Predicting the Need for Surgery in Patients with Lumbar Disc Herniation: A New Internally Validated Scoring System
Article information
Abstract
Study Design
Prospective study.
Purpose
To propose a scoring system for predicting the need for surgery in patients with lumbar disc herniation (LDH).
Overview of Literature
The indications for surgery in patients with LDH are well established. However, the exact timing of surgery is not. According to surgeons, patients with failed conservative treatment who underwent delayed surgery, often after 6 months post-symptom initiation, have poor functional recovery and outcome.
Methods
The current study included patients with symptomatic LDH. Patients with an indication for emergent surgery such as profound or progressive motor deficit, cauda equina syndrome, and diagnoses other than single-level LDH were excluded from the analysis. All patients followed a conservative treatment regimen (a combination of physical therapy, pain medications, and/or spinal epidural steroid injections). Surgery was indicated for patients who continuously experienced pain despite maximal conservative therapy.
Results
In total, 134 patients met the inclusion and exclusion criteria. Among them, 108 (80.6%) responded to conservative management, and 26 (19.4%) underwent unilateral laminotomy and microdiscectomy. The symptom duration, disc degeneration grade on magnetic resonance imaging (Pfirrmann disc grade), herniated disc location and type, fragment size, and thecal sac diameter significantly differed between patients who responded to conservative treatment and those requiring surgery. The area under the receiver operating characteristic curve of the scoring system based on the anteroposterior size of the herniated disc fragment and herniated disc location and type was 0.81.
Conclusions
A scoring system based on herniated disc/fragment size, location, and type can be applied to predict the need for surgery in patients with LDH. In the future, this tool can be used to prevent unnecessarily prolonged conservative management (>4–8 weeks).
Introduction
Lumbar disc herniation (LDH) generally follows a favorable natural course as most patients recover well within approximately 4–6 weeks after conservative therapy. Conservative treatment includes pain medication and physical treatment, with or without interventional pain therapy including epidural steroid injections. Patients with profound neurologic deficits such as cauda equina syndrome and progressive motor deficit and those with failed conservative treatment are surgical candidates [1].
The treatment of choice and the candidates for conservative treatment versus surgical intervention are well established. However, the timing of surgery is not. Nonetheless, some studies have revealed that patients with delayed surgery after conservative treatment and those with a waiting time of >12 weeks after symptom initiation had a worse functional recovery and outcome [2–5].
To achieve a better functional outcome and long-term pain relief, it can be helpful to predict which patients are likely to have failed conservative management. A previous study has published their results regarding the predictive value of clinical and imaging findings in determining the need for surgery in patients diagnosed with LDH [1]. Herein, we propose a scoring system for predicting the need for surgery in these patients. To the best of our knowledge, it is the first on the only scoring system that can predict the need for surgery in patients with LDH.
Materials and Methods
The current study included patients with a history of low back pain or related radicular leg pain. The exclusion criteria were patients who received standard treatment from a practitioner prior to presenting to our clinic, those with a history of prior lumbar surgery, those with diagnoses other than single-level LDH (such as multilevel LDH, lumbar canal stenosis, and spondylolisthesis), and those with an indication for urgent surgery, such as profound or progressive motor deficit and cauda equina syndrome.
After the initial presentation and gathering of baseline data, all patients followed a conservative treatment regimen and underwent lumbosacral magnetic resonance imaging (MRI). The conservative treatment included a standard course of physical therapy and pain medications (a combination of non-steroidal anti-inflammatory drugs, opioids, and gabapentin) for at least 4–6 weeks. In accordance with the standard practice of care, patients who continuously presented with radicular leg pain despite maximal conservative therapy and those who had confirmed single-level disc herniation on MRI were considered surgical candidates.
