Perioperative Intravenous Nefopam on Pain Management and Ambulation after Open Spine Surgery: A Randomized Double-Blind Controlled Study
Article information
Abstract
Study Design
This was a randomized double-blind controlled study.
Purpose
This study was designed to evaluate the effects of intravenous nefopam regarding its ability to reduce morphine consumption and postoperative pain and improve recovery in patients undergoing open spine surgery.
Overview of Literature
Multimodal analgesia, including nonopioid medications, is essential for pain management in spine surgery. Evidence regarding the use of intravenous nefopam in open spine surgery as part of enhanced recovery after surgery is lacking.
Methods
In this study, 100 patients undergoing lumbar decompressive laminectomy with fusion were randomized into two groups. The nefopam group received 20-mg intravenous nefopam diluted in 100-mL normal saline intraoperatively, followed by 80-mg nefopam diluted in 500-mL normal saline, administered as a continuous infusion postoperatively for 24 hours. The control group received an identical volume of normal saline. Postoperative pain was managed using intravenous morphine via patient-controlled analgesia. Morphine consumption in the first 24 hours was recorded as the primary outcome. Secondary outcomes, including postoperative pain score, postoperative function, and length of hospital stay (LOS), were assessed.
Results
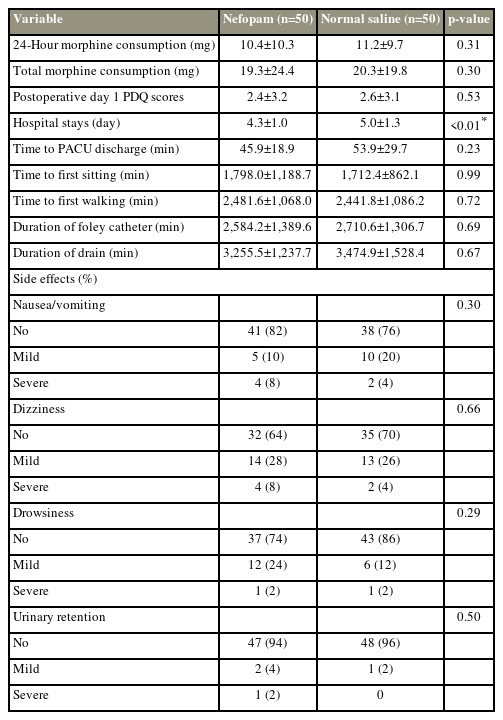
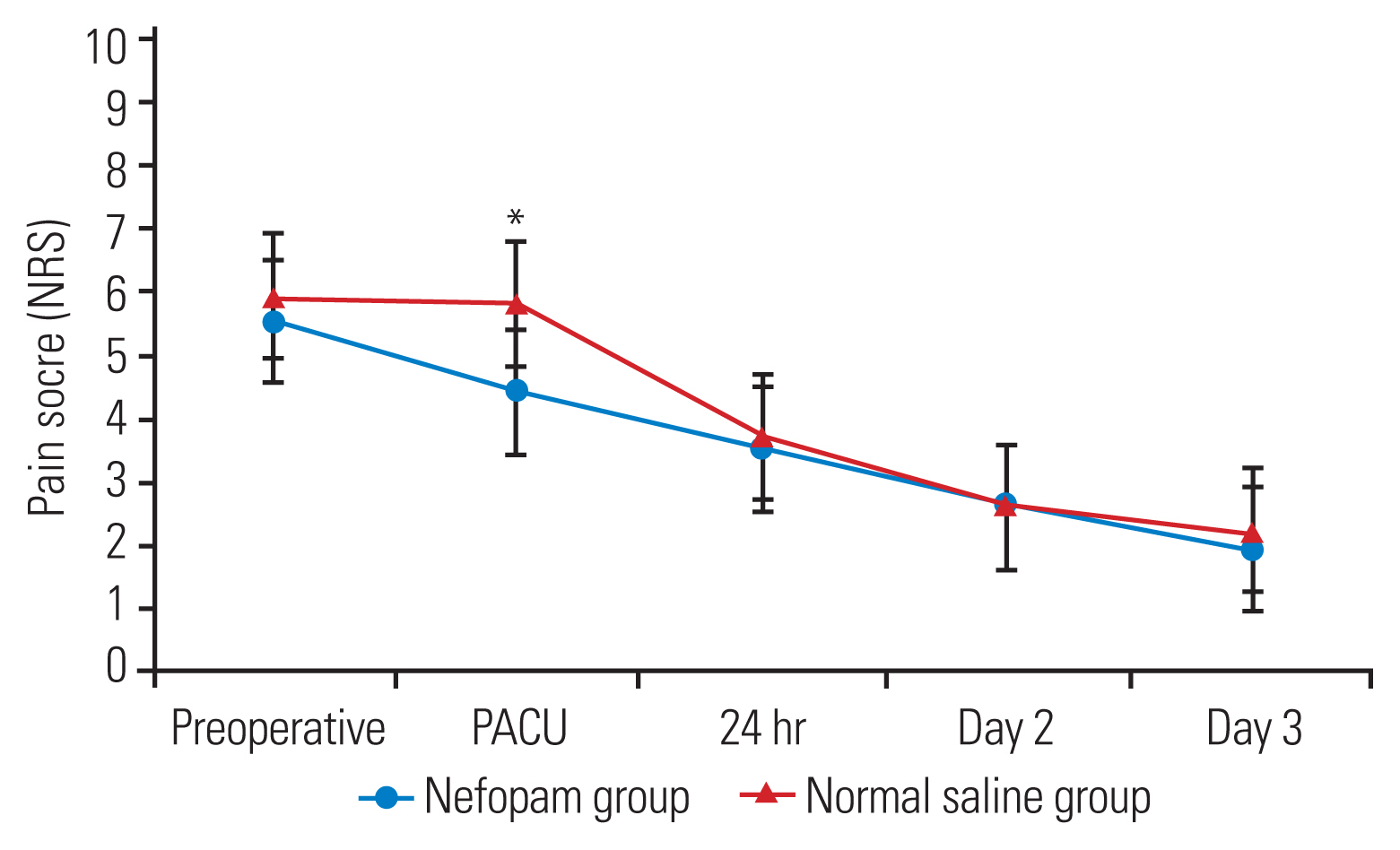
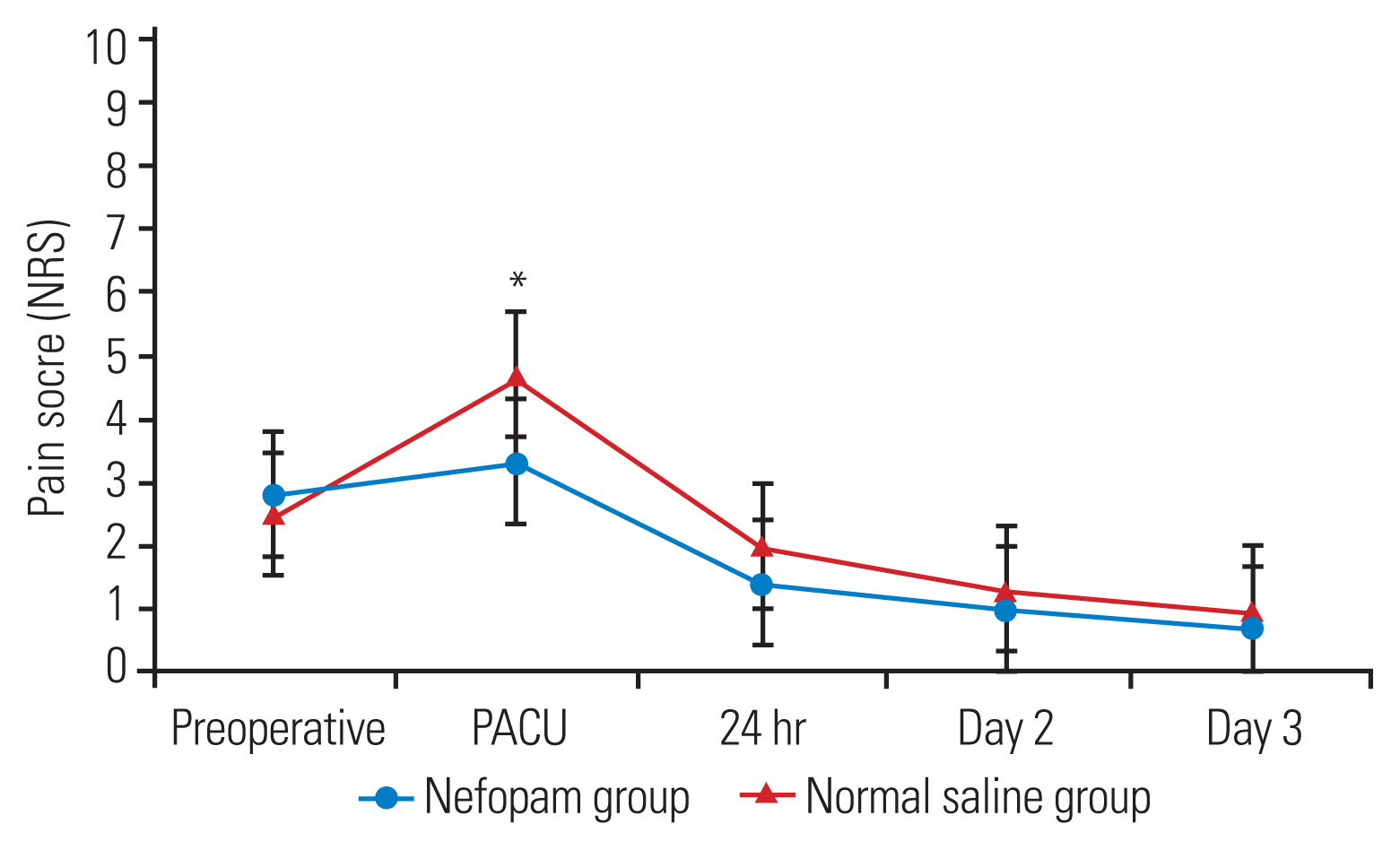
No statistically significant differences in the total morphine consumption and postoperative pain score in the first 24 hours postoperatively between the two groups. At the post-anesthesia care unit (PACU), the nefopam group demonstrated lower pain scores while at rest (p=0.03) and upon movement (p=0.02) than the normal saline group. However, the severity of postoperative pain between the two groups was similar from postoperative day 1 to day 3. LOS was significantly shorter in the nefopam group than in the control group (p<0.01). The time to first sitting and walking and PACU discharge between the two groups were comparable.
Conclusions
Perioperative intravenous nefopam demonstrated significant pain reduction during the early postoperative period and shortened LOS. Nefopam is considered safe and effective as a part of multimodal analgesia in open spine surgery.
Introduction
With the wide adoption of the enhanced recovery after surgery (ERAS) protocol, the importance of an alternative to opioids for postoperative pain management and as part of an opioid-free anesthesia protocol has significantly increased [1]. Opioid-free anesthesia provides numerous advantages over conventional techniques where the liberal use of opioids is often required for open spine surgery, particularly lumbar decompression with fusion, where significant postoperative pain is expected. The benefits of opioid-free anesthesia include less postoperative nausea and vomiting, dizziness, and bowel ileus. These complications can hinder recovery and attempts at early ambulation or rehabilitation. For this purpose, multiple nonopioid analgesics have been proposed, such as acetaminophen, ketamine, lidocaine, dexmedetomidine, and gabapentinoids, all of which have shown opioid-sparing effects and significant analgesic properties in major spine surgery [2]. Along with improvements in surgical techniques, the development of better analgesia is required to provide multidisciplinary patient care.
