Comparison of Intraoperative Low-Dose Ketodex and Fentanyl Infusion for Postoperative Analgesia In Spine Surgery: A Prospective Randomized Double-Blind Study
Article information
Abstract
Study Design
Prospective randomized double-blind study.
Purpose
To assess the analgesic effects of the combination of a low-dose ketamine and dexmedetomidine (ketodex) infusion and compare it with that of fentanyl for postoperative analgesia after spine surgeries.
Overview of Literature
Adequate pain management following spine surgeries is crucial. Approximately 57% of patients experience inadequate pain control in the first 24 hours following elective spine surgery, which is attributable to the extensive soft tissue and muscle damage.
Methods
The study included 60 patients graded American Society of Anesthesiologists I and II and scheduled for thoracolumbar spine surgery involving >3 vertebral levels. The patients were divided into two groups: group KD (ketodex) and group F (fentanyl). The primary objective was to compare the postoperative analgesic requirements among the groups. The secondary objectives included a comparison of the intraoperative anesthetic requirements, postoperative pain scores, hemodynamic parameters, side effects of the study drugs, and the duration of post-anesthesia care unit stay of both the groups.
Results
Ketodex use prolonged the mean time to first rescue analgesia (22.00±2.30 hours vs. 11.69±3.02 hours, p<0.001) and reduced the requirement of rescue analgesics in the first 24 hours postoperatively compared to fentanyl use (70.00±8.16 μg vs. 113.31±36.65 μg, p=0.03). The intraoperative requirement of desflurane was comparable between the groups (p>0.05). The postoperative pain scores were significantly lower in the group KD than in group F at most timepoints (p<0.05). Patients in group KD had a shorter post-anesthesia care unit stay than group F did (p<0.001).
Conclusions
Low-dose ketodex could be a safe substitute for fentanyl infusion when employed as an anesthetic adjuvant for patients undergoing thoracolumbar spine surgeries involving >3 vertebral levels to achieve prolonged analgesia without any opioid-related side effects.
Introduction
Effective amelioration of pain following spine procedures remains a fundamental challenge. Inadequate pain control is observed in approximately 57% of patients in the first 24 hours following elective spine surgery [1]. This profound pain is attributable to extensive soft tissue and muscle damage. Spine surgeries have a long-drawn-out recovery phase, and adequate pain relief contributes to a shorter hospital stay, early ambulation and recovery, and overall patient satisfaction [2,3].
Several pharmacological options exist for adequate perioperative pain management. However, each drug has its pros and cons limiting its broad applicability. Although opioids remain the cornerstone of perioperative analgesia, they produce numerous side effects, including respiratory depression, postoperative hyperalgesia, nausea, vomiting, ileus, and urinary retention [2]. A non-opioid drug that is equipotent to opioids with minimal or no side effects is yet to be discovered. The use of a combination of adjunct analgesics to alleviate pain is a viable approach. The enhanced recovery protocol following spine surgery focuses on insignificant opioid use and multi-modal analgesia to improve patient outcomes [3].
Ketamine, a non-competitive N-methyl-D-aspartate antagonist, is a suitable analgesic in subanaesthetic doses. It binds to the opiate receptors in the brain and spinal cord, inhibits the “wind-up” phenomenon and central sensitization, and reduces the development of chronic pain [4,5]. Perioperative ketamine has a consistent opioid-sparing effect, minimizes the development of tolerance, and reduces the postoperative pain scores for up to 24 hours following spine surgery [6]. Dexmedetomidine is a selective α2-receptor agonist, whose analgesic and hypnotic effects are exerted at the spinal and supraspinal levels. Furthermore, its antinociceptive effect is mediated by stimulation of α2 receptors located in the locus coeruleus. The intrinsic antinociceptive and anti-hyperalgesia properties of α2 agonists reportedly reduce postoperative opioid consumption following spine surgery [7,8].
Numerous studies have demonstrated the analgesic and opioid-sparing properties of dexmedetomidine and ketamine in spine surgeries [6–11]. However, to the best of our knowledge, no study has assessed the postoperative analgesia produced by the simultaneous, but separate, use of low-dose ketamine and dexmedetomidine (ketodex infusion) as intraoperative anesthetic adjuvants. We hypothesized that intraoperative administration of ketodex infusion would produce a significant postoperative opioid-sparing effect, reducing the requirement of anesthetic agents and postoperative rescue analgesics in patients undergoing thoracolumbar spine surgery.