The following data were collected: age, sex, occupation, symptom duration, neurologic examination findings, MRI data including LDH level, presence or absence of osteophytes, annular tears, Modic-type changes and their grade, Pfirrmann disc grade, disc herniation location in the axial plane (central, paracentral, foraminal, or extra-foraminal), disc herniation type (bulging, protrusion, or extrusion), nerve root compression (van Rijn classification), and the anteroposterior (AP) and mediolateral (ML) diameter of the thecal sac (both measured in the axial MRI plane). The classification of disc herniation type was based on the MRI results. Disc bulging was defined as disc tissues extending beyond the edges of the ring apophyzes via the disc circumference. Disc protrusion was defined as the distance between the edges of the disc herniation measuring less than the distance between the edges of the herniation base. In contrast, extrusion was defined as the distance between the edges of the disc fragment measuring greater than the distance at the base [6]. In cases of disc protrusion and extrusion, the AP and ML size of the herniated fragment (again assessed in the axial MRI plane) were also measured.
Based on these measurements, two ratios were calculated: (1) AP fragment ratio=AP herniated fragment size/AP thecal sac diameter and (2) ML fragment ratio=ML herniated fragment size/ML thecal sac diameter (Fig. 1). All MRI interpretation and measurements were performed individually by two neurosurgeons. Discordances were resolved by a neuroradiologist who was blinded from the study.
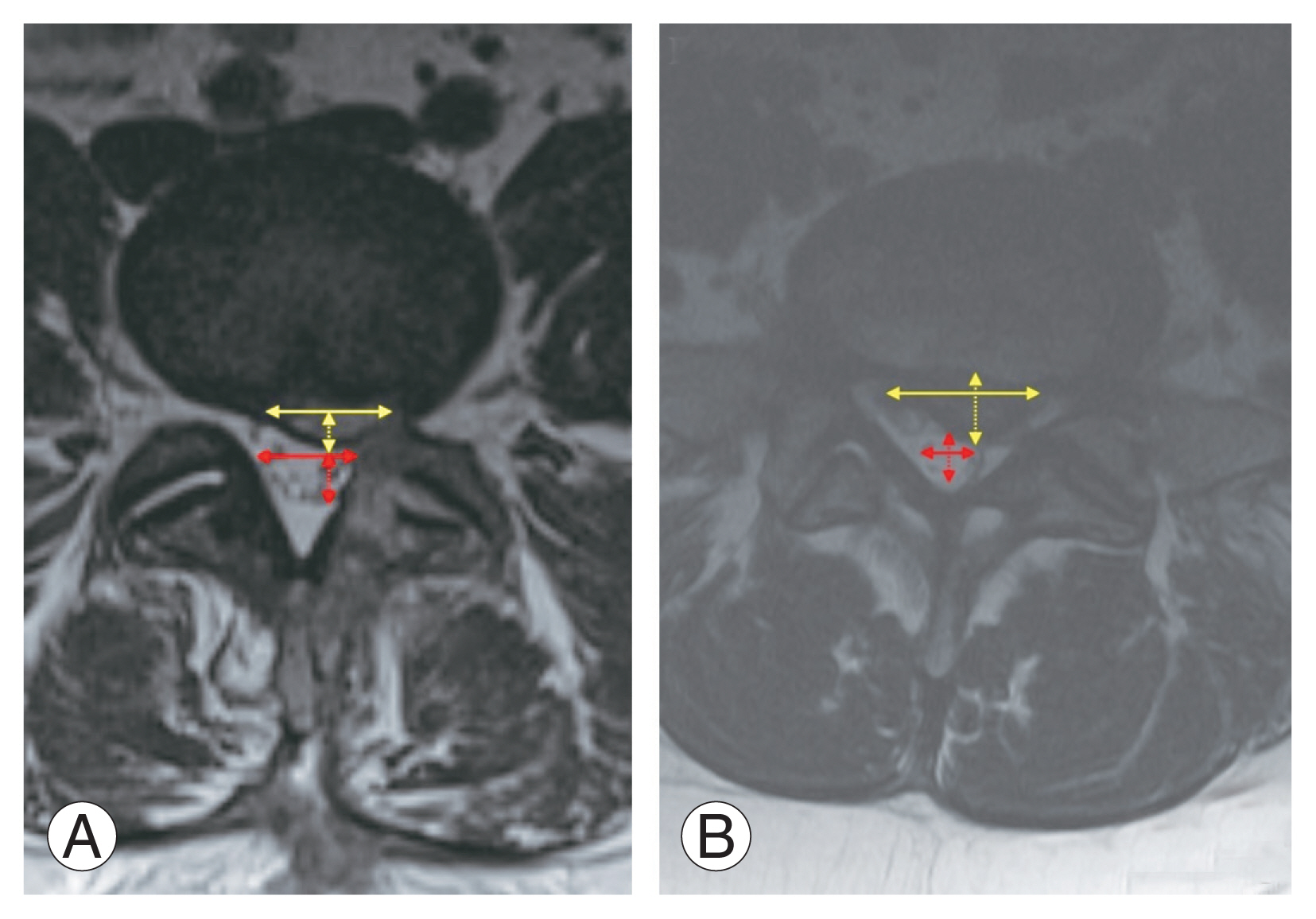
Axial T2 magnetic resonance imaging shows measurement of anteroposterior (AP) and mediolateral (ML) (solid yellow double-headed arrow) size of the herniated fragment (dashed and solid yellow double-headed arrow, respectively) and AP and ML diameter of the thecal sac (dashed and solid red double-headed arrow, respectively) on a disc protrusion (A) and extrusion (B).
Quantitative and qualitative data were analyzed with the independent samples t-test and the chi-square test, respectively. Based on the significant factors in the univariate and bivariate analyses, different scoring systems were created with a combination of various factors for predicting the need for surgery. The created scoring systems were then analyzed using the receiver operating characteristic curves (ROCs). The model with the highest area under the ROC curve (AUC) was selected, and a scoring system was assigned and proposed. A p-value of <0.05 was considered statistically significant. All analyses were performed with PASW SPSS Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). All patients provided consent for all diagnostic and therapeutic procedures. The current study was approved by the instituitional review board (IRB approval no., 139007).
