Assessment of the Initial Diagnostic Accuracy of a Fragility Fracture of the Sacrum: A Study of 56 Patients
Article information
Abstract
Study Design
Retrospective study.
Purpose
To investigate the clinical manifestations of a fragility fracture of the sacrum (FFS) and the factors that may contribute to a misdiagnosis.
Overview of Literature
The number of patients diagnosed with FFS has increased because of extended life expectancy and osteoporosis. Patients with FFS may report nonspecific symptoms, such as back, buttock, groin, and/or leg pain, leading to a misdiagnosis and a delay in definitive diagnosis.
Methods
Fifty-six patients (13 males and 43 females) with an average age of 80.2±9.2 years admitted to the hospital for FFS between 2006 and 2021 were analyzed retrospectively. The following patient data were collected using medical records: pain regions, a history of trauma, initial diagnoses, and rates of fracture detection using radiography, computed tomography (CT), and magnetic resonance imaging (MRI).
Results
Forty-one patients presented with low back and/or buttock pain, nine presented with groin pain, and 17 presented with thigh or leg pain. There was no history of trauma in 18 patients (32%). At the initial visit, 27 patients (48%) were diagnosed with sacral or pelvic fragility fractures. In contrast, 29 patients (52%) were initially misdiagnosed with lumbar spine disease (23 patients), hip joint diseases (three patients), and buttock bruises (three patients). Fracture detection rates for FFS were 2% using radiography, 71% using CT, and 93% using MRI. FFS was diagnosed definitively using an MRI with a coronal short tau inversion recovery (STIR) sequence.
Conclusions
Some patients with FFS have leg pain with no history of trauma and are initially misdiagnosed as having lumbar spine disease, hip joint disease, or simple bruises. When these clinical symptoms are reported, we recommend considering FFS as one of the differential diagnoses and performing lumbar or pelvic MRIs, particularly coronal STIR images, to rule out FFS.
Introduction
Fragility fractures are often the result of low-energy or minimal trauma combined with poor bone quality and are characterized by inadequate mineralization, as seen in people with osteoporosis [1,2]. In older adults, a single low-energy trauma often causes a fragility fracture of the pelvis (FFP), which includes the sacrum, pubis, ischium, and ilium [1]. Before FFPs were described in the literature, sacral insufficiency fractures (SIFs) were first reported in 1982 [3]. Insufficiency fractures are caused by multiple mild traumas and can occur in people with osteoporosis, bones with decreased elastic properties after irradiation, immobilized for a long time, and/or who have had corticosteroid therapy [1,4]. Fragility and insufficiency fractures are commonly caused by minor trauma in patients with weakened bones. Although the terms “fragility” and “insufficiency” regarding bone fractures of the pelvis or sacrum appear similar in many ways because they occur in weakened bones, they are often distinguished by the frequency of the trauma that causes them [1,2].
A definitive diagnosis of a fragility fracture of the sacrum (FFS) or a SIF can sometimes be missed, delaying early identification. This is because they are often associated with nonspecific symptoms, such as back, buttock, groin, and/or leg pain, and they are not always evident on radiographs in their early stages [4–11]. Fragility fractures may initially be misdiagnosed as other diseases. However, reports of previous studies indicate that the clinical manifestations and details underlying delayed diagnosis of FFS or SIF were limited due to the small number of patients (three to 42 patients); no study report documents the examination of more than 50 patients. The purpose of this study was to investigate the pitfalls associated with an early FFS misdiagnosis and the clinical manifestations of FFS.
Materials and Methods
This study was a single-center, retrospective case review of patients admitted to Seirei Sakura Citizen Hospital between 2006 and 2021. Data were collected from 59 patients. The inclusion criteria for this study were (1) hospitalization due to severe pain and (2) a final diagnosis of FFS. A fragility fracture is a low-energy trauma leading to osteoporosis fracture [2]. Patients with dementia and those exposed to high-energy trauma were excluded from the study because dementia can lead to inaccurate reporting of symptoms, and high-energy trauma can lead to injuries other than fragility fractures. We excluded three patients as a result of these. Thus, we included 56 patients in the present study.
