on the Risks for Thrombo-ischemic Events and Bleeding among Patients Undergoing Elective Spine Surgery
Article information
Abstract
Study Design
Retrospective matched analysis.
Purpose
To evaluate the effect of antithrombotic drug therapy on the rates of thrombo-ischemic or bleeding events 90 days following elective spine surgery.
Overview of Literature
Thrombo-ischemic and bleeding complications in patients undergoing spine surgery are major causes of morbidity. Many patients who pursue elective spine surgery are concurrently receiving antithrombotic therapy for unrelated conditions; however, at this time, the effects of preoperative antithrombotic use on postoperative bleeding and thrombosis are unclear.
Methods
Using an all-payer claims database, patients who underwent elective cervical and lumbar spine interventions between January 1, 2010, and June 30, 2018, were identified. Individuals were categorized into groups taking and not taking antithrombotics. A 1:1 analysis was constructed based on comorbidities found to be independently associated with bleeding or ischemic complications using logistic regression models. The primary outcomes were the rates of thrombo-ischemic events and bleeding complications.
Results
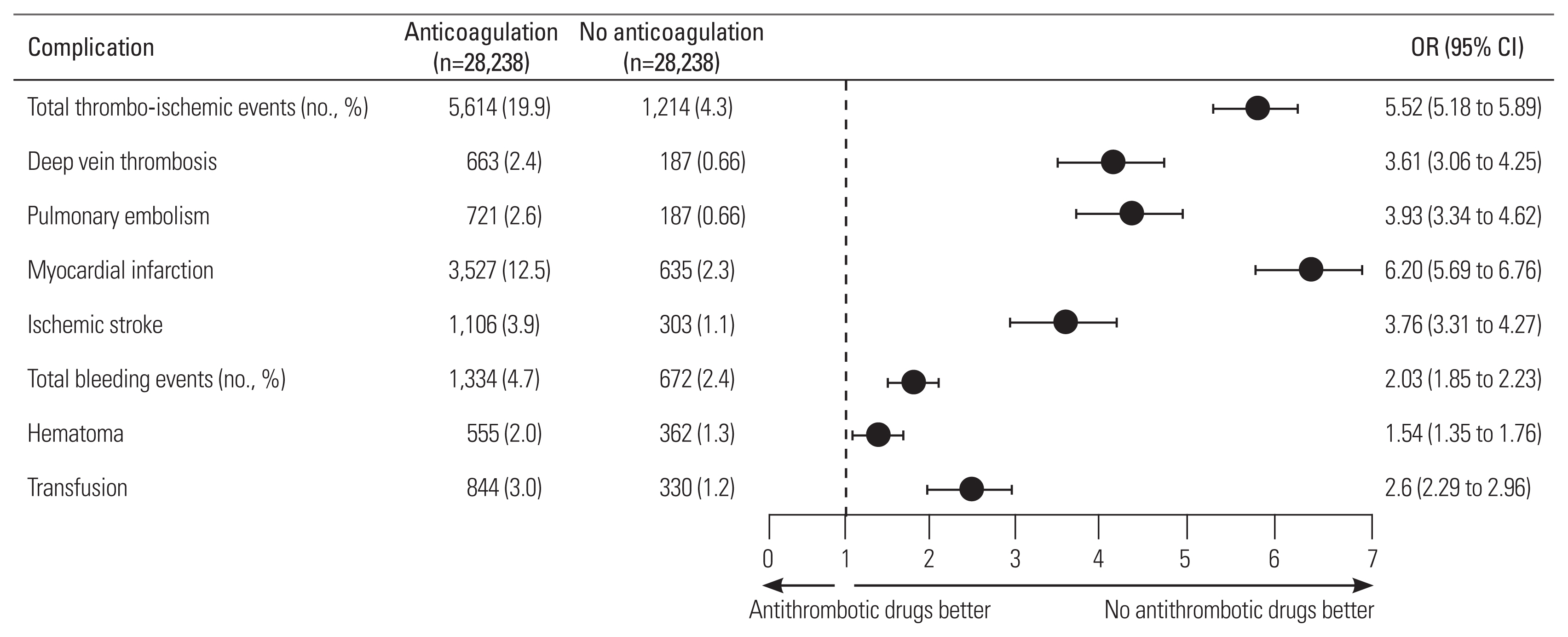
A total of 660,866 patients were eligible for inclusion. Following the matching procedure, 56,476 patient records were analyzed, with 28,238 in each group. The antithrombotic agent group had significantly greater odds of developing any 90-day thrombo-ischemic event after surgery: deep vein thrombosis (odds ratio [OR], 3.61; 95% confidence interval [CI], 3.06–4.25), pulmonary embolism (OR, 3.93; 95% CI, 3.34–4.62), myocardial infarction (OR, 6.20; 95% CI, 5.69–6.76), and ischemic stroke (OR, 3.76; 95% CI, 3.31–4.27). In addition, the antithrombotic agent group had an increased likelihood of experiencing hematoma (OR, 1.54; 95% CI, 1.35–1.76) and need for transfusion (OR, 2.61; 95% CI, 2.29–2.96).