Nefopam, developed in 1976, has since found a new role in pain management. Nefopam is considered a non-nonsteroidal anti-inflammatory drug and nonopioid analgesic. It has a multifaceted mechanism of action. The descending inhibitory pathway, derived from the brain stem toward the dorsal horn of the spinal cord, is activated by inhibiting norepinephrine, serotonin, and dopamine reuptake. Nefopam also acts as a sodium and calcium channel antagonist at the level of the dorsal horn of the spinal cord. These mechanisms result in antinociceptive and anti-neuropathic effects [3,4]. There is evidence that incorporating nefopam into an analgesics protocol can decrease morphine consumption by 13 mg in the postoperative period after major surgery [5–7]. The preoperative use of nefopam can also provide satisfactory analgesia, as was demonstrated in patients with severe preoperative pain scheduled for hip arthroplasty [5].
Evidence supporting the efficacy of nefopam as part of an ERAS scheme is currently limited in open spine surgery. Nefopam could significantly decrease postoperative paresthesia and dysesthesia in patients who underwent endoscopic lumbar discectomy [8]. However, in another study, postoperative morphine consumption was comparable to placebo for open spine surgery [9]. Therefore, this study was designed to evaluate the effects of nefopam on postoperative pain within the first 24 hours, time to recovery, and length of hospital stay (LOS) and assess its opioid-sparing properties in patients undergoing lumbar decompression and fusion.
Materials and Methods
1. Study design and participants
This study was approved by the Institutional Review Board (IRB) of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB number: 434/62), and complied with the Declaration of Helsinki guidelines. The Thai Clinical Trials Registry (number: TCTR20190615001) was applied before patient enrollment. The Consolidated Standards of Reporting Trials guidelines were followed throughout the study. Informed consent was obtained from all patients before enrollment. This study was conducted at the King Chulalongkorn Memorial Hospital, The Thai Red Cross Society, Bangkok, Thailand. The inclusion criteria included age between 20 years and 80 years, American Society of Anesthesiologists (ASA) physical status I–III, and undergoing elective lumbar decompressive laminectomy with fusion under general anesthesia. The exclusion criteria were patients with a history of chronic pain, coronary artery disease, severe liver and kidney impairment, epilepsy, and glaucoma; those currently using monoamine oxidase inhibitors; those with impaired cognitive function, and those who are allergic to the medication used in the study.
After enrollment, the painDETECT questionnaire (PDQ) was completed to identify preoperative neuropathic pain [10]. According to the questionnaire, patients with low back and leg pain are separated into three groups: neuropathic pain (score between 19 and 38, >90% more likely to have neuropathic pain), unclear (score between 13 and 18), and non-neuropathic pain (score between 0 and 12, <15% less likely to have neuropathic pain). The patients were then seen by a pain nurse and given an education program, after which they were seen by a physiotherapist who provided preoperative physiotherapy and rehabilitation. Randomization was performed using a computer using blocked randomization with randomly selected block sizes. The two study groups were the nefopam and control groups. The randomization was printed and stored in sealed envelopes. These envelopes were arranged by a research assistant and disclosed on the day of surgery by a nurse anesthetist. The solutions of nefopam and normal saline labeled as a study drug were volume- and color-matched and were prepared by the same nurse anesthetist.
The patients were not premedicated and received standard monitoring according to the ASA guidelines. All patients were given the same anesthetics protocol, which included 2–3 mg/kg of propofol and 0.15–0.2 mg/kg of cisatracurium intravenously for anesthesia induction and intubation. Desflurane with air and oxygen was used to maintain anesthesia. Then, 10-mg dexamethasone was administered intravenously to all patients for anti-inflammation immediately after induction, followed by 25-mg intravenous (IV) ketamine before the skin incision. IV fentanyl was permitted during intubation and throughout the operation (with a limit of 5 μg/kg). The last dose of fentanyl was not allowed to be administered less than 60 minutes before the extubation. The operation was performed by two surgeons (W.S. and W.Y.) using the same surgical technique. Near completion of the surgery, the patients were given the study drug, which was administered as an IV infusion in 30 minutes. The nefopam group received a mixture of 20-mg nefopam in 100-mL normal saline [11], whereas the control group received only the normal saline infusion. The anesthesiologists, surgeons, pain assessors, and patients were blinded to the study drug. At the end of the operation, the patients in both study groups also received 40-mg IV parecoxib (or 30-mg ketorolac for patients who were allergic to sulfa drugs) and 8-mg ondansetron. Additionally, 10-mL 0.25% bupivacaine was locally injected into the surgical wound and drain. After that, the patients were extubated and transferred to the post-anesthesia care unit (PACU). A modified Aldrete score of ≥9 was achieved before the discharge from the PACU.
