Ultrasonic Bone Scalpel versus Conventional Methods for Osteotomy in Posterior Surgery for Cervical Spondylotic Myelopathy: A Review and Meta-Analysis
Article information
Abstract
Posterior methods for cervical myelopathy include laminoplasty and laminectomy with/without fusion. A more recent innovation in these treatments is the use of an ultrasonic bone shaver for osteotomy. In this study, we examined the perioperative results after laminectomy/laminoplasty between conventional methods (rongeur/high-speed drill) vs. piezosurgery-based instruments. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed and the search was performed on four databases (PubMed, Scopus, EMBASE, and Google Scholar). Seven comparative studies were chosen after thorough screening by the authors and a meta-analysis was performed between piezosurgery and conventional technique to ascertain intraoperative and postoperative results after laminectomy/laminoplasty. The analysis includes four retrospective cohort studies and three randomized controlled trials published between 2015 and 2022. The mean age ranged from 55.5 to 64.2 years. Blood loss was significantly reduced in the piezosurgery group, other findings were not significant. On subgroup analysis, laminoplasty dramatically reduced blood loss and the rate of iatrogenic dural rips in the piezosurgery group. The use of ultrasonic bone shaver for osteotomy in cervical spondylotic myelopathy is related to significantly decreased blood loss and no significant increase in postoperative drainage, operative time, complication rate, and functional outcomes as compared to traditional techniques. We noticed significantly reduced blood and rate of dural tears in the laminoplasty subgroup with the use of ultrasonic bone shaver, which was not mirrored in the laminectomy subgroup. Careful intraoperative handling of the instrument can help prevent iatrogenic dural tears and nerve damage.
Introduction
Cervical spondylotic myelopathy (CSM) is a common cause of disability in the elderly population with an underlying etiology that includes cervical spondylosis, ossified posterior longitudinal ligament (OPLL), and congenital cervical stenosis [1–3]. Karadimas et al. [4] in a systematic review discovered that 20%–60% of patients on conservative treatment would have neurological deterioration without surgical intervention. The Japanese Orthopaedic Association (JOA) score considerably improved in patients who underwent surgery, according to a meta-analysis by Feng et al. [5]. An individual patient-based surgical method is the suggested mode of treatment for cervical myelopathy [6–8]. Surgical approaches to the condition might be anterior, posterior, or combination. Posterior procedures for CSM include laminectomy with or without fusion and laminoplasty. Some indications of posterior procedures include multilevel disease, and primarily posterior pathology such as OPLL [6]. During these procedures, osteotomy is typically performed using bone rongeurs, bone punches, and high-speed drilling [9,10]. These are related to complications such as thermal injury, and soft tissue entanglement, and can be challenging to control and time-consuming [11,12]. The use of ultrasonic bone scalpels (UBS) for oral and maxillofacial surgery and skull base surgery has been well established [13–15]; this instrument is now being commonly employed in spine surgeries as well [12,16,17]. The theoretical benefits of UBS include greater precision, lesser soft tissue damage, lower blood loss, and reduced operating time [16,17].
The use of UBS in posterior cervical methods is a relatively new development and very few comparative studies are published on this subject. We performed this review and meta-analysis to analyze the intraoperative findings and postoperative result among osteotomies performed using conventional methods and the UBS.
Methods
1. Search methodology
The literature search was carried out on 20th June 2022. It was conducted on four databases—PubMed, Scopus, EMBASE, and Google Scholar, and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed [18]. The search was conducted using the keywords “Cervical AND Myelopathy AND Ultrasonic OR Piezosurgery.” To locate further material, a secondary search of the shortlisted papers’ references using the same criteria was conducted.
2. Inclusion and exclusion criteria
Initial screening of search results was performed to exclude duplicate studies. Non-English literature, case reports, conference abstracts, cadaveric and animal studies, non-comparative studies, and reviews were excluded. Studies in which a comparison between conventional methods (bone rongeur/high-speed drilling) and UBS/piezosurgery instruments for cervical osteotomy via posterior approach were done were included in our analysis. The meta-analysis omitted publications on surgical technique, anterior approaches for CSM, and publications that included all spinal procedures with no distinct results.
3. Data collection and analysis
To ascertain the relevance of search results for our analysis, two authors (P.B. and V.K.) performed a screening of all results. Any conflicts between the two were resolved with input and analysis from the remaining co-authors. In our review for meta-analysis, studies that were deemed pertinent to our research topic and having an appropriate study design were included (Fig. 1).