Conclusions
Patients taking antithrombotic medications before elective surgery of the cervical and lumbar spine had increased risks of both ischemic and bleeding events. Spine surgeons should carefully consider these implications when appraising patients for surgery, given the lack of guidelines on perioperative management of antithrombotic agents.
Introduction
As the US population continues to age, adults aged >65 years account for an increasingly large proportion of those electing for spine surgery. These patients often have complex medical conditions, and some studies have suggested that >70% of them have multiple comorbidities [1,2]. Many comorbid conditions such as diabetes mellitus (DM) have well-researched guidelines for managing medications perioperatively; however, no similar “best practices” exist for patients on antithrombotic therapies, particularly for spine surgery [3]. This is a common clinical problem encountered in the United States. In 2012, the American College of Surgeons (ACS) estimated that 250,000 patients on anticoagulants face the prospect of an invasive surgical procedure annually [4].
The management of these patients is challenging because the risks of thrombo-ischemic events increase in the presence of antithrombotics; however, the risks of bleeding complications increase in their absence. Furthermore, the degree of these risks is enormously nuanced as it varies not only among patients and specific agent but heavily on operative variables such as complexity of the procedure, duration of postoperative immobility, and magnitude of correction [5,6]. In addition, spine surgeries are associated with large blood losses, with amounts ranging from 650 mL to 2 L, further complicating the picture [7].
Guidance available in the literature is sparse in the field of spine surgery. Some studies have assessed elective surgical procedures in general, and they have focused on delineating optimal intervals for interruption and resumption of specific antithrombotics and on the value of bridging therapies. The most notable is the ACS Clinical Practice Guidelines from 2012; however, even this document is limited by its reliance on a “disproportionately large number of methodologically weak observational studies” [4]. Given the relative paucity of well-designed trials, we aimed to examine the clinical implications of using preoperative antithrombotic agents among patients undergoing elective spine surgery in a large, matched analysis.
Materials and Methods
1. Study design and outcomes
This study was a retrospective cohort analysis of publicly available data. The main objective was to evaluate the independent effect of antithrombotic agents on the rates of thrombo-ischemic events or bleeding complications 90 days following index surgery. The study included thrombo-ischemic events such as deep vein thrombosis (DVT), pulmonary embolism (PE), myocardial infarction (MI), and ischemic stroke, while bleeding complications included hematoma and the need for transfusion. Patients were divided into groups receiving and not receiving preoperative antithrombotic agents.
Patients undergoing elective lumbar or cervical spine procedures were identified using the International Classification of Diseases 9 and 10 (ICD-9 and ICD-10, respectively) diagnosis and procedure codes and current procedural terminology (CPT) codes (Supplement 1). This set of patients was then further stratified using generic drug codes to identify individuals who had filled an outpatient prescription for one of the therapeutic antithrombotic drugs (Supplement 2) within a 90-day preoperative window. Given its over-the-counter availability, aspirin monotherapy was not included. In addition, low-molecular-weight heparin and unfractionated heparin were not included because we could not distinguish between prescriptions for comorbid conditions and perioperative prophylaxis. Furthermore, patients underwent received surgery for lumbar and cervical vertebral fractures and patients aged <18 years were excluded.
Patient comorbidities within 1 year of their procedure were identified through ICD-9/10 and CPT codes. These included atrial fibrillation, cancer, chronic kidney disease, coagulopathy, DM, hyperlipidemia, hypertension, liver disease, obesity, peripheral vascular disease, and smoking status.