Materials and Methods
This prospective, randomized, double-blind study was conducted at a tertiary hospital from March 2021 to November 2021. Consecutive adults (aged 18–60 years) of either sex who were classified as American Society of Anesthesiologists (ASA) physical status I and II and scheduled for thoracolumbar spine surgeries involving >3 vertebral levels were included in the study. Patients with sensory deficits at the level planned for surgery, severe hypertension, coronary heart disease, heart block, liver or renal disease, raised intracranial pressure, α2 agonist or β-blocker use, ASA III/IV classification, and allergy to study drugs, pregnant patients and non-consenting adults were excluded from the study.
1. Ethics
The study was approved by the Postgraduate Institute of Medical Education and Research, Chandigarh, India (approval no., NK/6924/MD/165; January 27, 2021) and registered with the Clinical Trials Registry-India (no., CTRI/2021/03/031999). Informed consent was obtained from each patient prior to study enrollment.
2. Study protocol
Each patient was assessed preoperatively using the 11-point Numeric Rating Scale (NRS) for pain, where 0 stands for “no pain” and 10 stands for “worst imaginable pain.” Standard NPO (nil per os) guidelines were followed, and patients were administered oral alprazolam (0.25 mg) and ranitidine (150 mg) the previous night. In the operation theater, standard ASA monitors, such as electrocardiogram (ECG) leads, pulse oximetry probe (oxygen saturation [SpO2]), non-invasive blood pressure (BP) cuff, and temperature probe, and additional monitors, such as train-of-four (TOF) monitor and bispectral index (BIS) electrodes, were attached.
3. Randomization and blinding
Computer-generated randomization and the sealed envelope technique were used to divide the eligible patients into group KD (receiving ketodex infusion) and group F (receiving fentanyl). The attending anesthesiologist managing the patient was aware of the intervention protocol and could not be blinded and was thus excluded from further study. The patient and the investigator collecting the data were blinded to the study intervention groups.
Patients in group KD were induced with ketodex (1 mg/kg of ketamine and 1 μg/kg of dexmedetomidine over 10 minutes) and propofol (1 mg/kg) and maintained with low-dose ketodex infusion (0.25 mg/kg/hr of ketamine and 0.3 μg/kg/hr of dexmedetomidine), O2/N2O, and desflurane. Ketamine and dexmedetomidine were administered simultaneously via two separate infusion pumps.
Patients in group F were induced with fentanyl (2 mcg/kg) followed by propofol 2 (mg/kg) and maintained with fentanyl infusion (1 μg/kg/hr), O2/N2O, and desflurane. A sustained BIS of 48±2 for >1 minute was considered as the end-point of induction. The patient was intubated 4 minutes after administering vecuronium (0.1 mg/kg) when the TOF was zero. The patients were positioned prone as per the planned procedure and the surgeon’s need.
Heart rate (HR), BP, ECG, SpO2, end-tidal carbon dioxide, BIS, TOF parameters, and temperature were monitored intraoperatively. Intraoperative anesthesia was maintained using desflurane in O2/N2O (1:1 mixture) to maintain a BIS of 40–60, and administration of vecuronium top-up (0.02 mg/kg) was guided by the TOF. If BIS was >60 and HR was >20% of baseline, the desflurane concentration was increased. If HR was >20% of baseline and the BIS was 40–60, 1 mcg/kg of fentanyl was injected and the dose was recorded. No additional non-opioid analgesics were administered intraoperatively in either group.
The infusions were continued until the application of the last skin suture. At this timepoint, the maintenance anesthetic agent desflurane was also stopped. The residual neuromuscular blockade was reversed with an injection of neostigmine (50 μg/kg) and glycopyrrolate (10 μg/kg) based on the TOF ratio of ≥0.6 and count of 4 and when the patient’s spontaneous breathing had resumed. Before extubation, the anti-emetic agent ondansetron (0.1 mg/kg) was administered. After extubation, the patients were shifted to the post-anesthesia care unit (PACU) for further monitoring.