At the ward, the patients were given a continued 24-hour infusion of the study drug. The nefopam group received a mixture of 80-mg nefopam in 500-mL normal saline [11], whereas the control group received only the normal saline infusion. According to a multimodal analgesia protocol, all patients were given 1,000-mg oral paracetamol every 6 hours, 90-mg etoricoxib once daily in the morning, and 75-mg pregabalin before bedtime as background pain control. Breakthrough pain was managed by IV morphine using patient-controlled analgesia with a setting of 1-mg bolus and 6-minute lockout without continuous infusion.
The intensity of postoperative pain at rest and upon movement at the PACU and on postoperative days 1, 2, and 3 was graded using a numerical rating scale, ranging 0–10. Pain while changing positions, sitting, and walking was considered pain on movement. The PDQ was redone on postoperative day 1. During the postoperative period, the patients were motivated by healthcare providers for early ambulation and prompt enteral nutrition. The study’s primary outcome was the amount of morphine consumption at the first 24 hours after surgery. Secondary outcomes were the total postoperative morphine consumption, the intensity of postoperative pain, the duration of PACU stay, the time from the end of surgery to sitting (first sitting), the time from the end of surgery to walking (first walking), the time to discontinuation of the drainage tube and foley catheter, and LOS (the time between the end of surgery until readiness for discharge). The patients were discharged by the attending surgeons, who were blinded to the study group, when they could walk without any assistance, had a pain score <3 of 10 without any requirement of IV pain medication, and had no sign of wound infection. Adverse events related to analgesics were recorded, including dizziness, drowsiness, nausea/vomiting, tachycardia, and hypertension.
2. Statistical analyses
The sample size was calculated using an average morphine consumption of 17 mg in patients undergoing spine surgery, with the estimation of 9 mg of morphine reduction as the minimum clinical difference. The power analysis was performed with 90% power and considered a 10% dropout rate; therefore, 100 patients were required in this study. Categorical data were analyzed with the chi-square test or Fisher’s exact test. Continuous data between the groups were analyzed using an unpaired t-test or the Mann-Whitney test using Stata ver. 14.0 (Stata Corp., College Station, TX, USA). All p-values of less than 0.05 were used to denote statistical significance.