Results
During the study period, 134 patients met the inclusion and exclusion criteria. Table 1 shows the clinical data of the patients. L4–L5 (57.8%) was the most commonly involved level on MRI, followed by L5–S1 (35.3%). Disc bulging, protrusion, and extrusion were observed in 32.8%, 54.5%, and 12.7% of patients, respectively. In terms of extruded subtype, 10 and seven patients presented with subligamentous and transligamentous extrusions, respectively. None of the patients in the target population had sequestrated disc herniation. In the whole cohort, 108 patients (80.6%) were responsive to conservative management, and 26 (19.4%) had failed conservative management. In total, 26 patients received a course of standard medical management with three different medications and a full course of physical therapy (4–6 weeks, at least 10 sessions). Eight patients had received lumbar epidural steroid injection, with failure to respond to treatment. The mean±standard deviation time from injection to surgery was 24.0±7.1 days. Persistent radicular leg pain was the indication for surgery in all patients. The patients underwent unilateral laminotomy and microdiscectomy at the corresponding level.
1. Factors predicting the need for surgery
Table 2 shows the factors predicting the need for surgery in the study cohort. The symptom duration significantly differed between patients who responded to conservative treatment and those who needed surgery (p=0.02). The rates of surgery after failure to respond to conservative management were 23.6%, 30.0%, and 33.3% in patients who had symptoms for <6, 6–12, and >12 months, respectively (p=0.81). Furthermore, there were significant differences in terms of disc degeneration grade on MRI (Pfirrmann disc grade), herniated disc location and type, presence of nerve root compression, AP size of the herniated fragment, and thecal sac diameter and fragment ratios of AP and ML between patients who responded to conservative treatment and those who needed surgery (p-values=0.008, <0.001, <0.001, 0.08, <0.001, 0.02, <0.001, 0.003, and <0.001, respectively). In contrast, sex, age, occupation, disc level, Modic-type changes and their grade on MRI, presence of osteophytes, annular tears, extrusion disc type (i.e., subligamentous versus transligamentous), and ML herniated disc fragment size were not significant predictors of the need for surgery (p-values=0.53, 0.08, 0.50, 0.43, 0.58, 0.37, 0.48, 0.73, and 0.12, respectively).
