Concurrent Presence of Thoracolumbar Scoliosis and Chiari Malformation: Is Operative Risk Magnified?
Article information
Abstract
Study Design
Retrospective review of Kids’ Inpatient Database (KID).
Purpose:
Identify the risks and complications associated with surgery in adolescents diagnosed with Chiari and scoliosis.
Overview of Literature
Scoliosis is frequently associated with Chiari malformation (CM). More specifically, reports have been made about this association with CM type I in the absence of syrinx status.
Methods:
The KID was used to identify all pediatric inpatients with CM and scoliosis. The patients were stratified into three groups: those with concomitant CM and scoliosis (CMS group), those with only CM (CM group), and those with only scoliosis (Sc group). Multivariate logistic regressions were used to assess association between surgical characteristics and diagnosis with complication rate.
Results:
A total of 90,707 spine patients were identified (61.8% Sc, 37% CM, 1.2% CMS). Sc patients were older, had a higher invasiveness score, and higher Charlson comorbidity index (all p<0.001). CMS patients had significantly higher rates of surgical decompression (36.7%). Sc patients had significantly higher rates of fusions (35.3%) and osteotomies (1.2%, all p<0.001). Controlling for age and invasiveness, postoperative complications were significantly associated with spine fusion surgery for Sc patients (odds ratio [OR], 1.8; p<0.05). Specifically, posterior spinal fusion in the thoracolumbar region had a greater risk of complications (OR, 4.9) than an anterior approach (OR, 3.6; all p<0.001). CM patients had a significant risk of complications when an osteotomy was performed as part of their surgery (OR, 2.9) and if a spinal fusion was concurrently performed (OR, 1.8; all p<0.05). Patients in the CMS cohort were significantly likely to develop postoperative complications if they underwent a spinal fusion from both anterior (OR, 2.5) and posterior approach (OR, 2.7; all p<0.001).
Conclusions:
Having concurrent scoliosis and CM increases operative risk for fusion surgeries despite approach. Being independently inflicted with scoliosis or Chiari leads to increased complication rate when paired with thoracolumbar fusion and osteotomies; respectively.
Introduction
First described by Hans Chiari in 1891, Chiari malformation type I (CM-I) is a hindbrain herniation that involves herniation of the cerebellar tonsils through the foramen magnum [1,2]. In 1894, Julius Arnold concurrently described herniation of the fourth ventricle and cerebellum through the foramen magnum, now named Arnold-Chiari Malformation [1]. These malformations are part of a spectrum of congenital hindbrain abnormalities and various types exist based on their primary etiology [3]. The most common type of Chiari malformation is the type I malformation and it is diagnosed on magnetic resonance imaging (MRI) with >5 mm descent of the cerebellar tonsils [3]. Patients with these conditions often present with multiple complaints including cranial nerve deficits, headache, neck pain, motor or sensory dysfunctions, and scoliosis [2].
Scoliosis is a spinal deformity often associated with CM-I. This association was first described in 1988 by Dauser et al. [4] in their report of seven patients with CM-I. The concomitant rate for the two conditions varies greatly with one study of 500 patients with CM-I reporting an 18% scoliosis rate while others report a rate as high as 50% [2,5]. While the exact pathogenesis of scoliosis in CM-I is still unknown, it has been associated with the presence of syringomyelia in patients with Chiari malformations [6]. Studies have reported a 25%–85% rate of scoliosis in CM-I patients with syringomyelia [6–9]. The presence of a syrinx has also been independently associated with scoliosis in a study of pediatric patients undergoing MRI [10]. While variations exist in the reported rates, CM-I with a syrinx is believed to have a higher prevalence of scoliosis than CM-I without a syrinx [3]. Furthermore, several studies have reported that the convex side of a scoliosis curve is often associated with the dominant side of an asymmetrically displaced cerebellar tonsil and the deviated side of an eccentric syrinx [11,12]. These reports suggest that tonsillar impaction, particularly on the dorsum of the cervicomedullary junction, is enough of an impetus to lead to scoliosis in patients with CM-I [3,12].
Several studies have reported cases of scoliosis in patients with CM-I without syringomyelia [6]. Most of these patients were small retrospective case series, with poor follow-up, in CM-I patients where only a handful of patients were found to have scoliosis without syringomyelia [6,13–15].
Posterior fossa decompression with or without spinal fusion is often performed to improve or stabilize scoliosis in patients with syringomyelia. Varying levels of success have been reported with some reporting scoliosis improvement in 38% and stabilization in 23% of patients who underwent a posterior fossa decompression for scoliosis in CM-I with syringomyelia [6–8]. Others have reported that decompression alone was adequate treatment for CM-I patients with syrinx and mild scoliosis less than 20° but patients with a curve greater than 20° required bracing or a spinal fusion in addition to the decompression [16].
While numerous studies report on decompression with or without fusion for CM-I patients with scoliosis there exists a paucity of literature on the risks and complication rates of these procedures. The purpose of this study was to utilize a large publicly-available pediatric database to investigate the risks and complication rates in patients with CM who underwent surgical interventions.