Postoperatively, all patients were administered injectable diclofenac (1.5 mg/kg) every 8 hours and the NRS scores were recorded every 4 hours for 24 hours. An NRS score of 4 was set as the threshold for tolerable postoperative pain [12]. In patients complaining of intolerable pain (NRS >4), rescue analgesia was administered (1 μg/kg of intravenous fentanyl). The time to the first rescue analgesia dose and perioperative analgesic requirements were recorded and compared among the groups. “Postoperative opioid requirements” was defined as the total rescue analgesic bolus doses administered in 24 hours.
The postoperative side effects of opioids, such as nausea, vomiting, respiratory depression, delirium, and shivering, were also recorded. Nausea was defined as a feeling of retching, requiring medications. Vomiting was defined as the forceful expulsion of gastric contents following nausea. Respiratory depression was defined as a respiratory rate (RR) <8/min, with brief apneic episodes lasting >30 seconds without any stimulus. Postoperative delirium was defined as a disturbed state of mind characterized by restlessness, illusions, incoherence, hallucinations, and the inability to comprehend time, place, and person in the PACU. Shivering was defined as the presence of visible tremors or muscular activity involving more than one muscle group or the entire body. The side effects of the other study drugs (ketodex infusion), which included tachycardia (HR >100/min), bradycardia (HR <50/min), hypotension (mean arterial pressure [MAP] <20% of the baseline), or hypertension (MAP >20% of the baseline) were also noted.
The primary objective of the current study was to compare the postoperative opioid requirement for the first 24 hours after administration of intraoperative low-dose ketodex and fentanyl infusion in patients undergoing thoracolumbar spine surgery. The secondary objectives were to compare the intraoperative anesthetic requirements, postoperative pain scores, hemodynamic parameters, any side effects of study drugs and the duration of PACU stay among groups.
4. Statistical analysis
The collected data was entered into Microsoft Excel 2019 (Microsoft Corp., Redmond, WA, USA). IBM SPSS ver. 24.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. The categorical and quantitative data were reported as frequency (%) and mean±standard deviation, respectively. The normality of the data was assessed using the Shapiro-Wilk test. Independent t-test was used to compare the means between the two groups. Cross-tabs were used to identify the distribution of categorical data in the two groups. A confidence interval of 95% was used to define the lower and upper boundaries of the values. Statistical significance was set at a two-tailed p<0.05.