2. Data source
This study used longitudinal data from the Mariner-53 database (PearlDiver Technologies, Colorado Springs, CO, USA). The database consists of medical and surgical claims of more than 53 million individuals between January 1, 2010, and June 30, 2018. Mariner 53 was constructed using networks of providers, and it is made up of all-payer types, including self-pay, private insurance, Medicaid, and Medicare.
3. Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Illinois at Chicago (UIC) Institutional Review Board and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the UIC Institutional Review Board, with a waiver of patient informed consent because the analysis posed minimal risk to participants.
4. Statistical analysis
Descriptive characteristics were calculated for the unmatched and matched patient populations. Patient cohorts were matched 1:1 using binomial logistic regression models to assess the independent effects of patient characteristics (age and comorbidities) on postoperative outcomes. All categorical variables including demographics and comorbidities were compared using chi-square tests. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to compare the incidence of postoperative bleeding and thrombo-ischemic complications between both groups. Additionally, multivariable logistic regression models and calculation of both binomial and Gaussian ORs and 95% CIs were created to compare 90-day thrombo-ischemic and bleeding events between the antithrombotic and non-antithrombotic prescription groups. Regression models were designed to adjust for age, each studied comorbidity, antithrombotic prescription status, drug classes, and each studied medication. p-values <0.05 were considered statistically significant. All data were analyzed using R ver. 4.0 (2020; The R Foundation for Statistical Computing, Vienna, Austria).
Results