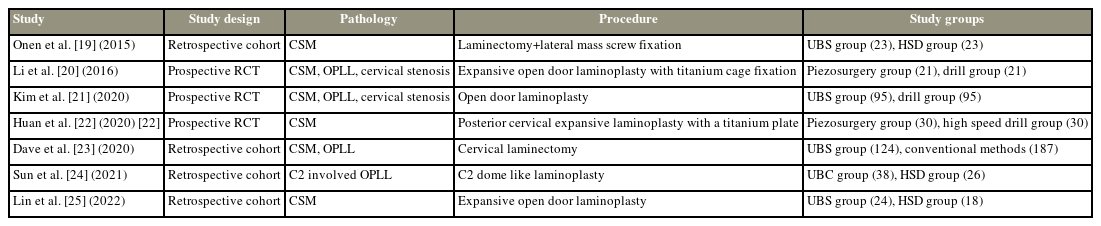
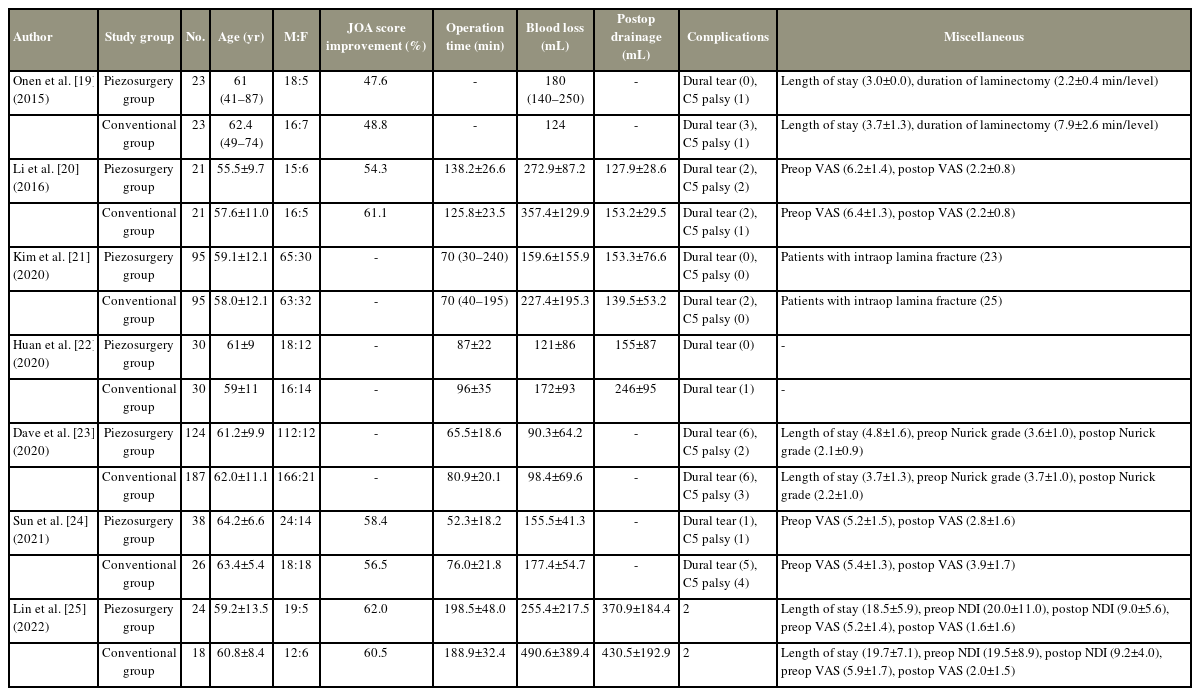
The studies which were chosen for review were thoroughly read and all data from these studies were extracted and entered into a structured format. Table 1 provides an overview of the study’s year of publication, design, pathology it examined, surgical technique, and study groups. Table 2 summarizes the mean age, gender distribution, and the reported intraoperative and postoperative findings. Various studies had given various labels for their study groups but for convenience, we have labeled patients who received osteotomy with UBS/piezosurgery instruments as the piezosurgery group and patients in whom high-speed drill or rongeur was used as the conventional group.
4. Search and screening results
After duplicates were eliminated, 246 articles were assessed. Based on preliminary screening based on title, study design, and language we were left with 42 publications for further evaluation. Following the reading of the abstracts for these, 19 further papers were chosen for a more thorough evaluation. Seven comparative studies published between the years 2015 and 2022 were then included in our final review and meta-analysis [19–25].