Radiography and computed tomography (CT) of the lumbar spine, hip joint, or pelvis were ordered depending on the patient’s symptoms and physical findings at admission. Magnetic resonance imaging (MRI) was performed during hospitalization before any fractures were diagnosed or if the cause of the pain could not be determined. All diagnoses were made by the orthopedic surgeon, who examined the patient upon admission. All diagnostic images were reviewed in consultation with other experienced orthopedic surgeons.
The CT was performed using a tube potential of 120 kV and a slice thickness of 1.25 mm (Revolution HD; GE Healthcare, Tokyo, Japan). Sagittal, coronal, and axial images were obtained using multiplanar reconstruction. MRIs of the lumbar spine mainly included T2 turbo spin echo (TSE) sagittal, T2 axial, T1 TSE sagittal, and T1 axial sequences (Discovery MR750w 1.5 Tesla; GE Healthcare). Sagittal or coronal short tau inversion recovery (STIR) images were included when those sequences were ordered. T2 TSE coronal, T2 axial, T1 TSE coronal, T1 axial, and STIR coronal sequences were often used in the hip joint or pelvis MRIs. The 1.5-Tesla MRI was used throughout our study, which lasted from 2006 to 2021. During this period, there were no changes to the imaging diagnostic tool. Our hospital’s response time for CT and MRI is typically 1 to 3 days. The radiographic, CT, and MRI images were examined by an experienced orthopedic surgeon. Because bone mineral density (BMD) of the lumbar spine was influenced by degeneration and did not accurately indicate osteoporosis, dual-energy X-ray absorptiometry (DEXA) was used to obtain BMD and T-scores of the femoral neck. The following patient information was retrieved from patients’ medical records: age, gender, medical history related to osteoporotic fragility fractures or spinal diseases, a history of trauma, pain regions, initial diagnosis, time from admission to a final diagnosis of FFS, and length of hospital stay. Additionally, CT and MRI sensitivities in fracture detection were examined using radiographs. Continuous variables were analyzed using the Student t-test. All analyses were performed using the IBM SPSS statistical software ver. 25.0 (IBM Corp., Armonk, NY, USA). The review board of Seirei Sakura Citizen Hospital approved the study protocol, including the review of patient records (approval code: 2021029). Informed consent was obtained from patients or their families.
Results
Patient demographic data are shown in Table 1. The mean age at hospital admission was 80.2±9.2 years. The BMD was 0.46±0.13 g/cm2, and the BMD T-score was −3.6±1.5. Twenty-two patients (43%) had a history of osteoporotic vertebral fracture, and 6 (11%) had a history of proximal femoral fractures. Forty-one patients (73%) presented with low back or buttock pain, 9 (16%) presented with groin pain, and 17 (30%) presented with thigh or leg pain. There was no history of trauma in 18 patients (32%).
Twenty-seven patients (48%) were diagnosed with sacral or pelvic fragility fractures during the initial visit using a radiograph or CT imaging. In contrast, 29 patients (52%) were initially misdiagnosed with lumbar spine diseases (23 patients), such as vertebral fractures or lumbar spinal stenosis, hip joint diseases, such as proximal femoral fractures (two patients) and hip joint arthritis (one patient), and buttock bruises (three patients) (Tables 2, 3). These patients were initially misdiagnosed, but MRI revealed sacral or pelvic fragility fractures. The time required to obtain a definitive diagnosis was 5.0±10.9 days, with 50 patients (89%) requiring less than 10 days and six patients (11%) requiring more than 10 days. The longest time required for a definitive diagnosis was 54 days (Fig. 1, Table 3). The length of hospital stay was 49.8±29.8 days in diagnosed cases and 42.4±23.2 days in delayed cases, respectively. The difference in hospital stay between these two groups was not statistically significant (p=0.30).