5. Sample size calculation
To the best of our knowledge, no previous study has used ketodex for postoperative analgesia in spine surgeries. Based on the literature review of proximate studies, the total postoperative fentanyl dose consumed during the 24 hours postoperatively in those who receive dexmedetomidine is 1,035.4±391.8 μg [10]. Assuming that the analgesic dose requirement for ketodex infusion would be 30% less than the dexmedetomidine dose, a minimum of 29 patients were required in each group to detect this difference with a 95% confidence level and 85% power. Hence, we recruited 30 patients for each group.
Results
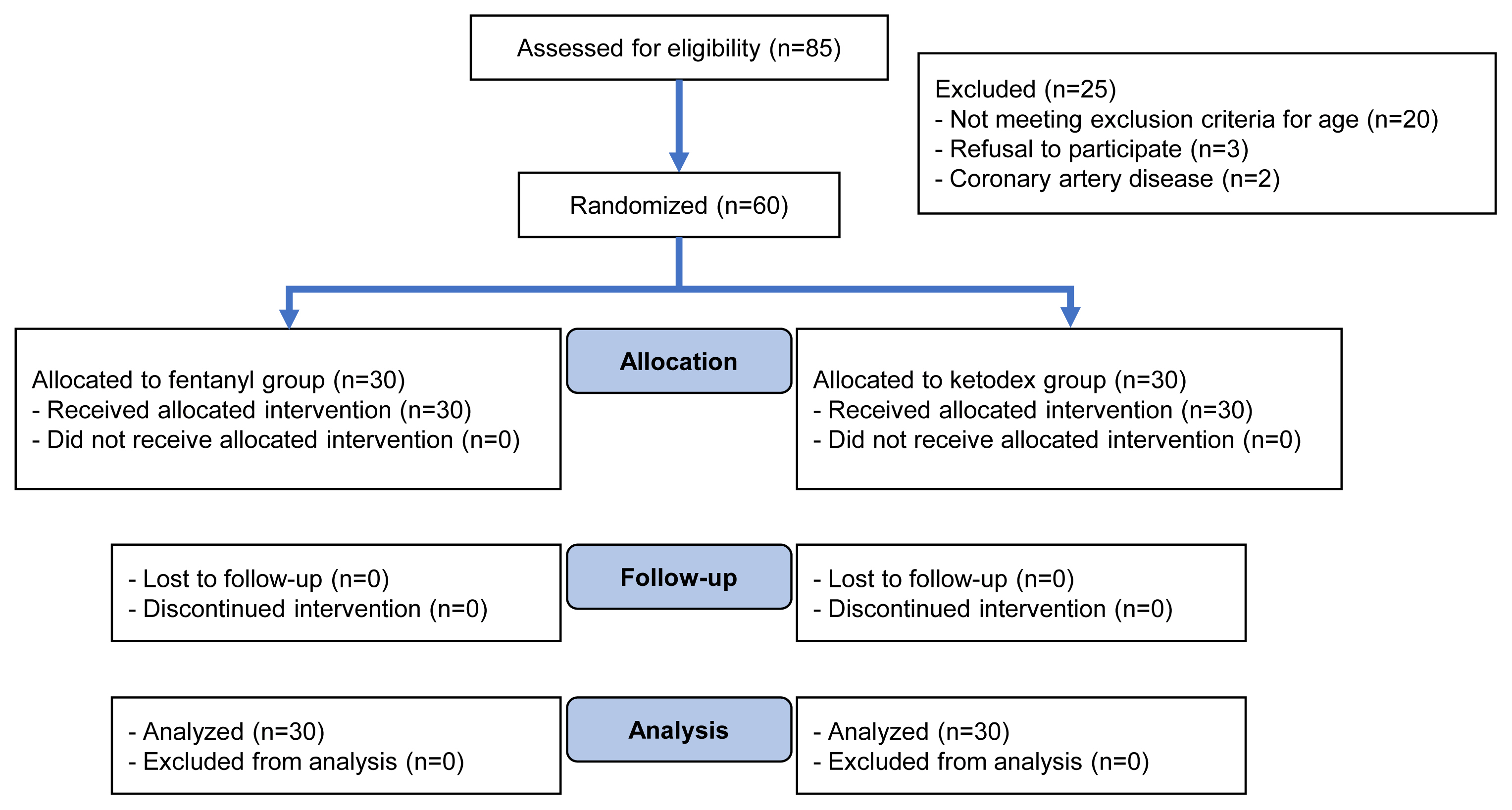
A total of 85 patients were assessed over the study period of which 60 patients (group KD, n=30 and group F, n=30) were finally analyzed (Fig. 1). The groups were comparable in their demographic characteristics, vertebral levels involved, and duration of surgery (Table 1).
1. Postoperative analgesia requirement and pain scores
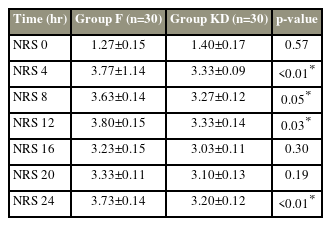
The mean time to the first rescue analgesia was significantly longer in group KD than in group F (22.00±2.30 hours versus 11.69±3.02 hours, p<0.001) (Table 2). The rescue fentanyl requirement in the postoperative period was significantly higher in group F than in group KD (113.31±36.65 μg versus 70.00±8.16 μg, p=0.03) (Table 2). The mean NRS score was significantly higher in group F at all postoperative timepoints, except when they were comparable at the 0, 16th, and 20th hour (Table 3).

Comparison of intraoperative anaesthetic requirements along with postoperative characteristics and complications
2. Intraoperative drug usage
Although the mean amount of desflurane used was higher in group F than in group KD, the difference was not statistically significant (Table 2).
3. Postoperative complications and post-anesthesia care unit stay
Significantly more patients in group F developed nausea (p=0.02) and vomiting (p=0.01) in the first 24 hours postoperative than those in group KD did. In group F, six patients (20%) developed respiratory depression (p=0.02) and two patients developed shivering. Four patients (13%) in group KD developed postoperative delirium. The mean PACU stay was longer in group F than in group KD (p<0.001).