Results
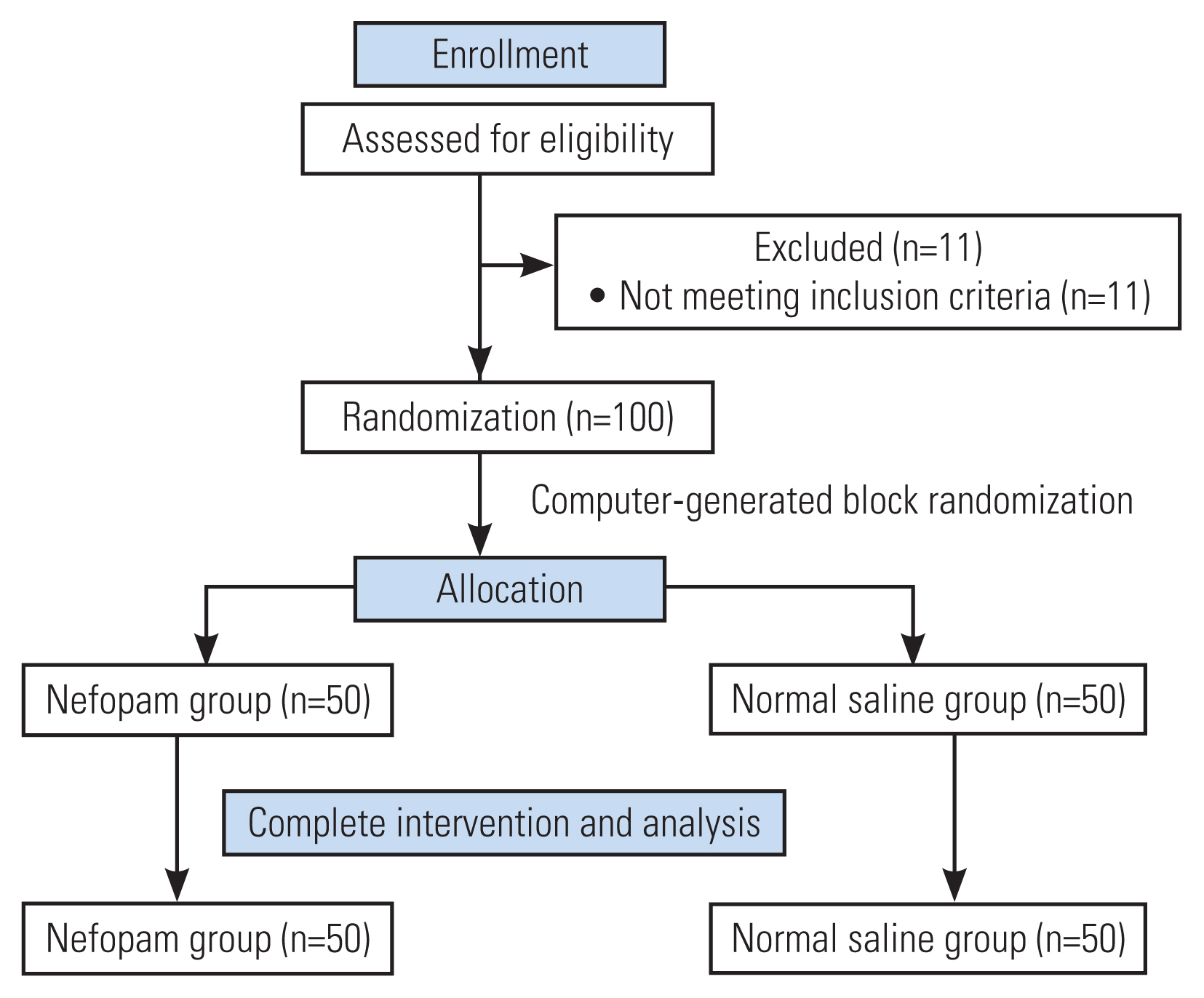
In this study, 100 surgical patients were prospectively randomized between August 2019 and March 2022 (Fig. 1). The demographic data and characteristics of the patients are demonstrated in Table 1. The parameters on the first 24 hours after surgery and total morphine consumption were comparable between the two study groups (Table 2). The patients in the nefopam group required a mean morphine dose of 10.4±10.3 mg during the first 24 hours postoperatively compared with those in the control group who required a mean morphine dose of 11.2±9.7 mg (p=0.31). In the PACU, the nefopam group showed lower pain scores both at rest (p=0.03) and upon movement (p=0.02) than the control group (Figs. 2, 3). However, the intensity of postoperative pain between the two groups was similar on postoperative days 1, 2, and 3. The PDQ representing the severity of neuropathic pain on postoperative day 1 showed significant improvement in both groups; however, the postoperative PDQ scores were not significantly different between the two groups. The LOS was significantly shorter in patients receiving nefopam than in those receiving normal saline only (p<0.01). However, no differences in the duration of PACU stay, time to first sitting, time to first walking, and time to foley catheter and drainage tube removal were observed between the two groups. IV nefopam caused severe nausea/vomiting and dizziness in 8% of the patients in the nefopam group compared with 4% of the patients in the control group. Nevertheless, the difference did not reach statistical significance (Table 2).
Postoperative pain score (resting pain). NRS, Numerical Rating Scale; PACU, post-anesthesia care unit.
Discussion
This study demonstrated that adding IV nefopam perioperatively in a multimodal analgesic regimen provided significant pain reduction for hours in the PACU. However, its analgesic effect did not continue into postoperative days 1, 2, and 3. Although 24-hour nefopam infusion following major spine surgery provided no beneficial effect on morphine consumption and postoperative functional outcomes, the LOS was significantly shorter. The nefopam group was admitted for a mean duration of 4.3±1.0 days compared with the control group, taking a mean duration of 5.0±1.3 days (p<0.01), which might result in overall cost savings.