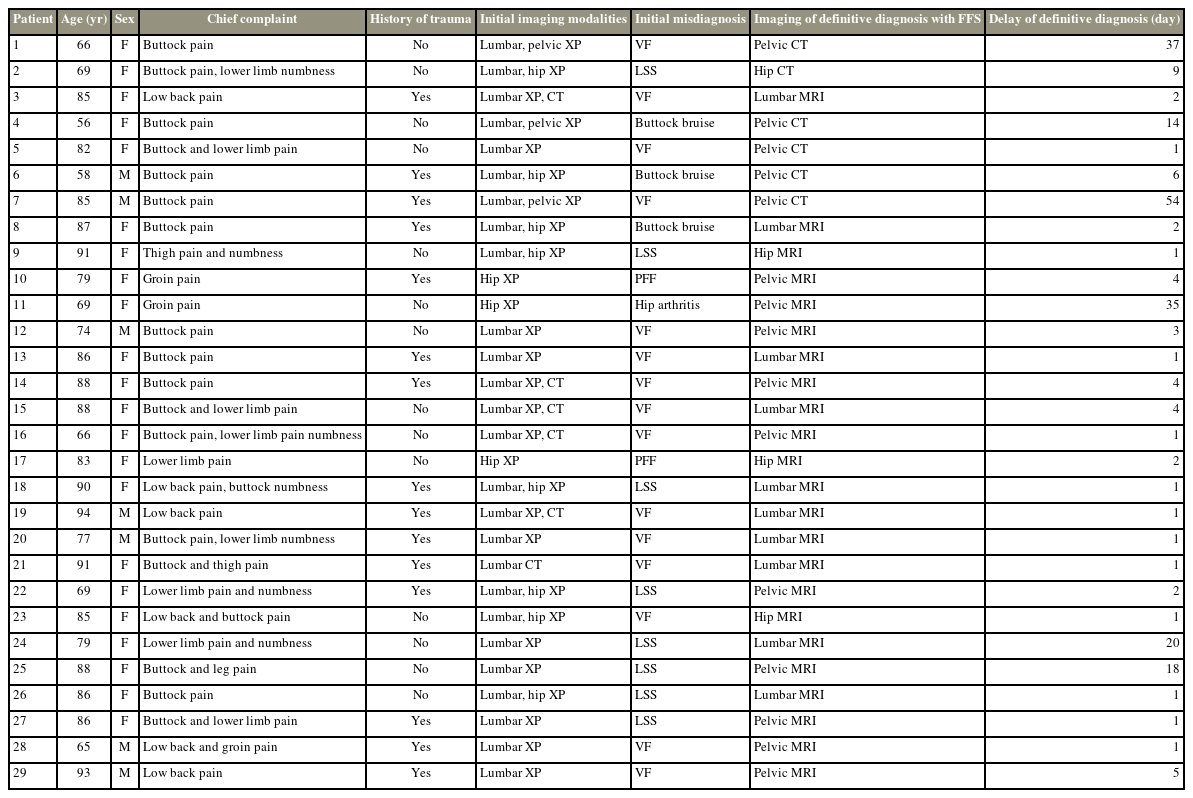
Demographic and clinical data for 29 patients initially misdiagnosed with lumbar spine diseases, hip joint diseases, or buttock bruises
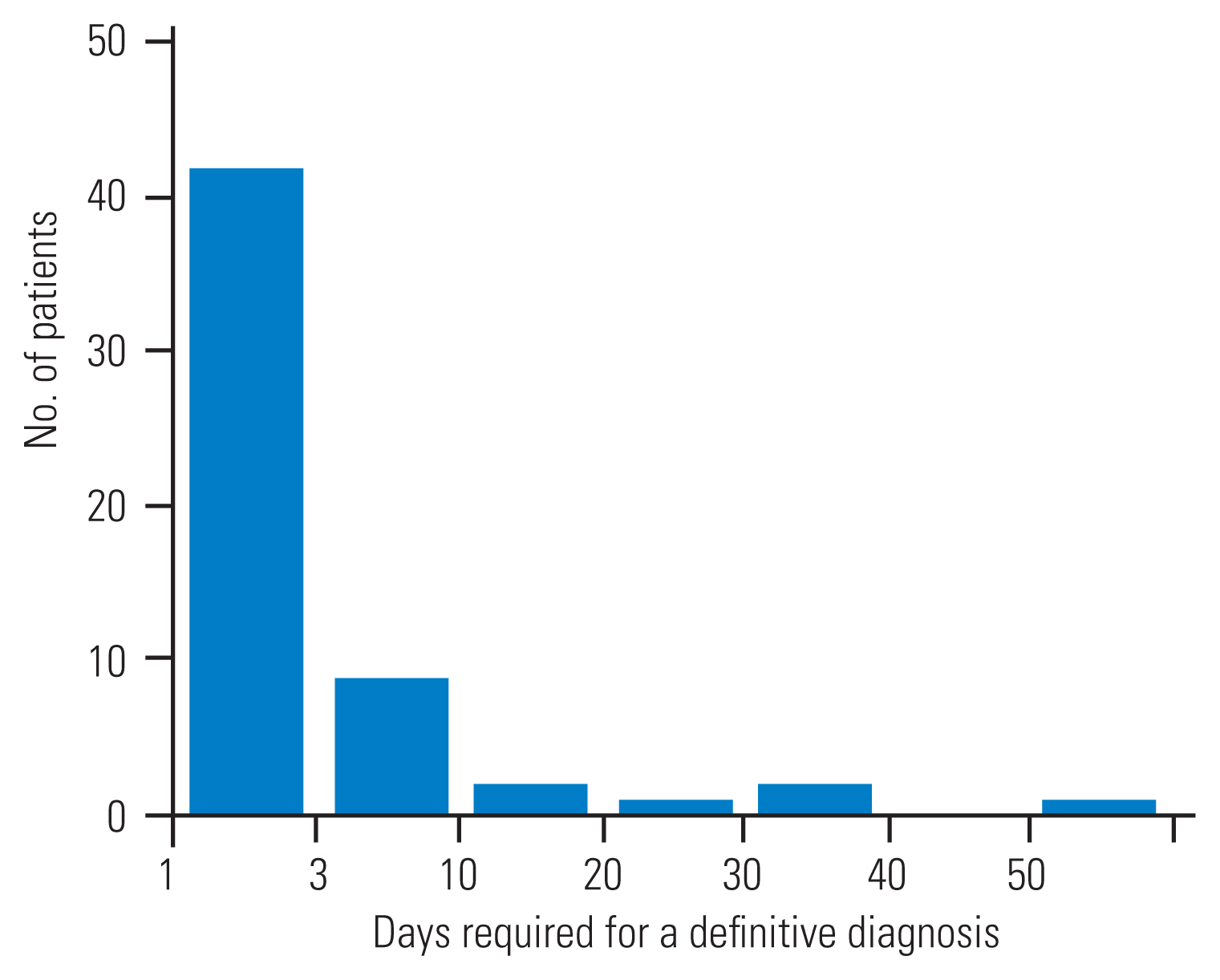
Graph illustrating the number of patients divided by the days required for a definitive diagnosis from first consultation. The time needed for definitive diagnosis was >10 days for six patients. The longest time was 54 days.
In total, 55 patients (98%) had radiographs obtained at the lumbar spine (46 patients), hip (21 patients), and pelvis (10 patients). Only one patient was diagnosed with a sacral fracture using a pelvic radiograph, and no patients were examined using a lumbar spine or hip radiograph.