4. Monitoring parameters
Intraoperatively the monitoring parameters were recorded every 5 minutes, and postoperatively they were recorded every 4 hours for 24 hours. The intraoperative SpO2 and BIS were comparable between the two groups at baseline and in the intraoperative period (p>0.05) (Table 2).
Intraoperatively, the systolic BP was significantly higher in group F than in group KD between 40 to 175 minutes (p<0.05); it was comparable at all other timepoints (p>0.05) (Fig. 2A). In the postoperative period, the mean systolic BP was significantly higher in group F than in group KD (p<0.05), except when it was comparable at the 16th postoperative hour (Fig. 2B).

Hemodynamic parameters and postoperative respiratory rate. (A) Intraoperative systolic blood pressure (SBP). (B) Postoperative SBP. (C) Intraoperative diastolic blood pressure (DBP). (D) Postoperative DBP. (E) Intraoperative mean arterial pressure (MAP). (F) Postoperative heart rate (HR). (G) Postoperative respiratory rate (RR). Group F, patients induced with fentanyl; Group KD, patients induced with ketodex.
Intraoperatively, the mean diastolic BP was significantly higher in group F than in group KD at 125 minutes and 130 minutes (p=0.05); it was comparable at all other timepoints (p>0.05) (Fig. 2C). The postoperative diastolic BP was comparable between the groups, except when it was significantly higher in group F than in group KD at the 4th, 12th, and 16th postoperative hour (Fig. 2D).
Intraoperatively, the MAP was significantly higher in group F than in group KD at all 5-minute intervals from 25 to 185 minutes and 195 to 220 minutes and at 230 minutes (p<0.05). The MAP did not significantly differ between the groups at all other timepoints (p>0.05) (Fig. 2E).
The mean HR at baseline and at intraoperative timepoints was comparable between the two groups (p>0.05). In the postoperative period, the mean HR was significantly higher in group F than in group KD at all timepoints (p<0.05) (Fig. 2F). The postoperative mean RR was significantly higher in group KD than group F at all timepoints, except when they were comparable at the 16th and 20th postoperative hour (Fig. 2G).
Discussion
Although ketamine and dexmedetomidine are conventional anesthetics, their combination use has been seldom assessed. In the realm of neurosurgeries, ketodex has been successfully used for concurrent electrocorticography and subcortical motor evoked potential mapping [13]. The present prospective randomized study was conducted to observe the postoperative analgesia effects of intraoperative low-dose ketodex infusion and compare it with those of the more commonly used fentanyl infusion in patients undergoing thoracolumbar spine surgery.
Empirical evidence supports the individual use of ketamine and dexmedetomidine for pain control [9–11]. Sub-anesthetic doses of ketamine inhibit central nociceptive system sensitization and produce fewer psychomimetic side effects [4,5]. Dexmedetomidine acts on the dorsal horn of the spinal cord and inhibits the release of substance P and other inflammatory cytokines during tissue injury [14]. Co-administration of these drugs could provide a synergistic analgesic effect while acting at different sites.
In our study, ketodex use almost doubled the mean time to first rescue analgesia and significantly reduced the requirement of postoperative analgesics in the first 24 hours as opposed to fentanyl use. This ketodex-induced prolongation of the mean time to first rescue analgesia may be attributable to the synergistic effect of the ketodex combination. This finding is significant as ketodex use could reduce the demand for rescue opioids, thereby minimizing various opioid-related adverse effects. We found that the postoperative NRS-pain scores were significantly lower in group KD than in group F at most timepoints, except when they were comparable at the 16th and 20th postoperative hour. This could be due to the additional postoperative administration of fentanyl in group F when the NRS score was >4 after 12 hours compared to almost 22 hours in group KD.