According to the latest ERAS protocol for postoperative pain management, a multimodal analgesia regimen aimed at reducing opioid consumption is strongly recommended for pain control following lumbar spinal fusion [12]. The data on the effectiveness of nefopam are scant. Nefopam has both antinociceptive and anti-neuropathic pain properties without effect on platelet aggregation or blood coagulation and no sedative effect [4], making it the drug of choice for perioperative multimodal analgesia for spine surgeries. This study demonstrated the short-term analgesic effect of nefopam in the PACU following its administration at the end of surgery, resulting in an early discharge from the PACU. These outcomes suggested that a bolus dose could provide a better analgesic effect, whereas the 24-hour infusion of nefopam may not reach the therapeutic window. Intermittent nefopam bolus administration every 6 hours instead of a 24-hour infusion might be an alternative that could improve pain control. This study did not use a weight-based dose as the recommended bolus dose is 20 mg by IV injection and then repeated every 6 hours with a maximum of 120 mg/day [11]. According to a study involving patients undergoing open spine surgeries, nefopam administered before the skin incision and at the end of surgery did not yield any difference in the amount of morphine consumption and the severity of postoperative pain [9]. Similarly, a study involving patients undergoing minimally invasive spine surgery showed that the inclusion of 24-hour nefopam infusion revealed no additional analgesic effect or improved functional outcomes postoperatively [13]. To date, no evidence shows any additional advantage of nefopam in spine surgeries. However, the analgesic effect of nefopam did not prolong for days after discontinuation, which may explain the observations that nefopam had no beneficial effect on postoperative pain intensity and opioid consumption on postoperative days 2 and 3.
Regarding the anti-neuropathic pain property of nefopam, perioperative nefopam significantly diminished neuropathic pain symptoms, such as dysesthesia and paresthesia, in patients who underwent percutaneous endoscopic lumbar discectomy [8]. Our results demonstrated that perioperative nefopam infusion was less beneficial for postoperative neuropathic pain reduction in patients undergoing open spine surgery. This was the first prospective randomized controlled study on the analgesic effect of nefopam in major spine surgery that focused on the recovery outcomes, including the capability of sitting and walking, tube removal, and the LOS. Our protocol was similar to the recommended ERAS scheme in implementing nonopioid multimodal analgesia, limited opioid use, and a prompt for early ambulation [1,12]. The addition of nefopam could decrease the LOS by 1 day, which could save costs and improve resource usage. Unfortunately, perioperative nefopam did not demonstrate any advantageous impact on early ambulation.
This study had some limitations. First, this study had a small sample size, although the power analysis was sufficient. Second, approximately one-third of the patients underwent more than three levels of spine surgery, which caused extensive soft tissue trauma and the need for intensive pain control. The 24-hour infusion of postoperative nefopam might not cover the entire noxious stimulus. Third, subgroup analysis of single- and multilevel fusion would offer further insight into the analgesic effect of nefopam. Future studies could extend nefopam administration to a 6-hour interval for up to 3 postoperative days to ensure its maximum analgesic effect, which may influence postsurgical functional outcomes.
Conclusions
Perioperative IV nefopam demonstrated significant pain reduction during the early postoperative period and shortened the LOS. However, it did not show an opioid-sparing effect, neuropathic pain reduction, or any postoperative functional improvement following open spine surgery. Nefopam is considered safe and effective as a part of multimodal analgesia in open spine surgery.
Acknowledgments
We thank the nurse anesthetists: Ms. Panatchakorn Pitugchaiyawong and Ms. Supreeda Dowkrajang, as well as the research assistances: Mr. Jirat Chantakhat.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: PC; resources: PC, NT, WS; methodology: PP, WS; data collection: WY, TT; data curation: WY, WL; formal analysis: WL; writing–original draft: PC; writing–review and editing: TT, PP; supervision: NT; project administration: WS; and final approval of the manuscript: all authors.