2. Scoring system for predicting the need for surgery
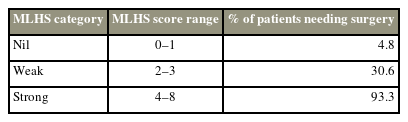
Based on significant surgical predictors (symptom duration, Pfirrmann disc grade, herniated disc location and type, nerve root compression, AP herniated disc fragment size, and AP and ML thecal sac diameter), numerous models were created and tested with the combination of 3–4 factors. ROC analysis showed that the best scoring system was based on the AP size of the herniated disc fragment and herniated disc location and type (Table 3), with an AUC of 0.81 (p<0.001). Fig. 2 shows the number of patients who responded to treatment versus those who are refractory to conservative management based on each score. Chi-square test analysis further showed that there was a significant propensity toward responding to conservative management in patients with scores of 0 and 1 and the need for surgery in those with scores of ≥4 (p<0.001). Therefore, the scoring system was categorized to three levels based on the power to predict surgery (scores of 0–1, 2–3, and ≥4) (Table 4).

The components of the proposed scoring system (microdiscectomy for lumbar disc herniation scoring system)
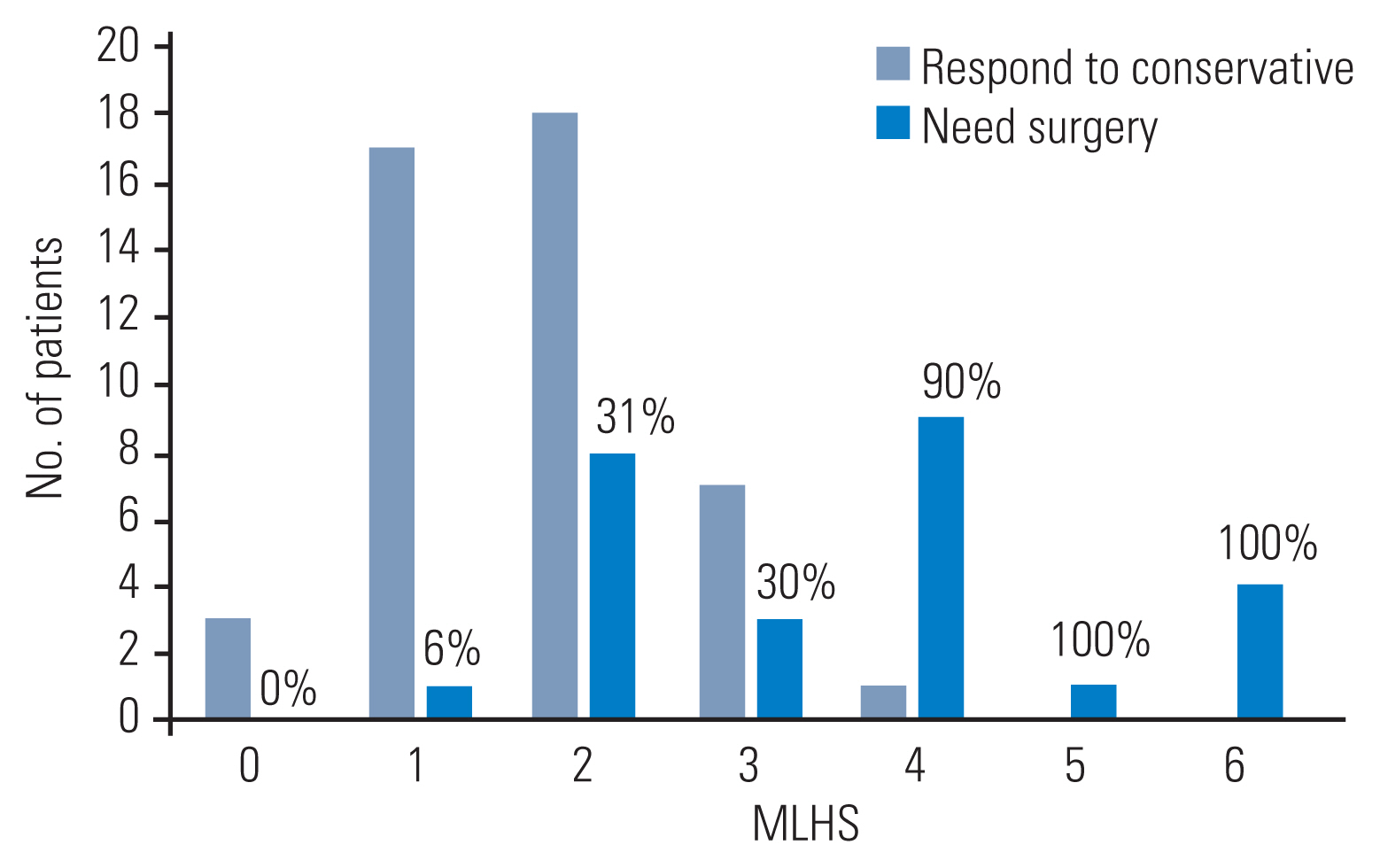
Patients who do or do not respond to conservative treatment, based on the proposed microdiscectomy for lumbar disc herniation scoring system (MLHS). The number over the bars shows the percent of patients needing surgery within each score.
Discussion
The initial treatment protocol for LDH generally comprises conservative therapy including a combination of pain medication, physical treatment, and epidural steroid injections. In general, surgery is recommended to patients with profound neurologic deficits (such as cauda equina syndrome and progressive motor deficit) or those who initially had failed conservative treatment [1]. In our study, the inclusion criteria included patients with persistent radicular leg pain despite full course of conservative management (three types of medications plus full course of physical therapy in all patients and epidural steroid injections in some).
The treatment of choice and indications for the available treatment modalities is well established in the current practice. However, the timing of surgery associated with the best long-term functional outcome is not. Hence, this has been a cause of concern highlighted in the study by Folman et al. [7], which reported that 10%–40% of patients who underwent surgery did not experience optimal expected pain relief. Several authors have shown that delayed surgery in cases with prolonged conservative treatment is associated with worse functional outcomes [2,3]. A randomized crossover