Fifty-two patients (93%) had computed tomography images taken at the lumbar spine (25 patients), hip (eight patients), and pelvis (19 patients). Fifteen (29%) of 52 patients could not be diagnosed with FFS using CT. Thirteen of the 15 misdiagnosed patients were examined using a CT image of the lumbar spine. Although a sacral fracture was missed at the initial examination, fractures were diagnosed retrospectively in 12 of 13 patients. A sacral fracture was not confirmed retrospectively in one patient. Most patients who underwent CT of their hip or pelvis were diagnosed with sacral or pelvic fragility fractures, whereas three patients’ fractures were not confirmed retrospectively.
Forty-five patients (80%) had magnetic resonance imaging at the lumbar spine (20 patients), hip (five patients), and pelvis (20 patients). Three of the 45 patients (7%) were not diagnosed with FFS following a lumbar spine MRI. Coronal STIR sequences were lacking in two patients, and the sacral fracture was not observed in the sagittal images. The sacral fracture was missed in one of them, although it was observed retrospectively in the T1 coronal images (Fig. 2). After a re-examination of an MRI or CT scan of the pelvis, these three patients were finally diagnosed with sacral fractures. Bone marrow edema in the sacral alar or body was detected in coronal STIR images in 17 of the 20 patients who underwent lumbar MRI (Fig. 3). All 25 patients who underwent hip or pelvic MRI were diagnosed with sacral fractures.
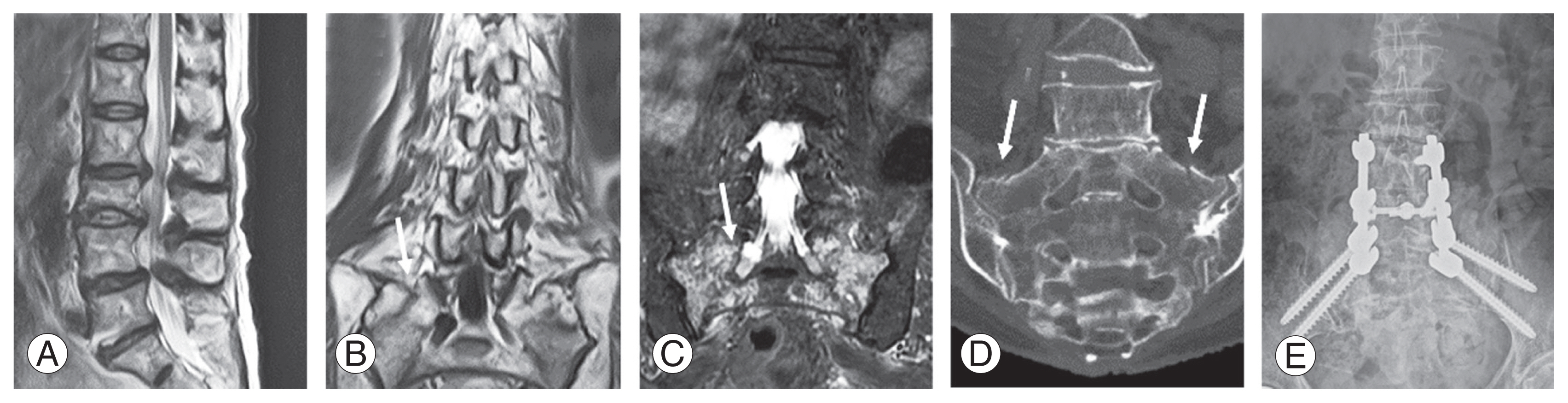
Case 1 (a misdiagnosis): An 88-year-old woman with no history of trauma was admitted after reporting buttock and leg pain. (A) A T2 sagittal magnetic resonance imaging (MRI) showed lumbar spinal canal stenosis. (B) A sacral fracture was observed on the T1 coronal image; this finding was initially missed, and the patient was originally misdiagnosed with lumbar canal stenosis (arrow). Despite conservative treatment consisting of a caudal block, her pain worsened. (C) A pelvic MRI and coronal short tau inversion recovery images revealed bone marrow edema at the sacral foramina (arrow), and (D) coronal computed tomography images of the pelvis showed fractures of the sacral ala (arrows). She was finally diagnosed with fragility fractures of sacrum 2 weeks after her initial visit. (E) Conservative treatment was ineffective; therefore, she underwent a spinopelvic fusion and her buttock and leg pain were relieved postoperatively.