5. Statistical analysis
Comparative analysis between the piezosurgery group (experimental) and conventional group (control) was performed using Review Manager (RevMan) ver. 5.4 software (The Cochrane Collaboration, London, UK). Comparison of operating time, blood loss, and postoperative drainage were compared using inverse variance weighted meta-analysis. Using Mantel-Haenszel analysis, odds ratios (OR) were generated to compare the frequency of dural tears and postoperative C5 palsy.
6. Risk of bias assessment
Methodological index for non-randomized studies (MINORS) was used to evaluate the risk of bias in included studies [26].
Observations and Results
1. Study characteristics
All publications included in the review were comparative studies. Of the seven studies included in our review, four were retrospective cohort studies (level 3 evidence) [19,23–25] and the remaining three were prospective randomized controlled trials (RCT) (level 1/2 evidence) [20–22]. A total of 755 individuals were involved in this research, 355 of whom underwent piezosurgery and 400 of whom underwent conventional osteotomy. The primary pathologies included were CSM, OPLL, and cervical stenosis (Table 1).
2. Patient characteristics
Li et al. [20] reported a minimum mean age of 55.5 years for the piezosurgery group, while Sun et al. [24] reported a mean age of 64.2 years. The minimum mean age of patients in the conventional group was 57.6 years [20] and the maximum was 63.4 years [24]. Male predominance was observed in the patient groups with both the piezosurgery group and traditional group having 3–4 times males as compared to females (Table 2).
3. Surgical procedure
Onen et al. [19] carried out cervical laminectomy in their study cohort. While using UBS the whole thickness of the bone was cut till the softness of the dura was felt. Similar steps were taken by Dave et al. [23], who used UBS to cut the entire thickness of the bone. In cases with severe canal compromise, they left the inner cortex intact and completed the osteotomy with the help of a thin osteotome. Both authors have emphasized the importance of not applying any undue pressure to the instrument. Laminoplasty was the method performed by the other five authors in our review. Using a spatula blade, Huan et al. [22] finished the osteotomy in with additional osteotomies up to the inner cortex. A dome-like laminoplasty was conducted by Sun et al. [24], which included single open-door laminoplasty from C3 downwards, along with a C2 dome laminectomy.
4. Comparative analysis (piezosurgery versus conventional groups)
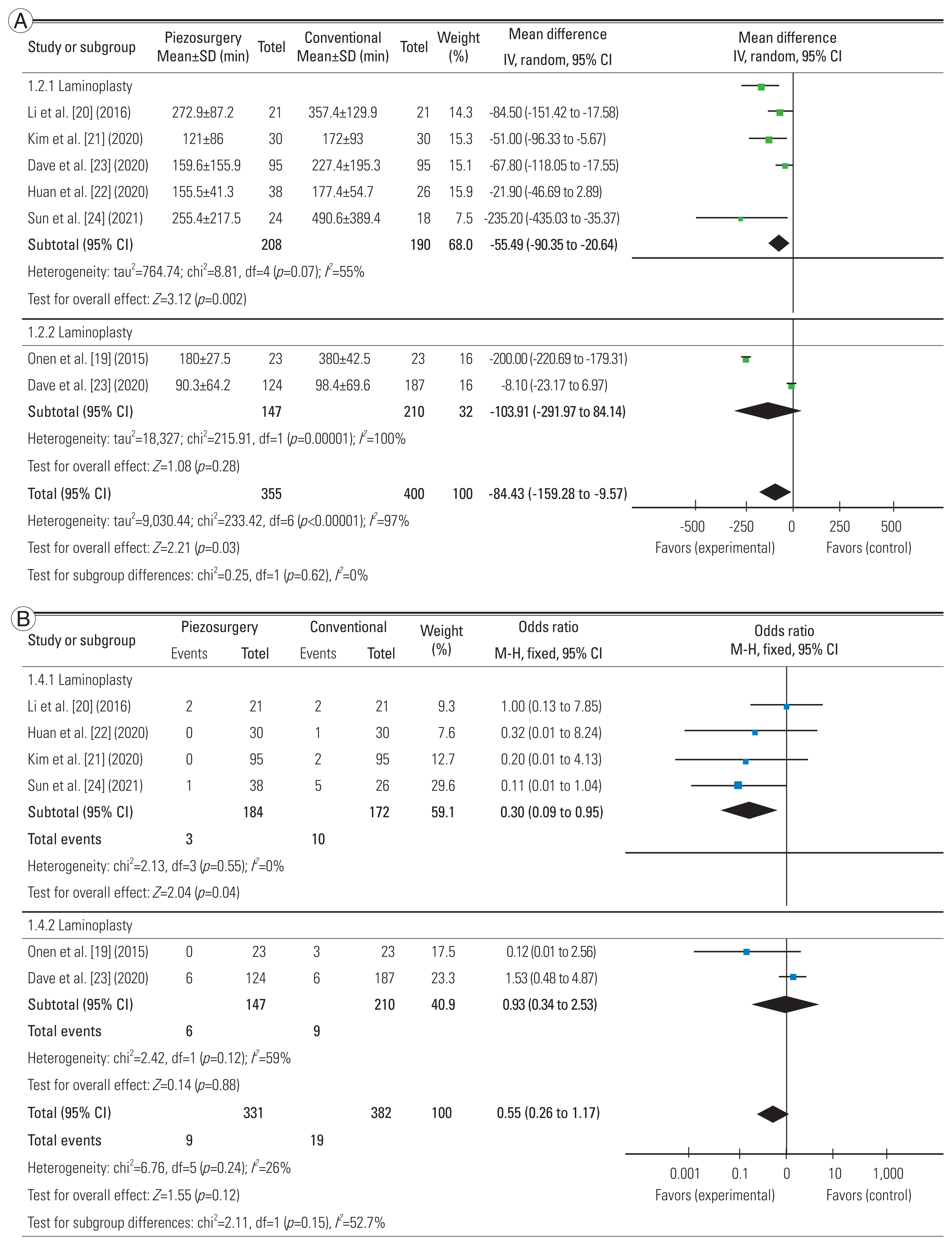