study of 142 patients showed that those opting to do surgery within 12 weeks of symptom initiation had significantly greater recovery and outcomes at 6 and 12 months after surgery, respectively [4]. Another study of 291 patients revealed that patients with surgery waiting times >12 weeks were 70% more likely to experience worse pain at 6 months after surgery compared with those with a waiting time of <12 weeks [5]. Two systematic reviews with 21 and 11 studies revealed that a longer duration of preoperative leg pain led to poorer outcomes in patients undergoing surgery for LDH. However, due to the broad time frame of different studies, 6 months was the optimal time for surgical intervention [8,9]. Further, early surgery is associated with a faster recovery of leg pain and rate of perceived recovery [10]. Dedicated studies have recommended surgery after 6–8 weeks of failed conservative management in indicated cases [7].
Our study showed that patients who finally underwent surgery had a longer symptom duration, higher Pfirrmann’s grades (an indicator of disc degeneration grade), nerve root compression, and larger disc fragments on MRI. In addition, surgery was significantly dependent on herniated disc location and type. Regarding location, if the herniation was further from midline, the likelihood of surgery was higher. Hence, the proximity of the herniated disc fragment to the nerve root is a major predictor of surgery. In addition, consistent with our observation, patients with nerve root compression (based on the van Rijn classification) had a higher rate of failed conservative management [11]. Bokov et al. [12] reported that nerve root compression in LDH was a possible link to chronic neuropathic pain. Moreover, extruded herniation types were the most prevalent in patients who underwent surgery, followed by protruded types (Table 2). However, the current study used the general disc herniation types of bulging, protrusion, and extrusion. In our study, there was no statistically significant difference in the rate of conservative treatment failure for the extrusion subcategories of transligamentous versus subligamentous. Of note, we did not observe any disc sequestration in our patient cohort. Hence, this could not be included in the analysis. Moreover, due to the limitations of data recording, transligamentous versus subligamentous were based on MRI findings, not actual observation and visualization during surgery, which might introduce some inaccuracies. There is evidence that transligamentous and sequestered subtypes have a higher rate of non-successful conservative management. This can be a topic of focused studies in the future [13,14].