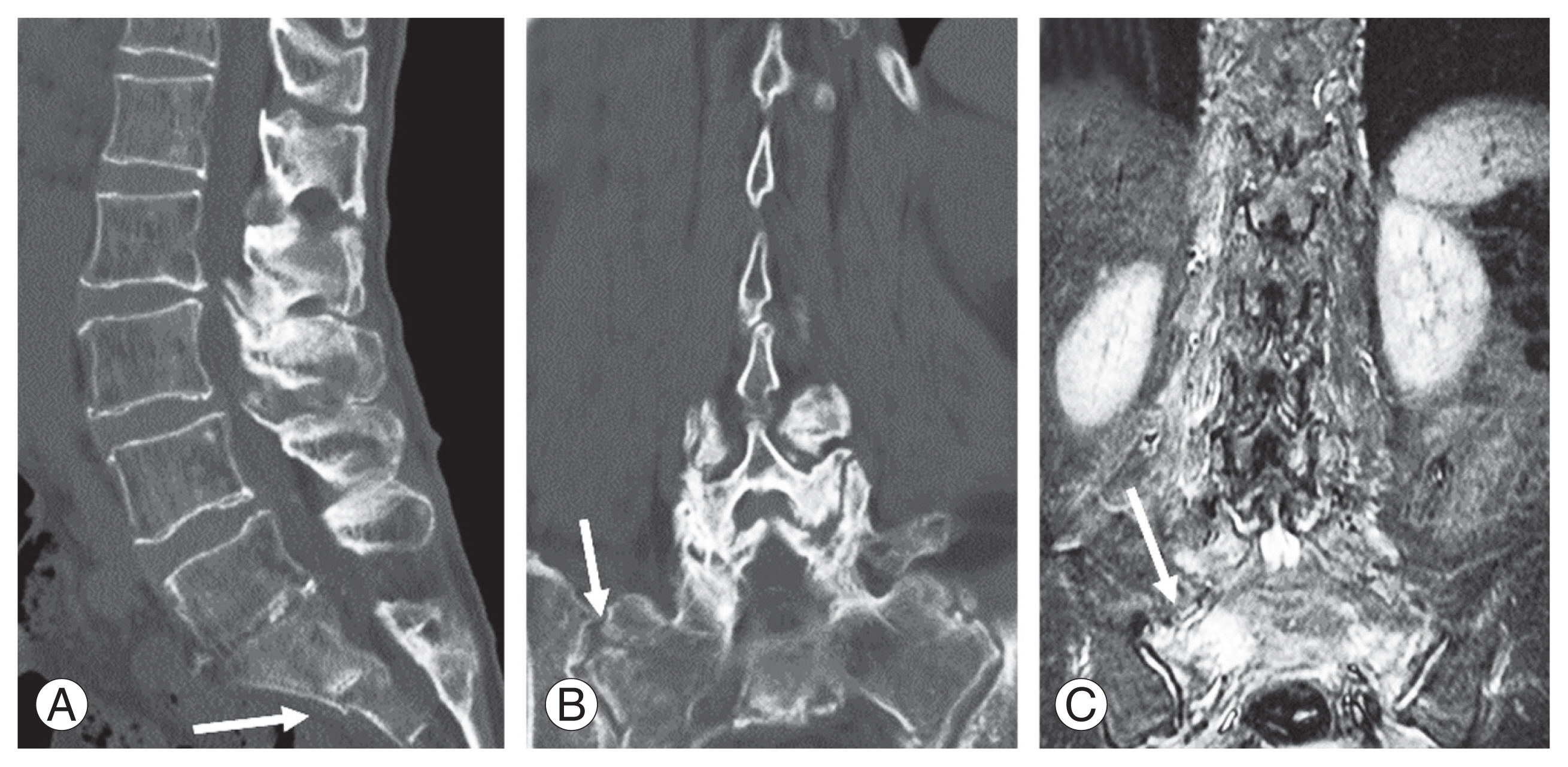
Case 2 (a correct diagnosis): An 88-year-old woman was admitted after reporting buttock pain. (A, B) Multiplanar reconstruction computed tomography of the lumbar spine showed a sacral fracture extending to the sacroiliac joint (arrow). (C) In addition, coronal-short tau inversion recovery magnetic resonance imaging of the lumbar spine revealed bone marrow edema of the sacrum extending to the sacroiliac joint, which was a correct diagnosis of fragility fractures of sacrum upon admission (arrow).