All seven studies were included in the comparative analysis. According to statistical analysis, the piezosurgery group experienced significantly less blood loss than the control group, with a mean difference (MD) of 84.4 mL (95% confidence interval [CI], 9.6 to 159.3; p=0.03). The amount of postoperative drainage (MD, 31.8 mL; 95% CI, −9.6 to 73.1; p=0.13) and operative time (MD, 6.5 minutes; 95% CI, −3.7 to 16.8; p=0.21) were also lower in the piezosurgery group, but this did not reach statistical significance. A similar insignificant difference was observed in the rate of dural tears (OR, 0.55; 95% CI, 0.26 to 1.17; p=0.12) and postoperative C5 palsy (OR, 0.67; 95% CI, 0.24 to 1.89; p=0.45). Although percentage increases in JOA scores could not be statistically tested, none of the studies that reported this variable revealed a significant difference between the two groups [19–22,24,25] (Fig. 2A–E).

Comparative analysis between piezosurgery (experimental) and conventional (control) groups. (A) On the basis of mean operative time. (B) On the basis of estimated blood loss. (C) On the basis of postoperative drainage. (D) On the basis of rate of dural tears. (E) On the basis of postoperative C5 palsy. SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom; M–H, Mantel-Haenszel.
5. Subgroup analysis
Subgroup analysis could be conducted for two parameters—blood loss and rate of dural tears. We analyzed for differences based on procedure—laminectomy/laminoplasty. Significantly reduced blood loss and lower risk of dural tears were observed in the piezosurgery groups when laminoplasty was performed (p<0.05). The same significance was not seen in laminectomy procedures in the research by Onen et al. [19] and Dave et al. [23] (Fig. 3A, B).
6. Risk of bias
MINORS scoring was done for studies included in the review [26]. All of the included studies were rated out of 24 points because they were all comparative. Most of the included studies had a moderate risk of bias, with two of the prospective RCTs performed by Li et al. [20] and Kim et al. [21] respectively having a low risk of bias (Table 3).
Discussion
This meta-analysis was performed to compare the perioperative results after spinal osteotomies (laminectomy/laminoplasty) for CSM using piezosurgery instruments and conventional methods (high-speed drill/rongeur). We discovered that there was a considerable variation in the amount of blood lost during surgery between the two osteotomy procedures. Further, we discovered that this difference along with a decreased rate of intraoperative dural tears with piezosurgery was significantly greater in the laminoplasty group compared to the laminectomy group.
The mean age reported in the study cohorts ranged from 55.5 to 64.2 years, with a male preponderance of subjects. There are very small number of research on the epidemiology of CSM, but the majority of published literature emphasizes an increased incidence with increasing age and greater frequency among males, which is consistent with the results of our review [2,27,28].