Between January 1, 2010, and June 30, 2018, 754,274 individuals undergoing elective cervical or lumbar spine interventions were identified. Of these individuals, 19,940 underwent surgery for lumbar/cervical fracture repair and were thus excluded from the analysis. Moreover, 73,468 individuals were also excluded for being <18 years old at the time of surgery. Ultimately, the study included a total of 660,866 individuals who underwent elective spine surgery (Fig. 1). The descriptive characteristics of this unmatched population are summarized in Supplement 3. Models comparing the risks of bleeding complications and thromboembolic events in the unmatched population are summarized in Supplements 4 and 5.
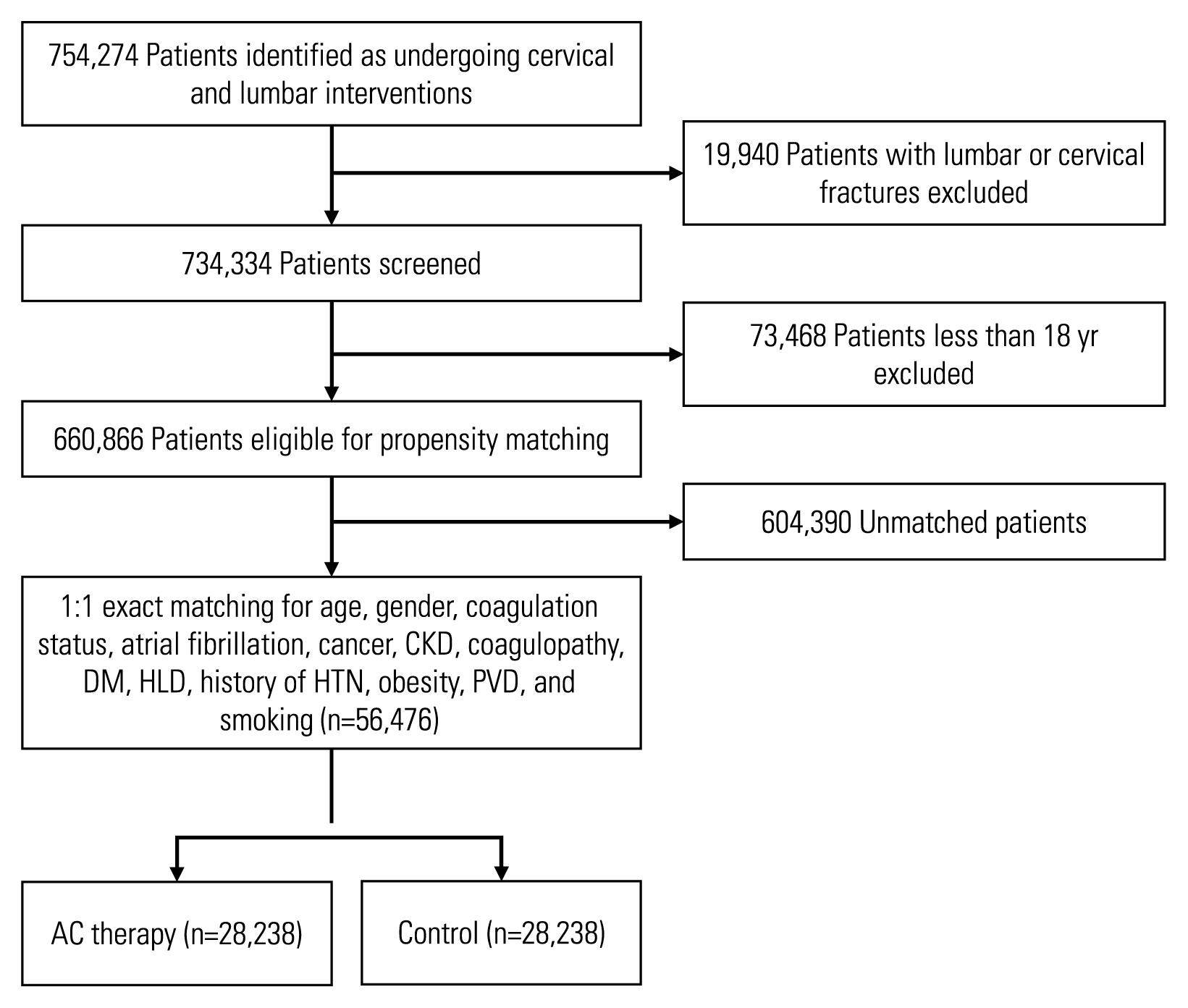
Patient selection. CKD, chronic kidney disease; DM, diabetes mellitus, HLD, hyperlipidemia; HTN, hypertension; PVD, peripheral vascular disease; AC, anticoagulation.
Following a 1:1 matching procedure based on patient age and comorbidities, 56,476 patients remained, including 28,238 and 28,238 patients in the antithrombotic and non-antithrombotic groups, respectively. No statistical difference in patient age was found between the groups. Each cohort predominantly consisted of individuals aged >65 years (n=14,886 [52.7%]). The most prevalent comorbidities in the matched cohort were hypertension (83.56%), DM (49.89%), and hyperlipidemia (44.93%) (Table 1).
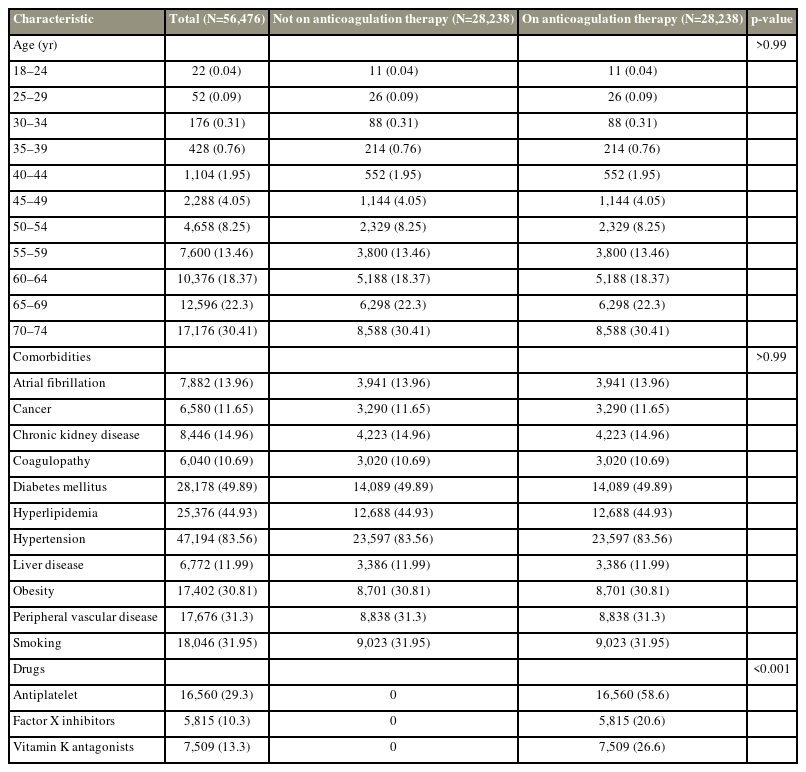
Patient demographics of matched patients undergoing elective lumbar and cervical spine interventions who were on anticoagulation therapy versus not prior to surgery
1. Impact of antithrombotic medications on thrombo-ischemic events
Among our matched population of patients undergoing elective spine surgery, a total of 6,828 ischemic events were recorded 90 days following surgery. Patients taking antithrombotic agents have a significantly increased likelihood of developing any 90-day thrombo-ischemic event after surgery: DVT (2.4% versus 0.7%; OR, 3.61; 95% CI, 3.06–4.25), PE (2.6% versus 0.7%; OR, 3.93; 95% CI, 3.34–4.62), MI (12.5% versus 2.3%; OR, 6.20; 95% CI, 5.69–6.76), and ischemic stroke (3.9% versus 1.1%; OR, 3.76; 95% CI, 3.31–4.27) (Fig. 2).