Fracture patterns were as follows: H- or U-shaped (35 patients), unilateral sacral alar (16 patients), and bilateral sacral alar (five patients). Fractures or bone marrow edema of the sacrum spreading to the sacroiliac joint surface was observed in 31 patients (55%) (Fig. 3B, C).
Six patients exhibited a concomitant fresh thoracolumbar vertebral fracture on lumbar MRI, four of whom were included in the cohort of misdiagnosed patients.
Discussion
We found that 52% of the patients with FFS were misdiagnosed with lumbar spine disease, hip joint disease, or simple bruises, all of which delayed a definitive diagnosis of FFS. Patients with FFS experienced lower limb symptoms, such as groin, thigh, and leg pain. To the best of our knowledge, this is the first study report in English that summarizes the probability of initial misdiagnoses and the cause of delayed definitive diagnoses in more than 50 patients with FFS. Previous English-language papers reviewed only three to 42 patients with an SIF or FFS, and the probability of initial misdiagnosis was not investigated [5,7–9,12]. Therefore, our report is unique in that the number of patients evaluated exceeds those previously reported, and we summarize the probability of initial misdiagnosis.
The present study revealed that half of the patients with FFS were initially misdiagnosed. There were 23 patients misdiagnosed with lumbar spine diseases, three with hip joint diseases, and three with buttock bruises. The cause of the initial misdiagnosis of the lumbar spine or hip joint disease is that some patients with FFS reported thigh, leg, or groin pain or presented without a history of trauma (32%). Previous reports of patients with FFS with pain and decreased sensation in the lower extremities show that 20%–80% have no history of trauma [5–8,11,12]. Our results are consistent with previous reports. In general, orthopedic surgeons typically consider neural symptoms, such as those reported as a result of lumbar spine diseases, in elderly patients with severe thigh and leg pain that requires hospitalization despite no history of trauma [13]. Furthermore, patients with lumbar vertebral fractures may not have a history of trauma [14]. Therefore, we postulated that patients with FFS may have been misdiagnosed with lumbar spinal diseases, such as lumbar spinal stenosis or lumbar vertebral fracture. Four of the misdiagnosed patients had a concomitant fresh thoracolumbar vertebral fracture, and our initial focus on detecting vertebral fractures led us to overlook the presence of concomitant SIF.
In the present study, 11% of patients with FFS did not receive a definitive diagnosis for 10 days; previous reports have also observed 14- to 92-day delays to diagnosis [10]. The delay in diagnosis was due to the failure to consider the possibility of FFS despite patients having persistent symptoms.
We postulated that there may be two mechanisms causing leg pain in patients with FFS. The first mechanism might be lumbar (L) or sacral (S) nerve root irritation (at L5, S1, or S2) caused by a bone fragment as a result of an FFS. The present study mainly observed H- or U-shaped fractures when patients experienced FFS. These fractures, which included S1 or S2 lesions, impacted the sacral ala or sacral foramina. Therefore, we considered stimulation of the L5 nerve root caused by bone fragments at the sacral ala and S1 and S2 nerve root irritation at the sacral foramina as the cause of the leg pain. Previous reports showed that 28% of patients with Denis classification type II sacral fractures had sciatic nerve symptoms associated with L5, S1, and S2 lesions and that some patients with FFS had neurological complications [4,6,15,16].