Our results were in concordance with those of the study by Garg et al. [9], who compared ketamine and dexmedetomidine infusions for analgesia following spine surgery. Patients who were administered ketamine were pain-free for longer than those who were administered dexmedetomidine (860 minutes versus 580 minutes). Furthermore, the rescue morphine requirement was lower in the ketamine and dexmedetomidine groups than in the placebo group. Thus, it suggested that infusions of low-dose ketamine and dexmedetomidine provide agreeable postoperative analgesia with minimal side effects. Our study differed from that by Garg et al. [9] in that the study drugs were administered intraoperatively in our study; the study drugs were administered in the 24 hours postoperative period in the study by Garg et al. [9].
Mitra et al. [10] assessed the use of low-dose ketamine and dexmedetomidine as an anesthetic adjuvant in lumbar spine instrumentation surgery for postoperative analgesia. The drugs were administered intraoperatively after placing the patient in the prone position. Similar to the observations in our study, the total fentanyl administration in the first 24 hours following surgery was less in the dexmedetomidine and ketamine groups than in the saline group. They concluded that opioid consumption, pain scores, inhalational agent administration, and hospital stay were comparable with ketamine or dexmedetomidine use [10]. Both drugs have proved to be effective in reducing postoperative pain after posterior spinal fusion surgery [11]. However, ketamine and dexmedetomidine were administered separately in previous studies; they were concurrently administered in our study.
The opioid-sparing effects of low-dose ketamine (defined as a continuous intravenous administration rate of ≤20 μg/kg/min) were evaluated by Kim et al. [15] after lumbar spinal fusion surgery. After a ketamine loading dose of 0.5 mg/kg, a continuous ketamine infusion (1 or 2 μg/kg/min) was administered before the skin was incised and continued until 48 hours postoperatively. They determined that low-dose ketamine (2 μg/kg/min) significantly reduced the total amount of fentanyl administered during the 48 hours postoperative period without increasing the incidence of adverse effects [15]. These results are in concurrence with those of our study. We used the drugs as an anesthetic adjuvant, unlike in this study where the drugs were infused prior to skin incision and continued until 48 hours postoperatively. A careful look at these studies reveal the heterogeneity in the administration timeline of the study drugs (only postoperative, intraoperative, or perioperative), making explicit comparisons difficult [9–11,15].
A recent meta-analysis indicated that only postoperative or intraoperative and postoperative administration of low-dose ketamine reduces opioid consumption and prolongs the postoperative analgesia time up to 48 hours after spine surgery [4]. However, our study demonstrated that the intraoperative use of ketodex was suitable. This could be ascribed to the additional analgesic effect of dexmedetomidine in ketodex, obviating the need for postoperative infusions, which are often cumbersome. Moreover, the results of the meta-analysis cautiously interpreted considering the heterogeneous studies included, methodological quality of the included studies, and different ketamine doses used.
The intraoperative anesthetic requirement in the form of desflurane was congruent in both groups. This may be attributable to the sub-anesthetic ketodex dose used, producing inadequate hypnotic plasma levels and only decreasing the perioperative pain score. Additionally, it may have resulted in relatively higher BIS scores, which were used to guide the maintenance of anesthesia, in the ketodex group despite adequate anesthetic depth. Both ketamine and dexmedetomidine are known to interfere with BIS values, with ketamine increasing these values. Higher BIS values in the ketodex group might have provoked the overuse of desflurane to maintain a BIS value of 40–60.
The overall rescue fentanyl administration was higher in group F than in group KD in our study. The PACU stay was significantly longer in group F than in group KD (49.83±9.86 minutes versus 30.83±7.99 minutes, p<0.001). This could be explained by the unfavorable postoperative effects of opioids, which require monitoring and treatment, thereby prolonging the PACU stay. Patients in group F experienced nausea and vomiting, while only one patient in group KD suffered from mild nausea. This could be attributable to the opioid-sparing effect of the ketodex infusion; a meta-analysis of 24 trials concluded that dexmedetomidine reduces the risk of postoperative nausea and vomiting due to a reduction in the administration of perioperative opioids [16]. More patients experienced respiratory depression in group F than in group KD; patients in group KD maintained a regular RR and showed no signs of respiratory depression. One factor, which could have contributed to the higher incidence of postoperative respiratory depression in our study, was the continuation of fentanyl infusion until the last skin suture was placed. Although opioids are currently the preferred choice for intraoperative analgesia in multiple level spine surgeries, the search for a better analgesic without the side effects of opioids is ongoing.