2. Impact of antithrombotic medications on bleeding events
In the 90-day postoperative period, 2,006 bleeding events were observed in the matched population. Individuals taking antithrombotic agents had an increased likelihood of experiencing a bleeding event overall (4.7% versus 2.4%; OR, 2.03; 95% CI, 1.85–2.23), hematoma (2.0% versus 1.3%; OR, 1.54; 95% CI, 1.35–1.76), and need for transfusion (3% versus 1.2%; OR, 2.61; 95% CI, 2.29–2.96) (Fig. 2).
Discussion
In this retrospective cohort study, data from 56,476 patients were matched and analyzed. Significantly increased rates of 90-day thrombo-ischemic events and bleeding complications were recorded among patients prescribed preoperative antithrombotic therapy. More specifically, the risks were uniformly higher for DVT, PE, MI, ischemic stroke, hematoma, and the need for transfusion in the antithrombotic group.
Although seemingly paradoxical, the increase in thrombo-ischemic events in the antithrombotic group likely has to do with the interruption of antithrombotics before surgery and the inherent invasiveness of spine procedures. Unfortunately, we could not determine the details of antithrombotic interruption before the surgery from our database analysis. However, literature has shown a higher incidence of thrombo-ischemic events in patients with spine disorders, such as an estimated incidence for DVT in non-spinal cord injury trauma patients at 6.0% (range, 0%–19%) [8]. Although poorly understood, unique aspects of spine surgery may likely explain this increased risk because studies following non-spine surgical procedures have reported a <1% incidence of perioperative ischemic events for those taking antithrombotic medications preoperatively [9,10].
The literature is clear on bleeding complications. Recently, Kim et al. [11] demonstrated that preoperative anticoagulation was a risk factor for postoperative hematoma in a retrospective analysis of 310 patients undergoing endoscopic spine surgery. Yi et al. [12] found similar results in their retrospective review of 3,720 spine surgeries and revealed preoperative anticoagulation therapy as a risk factor for postoperative epidural hematoma. Likewise, in patients on chronic anticoagulation therapy with warfarin, Young et al. [13] reported an increased need for transfusion following lumbar spine surgery. These findings underpin the difficulty of perioperative antithrombotic management. Although some antithrombotics require bridging therapy or earlier interruption to obtain good outcomes, the ideal timelines are unclear. Without consistent, evidence-based methodology, surgeons resort to personal experience and other empiric strategies to minimize adverse events, with visibly dubious outcomes.
Currently, the half-life of antithrombotics and operative variables such extent of surgery and involvement of highly vascular organs drive expert recommendations for antithrombotic management [14]. These factors are usually combined with the HAS-BLED score, which is a short scale based on patient factors such as comorbid renal impairments or hypertension, with moderative predictive value in bleeding risk quantification. To stratify the risk for thrombo-embolic events, the CHADS2 score was also used, although it has not been validated for perioperative risk stratification [15]. The results of these efforts highly vary and gain inconsistent expert recommendations [16,17]. For example, a recent study considered employing an optimal interval for the interruption of newer direct oral anticoagulants and concluded that the optimal interval may range from 1 to 5 days preoperatively, even in surgeries with similar bleeding risks [18].