Early reports on the use of ultrasonic bone curettes highlighted the usefulness of this instrument in spinal surgery at all vertebral levels [17,27,28]. The lightweight design, lack of operational field obstruction, and capability to utilize the shaver on the inner cortex without worrying about damaging the dura and epidural venous plexus were all highlighted as advantages of the single-handed operation. In addition, due to the absence of any spinning components, there was no fear of entanglements of cotton pieces and no significant heat generation locally. Initial studies indicated little to no intraoperative dural tears with the use of UBS, but subsequent research has demonstrated that they have a similar safety profile to traditional osteotomy techniques [16]. Hazer et al. [29] in a study on the technical aspects and safety profile of UBS in spine surgery speculated that the constant application of the shaver to a single point could be the cause of the iatrogenic dural tears with its use. They suggested lowering the energy output of the device and preventing use at a single point over 10 seconds. Dural injury with the use of UBS is reported in cases of severe canal compromise, and the application of suction from the tip of the instrument on the dura [27]. Although early studies revealed that the use of UBS reduced the incidence of dural tears and postoperative nerve root injury [12,27], these results have not been replicated and supported by more current research [16]. Our analysis also showed no considerable difference in the rate of dural tears and postoperative C5 palsy between the two groups.
On statistical comparison, the amount of blood loss significantly favored the use of piezosurgery instruments for osteotomy with a MD of 84.4 mL (95% CI, 9.6–159.3). The literature concerning this finding is mostly consistent with five out of seven studies in our cohort reporting significantly decreased blood loss in the piezosurgery group. Similar findings have been reported in other trials on the effects of using UBS in cervical, thoracic, and lumbar procedures, with much more blood loss in the traditional groups [17,29–32]. The possible reasons for decreased blood loss with the use of piezosurgery include lower trauma to the surrounding soft tissue due to tissue selectivity, heat generation during usage which results in micro vascular coagulation, and an air-water cavitation effect that closes the small blood vessels [20,25,33]. Although the meta-analysis showed a similar tendency of less postoperative drainage, this was not statistically significant. Findings of lower postoperative drainage have been reported in the literature before [34] and were statistically significant in the evaluation by Li et al. [20] but no other articles from our analysis revealed significance.
The literature with regards to operative time with the use of UBS is conflicting with reports ranging from decreased operative time with UBS to those significantly favoring the conventional techniques [30–32,35], Although this was not statistically significant, Li et al. [20] and Lin et al. [25] reported shorter surgical times using conventional procedures in our population. Wahlquist et al. [32] attributed the quicker surgical time to the decreased blood loss leading to improved operative field visualization and less time spent on obtaining hemostasis. Simultaneously, the surgeon’s inexperience with the instrument and the need for prolonged application of the shaver to the bone might be the reason for the increased operative time. Although a combination of these factors along with individual patient-related factors may be at play, our meta-analysis indicates that there is no significant difference in the operating time with the use of these two procedures.
A unique finding which we noticed in the subgroup analysis was the significantly decreased blood and rate of dural tears in the laminoplasty subgroup with the use of UBS, which was not mirrored in the laminectomy subgroup. This seems to imply that the use of UBS has a more significant benefit when doing laminoplasty; however, we were unable to determine the basis for this conclusion. It could be because of just two studies in the laminectomy subgroup, which necessitates the need for further prospective studies to ascertain whether this finding is reproduced or not.
Very few comparative studies have been published to examine the use of piezosurgery and conventional methods for posterior cervical procedures for CSM. Additionally, our meta-analysis has limitations due to the variability of study designs, operating procedures, and surgical techniques. Despite these limitations, the studies included in our review had a moderate to low risk of bias, and analysis showed valuable findings which can help in decision-making for spine surgeons with regard to the use of UBS. This analysis should form the basis of potential future research in the form of prospective RCTs to confirm these initial findings.
Conclusions
According to our meta-analysis, we ascertained that the use of UBS for osteotomy in CSM is related with significantly reduced blood loss and no significant increase in operative time as compared to traditional methods. Along with the ease of handling of the instrument, these results make UBS a viable alternative for osteotomies in the cervical spine. Simultaneously, it is essential to be well-versed in the use of this instrument to prevent iatrogenic dural tears and other complications.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
PB assisted in conducting the literature search, performed the statistical analysis, and drafted the initial version of the article. VK contributed to the study’s conceptualization, edited the drafts, and provided final approval. AJV participated in conceptualizing the review, conducting the literature search, and editing the drafts. AG aided in the literature search and contributed to drafting the initial version of the article. SSD contributed to the study’s conceptualization, edited the drafts, and provided final approval.