Opioids and dexmedetomidine inhibit shivering; this was evidenced in our study by the statistically insignificant incidence of shivering among our groups. More delirium episodes were observed in group KD than in group F; however, it was statistically insignificant. This may attributed to the psychomimetic potential of ketamine used in group KD. The concurrent use of dexmedetomidine and propofol counteracts the psychomimetic effects of ketamine.
The intraoperative hemodynamics (HR, systolic BP, and MAP) were marginally lower in group KD than in group F at most timepoints. Although statistically significant, both groups had stable vital parameters without substantial fluctuations. This is desirable during spine surgeries. The marginally lower HR and MAP values in group KD may be ascribed to the sympatholytic action of the vastly potent dexmedetomidine. Although we did not measure pain using an intraoperative pain monitoring device, the lower HR and MAP scores indicate a superior analgesic efficacy that was obtained by the synchronized action of ketamine and dexmedetomidine. Rahimzadeh et al. [17] also demonstrated that dexmedetomidine significantly lowered the intraoperative BP and HR compared to remifentanil in posterior spinal fusion surgeries. A prospective randomized controlled trial compared the effects of intraoperative dexmedetomidine and lidocaine infusion on hemodynamics, fentanyl requirements, and postoperative analgesia among patients undergoing lumbar fixation surgery. Akin to our study, the MAP and HR were lower in the dexmedetomidine group than in the lidocaine group [18]. In our study, none of the patients experienced dexmedetomidine-induced bradycardia or severe hypotension; this may be imputable to the concurrent administration of ketamine, which counterbalances the parasympathetic override of dexmedetomidine.
The intraoperative trend of lower hemodynamic parameters in the ketodex group continued for 24 hours postoperatively. The better analgesia provided by the ketodex synergism and sympatholytic action of dexmedetomidine and lesser episodes of nausea, vomiting, and other adverse effects that are usually associated with opioids, could allow a pleasant PACU stay. This has led to the early discharge from PACU with minimal side effects and overall better patient satisfaction. None of the patients experienced any adverse effects of ketamine or dexmedetomidine in the perioperative period. This may be due to the low dose of the two-constituent drugs used in the ketodex infusion.
Our study had a few limitations. The sample size was small, and we had no record of the severity of preoperative pain. The attending anesthesiologist could not be blinded to the study intervention groups and thus, remains an inevitable source of bias. The ketodex and fentanyl infusion doses used in our study was dissimilar and based on doses used in previous studies and our routine perioperative protocol. The use of BIS to assess the anesthetic depth may have led to ketamine and dexmedetomidine inordinately increasing its value, leading to study protocol-driven attempts to limit it, thereby altering desflurane administration. The hemodynamic parameters (HR and MAP) were used to guide the rescue analgesic doses. Using an intraoperative pain monitoring device would have added more objectivity to this measure.
Conclusions
In summary, use of ketodex as an anesthetic adjuvant during spine surgeries offers a hemodynamic profile comparable to fentanyl, a commonly used opioid. Although the consumption of maintenance agents was not majorly affected, the dose of concurrently administered opioid analgesics was reduced. Postoperatively, the patients who were administered ketodex reported less pain, required minimal rescue opioids, and experienced fewer side effects leading to speedy discharge and better patient satisfaction. Thus, low-dose ketodex infusion can be a safe substitute for fentanyl infusion when employed as an anesthetic adjuvant during general anesthesia for patients undergoing thoracolumbar spine surgeries for >3 vertebral levels to achieve prolonged analgesia without any opioid-related side effects.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Concept of study: Ankur Luthra; study design: Ankur Luthra, Navneet Singla; data acquisition: Ankur Luthra, Pruthviraj Deshpande; providing data: Vishal Kumar; data analysis: Priya Thappa, Nidhi Singh, Pruthviraj Deshpande, Shyam C. Meena, Vishal Kumar; data interpretation: Priya Thappa, Ankur Luthra, Shyam C. Meena, Vishal Kumar, Navneet Singla; drafting of manuscript: Priya Thappa, Nidhi Singh, Rajeev Chauhan, Shyam C. Meena; result formation: Shyam C. Meena; and final approval of version of manuscript: Ankur Luthra, Rajeev Chauhan