Based on these results, we further proposed a scoring system to predict the need for surgery in patients with symptomatic LDH (microdiscectomy for lumbar disc herniation scoring system, MLHS). MLHS comprises three simple components including herniated disc/fragment size, location, and type, with a good accuracy (0.81) based on ROC analysis. Our results confirmed that a MLHS score of ≥4 (range, 0–8) is associated with surgery in >90% of cases.
Few studies have proposed the use of scoring systems for in LDH in clinical settings. Lee et al. [15] suggested a scoring system based on disc degeneration, back muscle atrophy, facet joint degeneration, ligamentum flavum thickness, and interspinous ligament degeneration to predict symptom aggravation in patients with LDH. This scoring system can predict imaging progression in LDH, but not the need for microdiscectomy. Moreover, Boden et al. [16] proposed an 11-item questionnaire (Spine Surgery Likelihood-11 [SSL-11]) to triage patients with low back and leg symptoms who require surgery. SSL-11 identified a high likelihood for surgery group, in which 58% of patients underwent surgery [16]. This scoring system is more appropriate as a triage tool in spine clinics but is not designed to predict the need for surgery. To the best of our knowledge, MLHS is the first and only scoring system proposed to predict the need for surgery/microdiscectomy in patients with LDH. We believe that in patients with LDH, the proposed scoring can better help neurosurgeons and spine surgeons to decide regarding the need for surgery and chose patients for timely rather than unnecessarily late surgical treatment when patients fail standard conservative management beyond 6–8 weeks. However, we are not able to make a conclusion about the best timing or a timing beyond which surgical intervention is not successful based on our results. Therefore, this should be evaluated in further studies.
Our study had some limitations. First, patient recruitment might be a possible constraint. In surgical clinics, it could be a possible source of selection bias as patients were more likely to undergo surgery. The authors attempted to address this issue by recruiting only new patients and those who had not received a standard treatment prior to the visit at our clinic. The prospective nature of the study and the strict inclusion and exclusion criteria helped reduce selection bias. As indicated in our results, 19.4% of our patients underwent surgery, which is in accordance with the natural course in studies with a surgical rate of approximately 20%. Thus, bias did not affect the conclusion [1,17]. Second, due to manageable and tolerable symptoms, patients used over-the-counter medications or performed physical exercises at home prior to presenting to the clinic. This was reflected by significant variations in the length of symptoms. However, all patients received standard treatment only after they were enrolled in the study. Moreover, we did not control for specific medication options and types (such as steroids, non-steroidal anti-inflammatory drugs, and acetaminophen). However, all patients received at least three different medications before selecting surgery, which is in accordance with the standard of care. Therefore, further research should be performed to externally validate the scoring system in patients presenting to a non-surgical clinic (such as family medicine or physical medicine and rehabilitation clinics).
Conclusions
A scoring system based on herniated disc/fragment size (score of 0–3 based on the AP size of the herniated disc fragment), herniated disc location (0 for central, 1 for paracentral, and 3 for foraminal and extraforaminal), and herniated disc type (0 for bulging, 1 for protrusion, and 2 for extrusion) can be applied to predict the need for surgery in patients with LDH. In the future, this tool can be used to prevent unnecessarily prolonged conservative management (>4–8 weeks) in patients who are refractory to conservative management.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Authors’ Contributions
Study concept and design: RML; statistical analysis: RML, HS; manuscript preparation: RML; manuscript editing: RML, HS, UOE, AS, AJG, SV; and final approval of the manuscript: all authors.