Bridging is similarly controversial; however, several randomized clinical trials have investigated its value in antithrombotic agent management following surgery to minimize bleeding and thrombo-ischemic risk in a non-spine setting. For example, the PAUSE and BRIDGE trials focused on patients who had atrial fibrillation and were taking direct oral anticoagulants and warfarin, respectively. They revealed the limited role of heparin bridging following high-risk bleeding procedures [9,10]. However, these findings are not yet transplanted into clinical practice.
The timing of postoperative resumption of antithrombotic agents remains unclear. In addition, to the limited observational and small cohort studies, no strong data can be relied upon. The current ACS guidelines reflect this ambiguity by leaving it to surgeons to assess and balance patient risk factors—an inexact recommendation to say the least (Table 2) [19]. Baschera et al. [20] echoed this lack of clarity in their nationwide survey of orthopedic and neurosurgery attendings—they found wide variations in antithrombotic agent restart times, specifically with orthopedic surgeons recommending restarting platelet inhibitors earlier than their neurosurgeon colleagues.
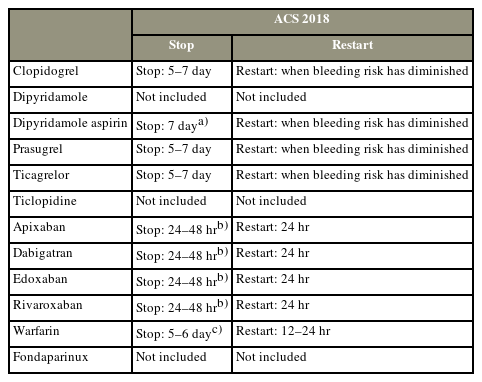
Guidelines for anticoagulation therapy use and recommendations to stop before and restart after intermediate-risk spine surgeries
This study has several shortcomings. First, the retrospective design and non-uniform procedures introduced heterogeneity in each group, which may not have been completely accounted for by the variables included in the analysis. All clinical data available in the Mariner database concerning diagnoses and adverse events were coded using ICD-9, ICD-10, and CPT codes. Misclassification errors are certainly present in the analysis; however, they typically appear at random and are less likely to bias the results, particularly given the large sample size of the matched analysis in this study. Second, the lack of granularity within the database made deriving firm conclusions regarding the duration of preoperative interruption timing and postoperative resumption interval of antithrombotic agents difficult. This information would have been useful to better stratify more nuanced treatment regimens. Similarly, future analysis should account for the medication type and type of surgery because significant heterogeneity may affect the risks of bleeding versus thrombosis. These are significant study limitations; however, our findings likely reflect the ambiguity in antithrombotic agent management for patients undergoing spine surgery. We hope our study inspires interest in further research to develop clear guidelines.
Conclusions
Given the rapidly aging US population, older patients with comorbidities make up an increasing proportion of those seeking elective spine surgeries. Surgeons are faced with the challenge of balancing operative variables with the potential risks of halting or continuing antithrombotic therapy. This study offers evidence of the increased rates of postoperative thrombo-ischemic events and bleeding complications within 90 days after surgery among patients prescribed preoperative antithrombotic agents. A better understanding of the medications used to decrease clotting, timing of withdrawal in the perioperative window, and definition of which patients are more likely to benefit from antithrombotic medications may be improved in the future.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Manuscript drafting, writing, and statistical analysis: Syed Khalid, Pranav Mirpuri, Sai Chilakapati, Angelia Kwak, Devon Mitchell; project conception and supervision: Owoicho Adogwa, Ankit I. Mehta: and final approval of the manuscript: all authors.
Supplementary Materials
Supplementary materials can be available from https://doi.org/10.31616/2023.0125.
Supplement 1. Patient characteristics by CPT and ICD-9 and ICD-10 diagnostic and procedure codes.
asj-2023-0125-Supplementary-1.pdfSupplemental 2. Drugs included and respective categories.
Supplemental 3. Patient demographics of unmatched patients undergoing elective lumbar and cervical spine interventions who were on anticoagulation therapy versus not prior to surgery.
asj-2023-0125-Supplementary-2-3.pdfSupplemental 4. Risk of adverse ischemic events 90 days following elective surgery.
Supplemental 5. Risk of adverse bleeding events 90 days following elective surgery.
asj-2023-0125-Supplementary-4-5.pdf