For the second mechanism, we considered the possibility of referred pain from the sacroiliac joint. In the present study, 55% of patients with FFS had fractures or bone marrow edema spreading to the sacroiliac joint surface. Previously, more than 60% of patients with sacroiliac joint disorders have reported leg symptoms, such as pain, numbness, and tingling [17]. Therefore, lower limb pain in patients with FFS may be caused by these mechanisms.
The mean BMD T-score of the femoral neck was −3.6 based on the patient’s medical history with an FFS. Additionally, 39% of patients had a history of osteoporotic vertebral fractures, with 11% having a proximal femoral fracture. This suggests that patients with FFS had severe osteoporosis underlying their pathology [18]. Previously, patients over 80 years old with severe osteoporosis were more likely to develop FFS [9]. We recommend that elderly patients with back or leg pain be examined for DEXA, as FFS is one of the potential differential diagnoses.
Our study revealed that 54 of 55 patients (98%) were not diagnosed with FFS using a radiograph; 15 of 52 patients (29%) were not analyzed using CT; and three of 45 patients (7%) were not analyzed using MRI. Previously, the rate of FFS misdiagnosis was 85% using radiography, 12% using CT, and 0% using MRI [19]. We believed that the misdiagnosis rate in our study was higher than previously reported because pelvic CTs were not obtained for half of the patients and coronal STIR plane images (containing the sacrum) were missing in three patients with lumbar spine MRIs. Thirteen of the 15 patients were misdiagnosed using CT, and all three cases of misdiagnosis based on lumbar spine MRIs, but not the sacral spine, were the primary cause for our re-evaluations. A high rate of missed sacral fractures due to lumbar CT or MRI, even though they were retrospectively confirmed, strongly suggests that doctors must examine the sacrum using these types of scans rather than automatically assuming lumbar spinal disease.
On the other hand, FFS was successfully diagnosed using lumbar MRIs with coronal STIR sequences. The use of coronal STIR sequences in lumbar MRIs for diagnosing FFS has already been reported [19]. Therefore, even if a patient has no history of trauma, reports back or leg pain, and is diagnosed with a lumbar spine disease during the initial examination, it is essential to consider the possibility of a pelvic fracture. In such cases, we recommend obtaining coronal STIR images and focusing on the sacrum.
The length of hospital stay did not significantly differ between cases with an accurate diagnosis and those with a delayed diagnosis, as conservative treatment was primarily used in both groups. However, it has recently been shown that using surgical intervention as a treatment option could decrease the length of hospital stay with precise diagnosis [1,2].
The present study had some limitations. First, this study was retrospective in design; hence, there was no information concerning exacerbations of symptoms associated with activities, such as walking and positions, such as standing or sitting, and particular physical findings at the pelvis, including percussion tenderness at the sacrum. When this information is accessible, future studies might help distinguish between FFS and lumbar spinal disease. Second, the treatment and clinical course were not well investigated. Understanding the effect of an initial misdiagnosis and the delay it causes in obtaining a final diagnosis on treatment results highlights the need to examine the possibility of FFS in the early stages of care.
Conclusions
In conclusion, 46% of patients with FFS in the present study had lower limb symptoms, such as groin and/or leg pain, whereas 32% had no history of trauma. Additionally, 52% of patients with FFS were initially misdiagnosed with lumbar spine disease, hip joint disease, and bruises. Therefore, when these clinical symptoms are reported, we recommend considering FFS as one of the differential diagnoses and performing lumbar or pelvic MRIs, particularly coronal STIR images, to rule out FFS.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Study conception: YI; acquisition, analysis of data: RU, YI, NY; manuscript writing: RU; supervision: YI; writing–review & editing: TK, TS, SK, KU, DK, TA, YS, SM, SO, KN; and all authors read and approved the final manuscript.