Materials and Methods
1. Data source
The Kids’ Inpatient Database (KID) is the largest publicly-available all-payer pediatric (age <21 years at admission) inpatient health care database in the United States. The Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project (HCUP) created KID. KID sampling includes complicated and uncomplicated births, as well as other pediatric inpatient procedures from community, non-rehabilitation hospitals. The KID contains 107 data elements, using International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) format to code all of the diagnoses and procedures. With over 3 million hospital stays per 3-year database, it is designed to allow accurate calculation of medical condition incidences using HCUP-provided trend weights [17]. Under the Health Insurance Portability and Accountability Act (HIPAA) of 1996, review by an institutional review board is not required for use of the current data set. A detailed overview of the KID design is available at https://www.hcup-us.ahrq.gov/kidoverview.jsp.
2. Patient sample
The KID was queried for patients with E-codes (ICD-9-CM codes) pertaining to Chiari malformation syndrome and development of scoliosis from 2003–2012 (Appendix 1). Given the nature of the KID database, all patients included were operative thus we identified our patient groups based on their ICD-9 diagnosis codes: (1) those who were diagnosed with concomitant CM and scoliosis (CMS group), (2) those with only CM (CM group), and (3) those with only scoliosis (Sc group). Procedure codes were not specific enough to our diagnoses to discern which patients received an operation for their specific diagnosis.
3. Statistical analysis
Descriptive and univariate analyses, including Kruskall-Wallis testing with post-hoc Mann-Whitney U tests identified differences between the three groups for demographic variables, incidence, surgical strategy, postoperative complications, and comorbidity profile. The latter was stratified by body systems (neurological, musculoskeletal, pulmonary, cardiovascular, renal). Groups were compared using t-tests and chi-square tests for continuous and discrete variables, respectively. Multivariate logistic regressions were used to assess association between surgical characteristics/diagnosis with complication rate. Receiver operating characteristic (ROC) curves identified cut-offs based on the procedures that were significant for developing postoperative complications in order to reduce this risk based on individual baseline Charlson comorbidity index (CCI). All statistics were done using IBM SPSS Statistics ver. 23.0 (IBM Corp., Armonk, NY, USA). A statistical cut-off value of p<0.05 was considered significant.
Results
1. Cohort overview
A total of 90,707 patients were identified, of which 61.8% were in the Sc cohort, 37.0% in CM cohort, and 1.2% in the CMS cohort. Sc patients were older (Sc 13.6 years versus CM 5.9 years versus CMS 11.2 years, p<0.001), had a higher invasiveness score (Sc 2.4 versus CM 0.13 versus CMS 1.8, p<0.001), and more comorbidities with higher CCI (Sc 0.89 versus CM 0.55 versus CMS 0.8, p<0.001) (Table 1).
2. Surgical overview
CMS patients had significantly higher rates of surgical decompression (36.7%, p<0.001). Sc patients had significantly higher rates of fusions (35.3%, p<0.001) and osteotomies (1.2%, p<0.001). However, CMS patients had the highest surgical rate amongst the cohorts (CMS 55.4% versus SC 36.6% versus CM 11%, p<0.001) (Table 2). Scoliosis patients had the greatest rate for larger fusions (16.4% versus CMS 10.9% versus CM 0.1%, p<0.05).
3. Overall complication rate
Patients in the Sc cohort had the higher overall complication rate at 27.2% (p<0.001). These patients also had significantly greater rates of gastrointestinal and infection complications compared to the other two groups (p<0.001) (Table 3). However, CMS patients had the greatest rates of cardiovascular (2.6%), respiratory (3.0%), and dysphagia (1.4%) complications (p<0.001).
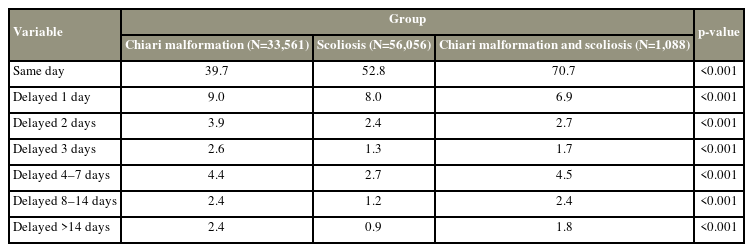
4. Time to procedure
CMS patients underwent more same day surgeries than the Sc and CM patients (CMS 70.7% versus Sc 52.8%, CM 39.7%, p<0.001). CM patients were more likely to experience a delay in surgery from the date of admission (Table 4). Patients in the CMS cohort undergoing surgery for scoliosis and CM had increased odds of postoperative complications if their surgery was delayed 14+ days (odds ratio [OR], 3.5; 95% confidence interval [CI], 1.4–8.8; p=0.007). For the CM cohort, all delays were identified to significantly increase a patient’s odds of having a postoperative complication. However, for Sc patients undergoing surgery, a surgical delay 3 days was found to increase the odds of complications 1.4× and delay of 14+ days to 1.9× (all p<0.001).
5. Postoperative complications based on surgery
Controlling for age and invasiveness, the OR for developing postoperative complications in Sc patients undergoing a spine fusion was 1.8 (95% CI, 1.08–3.2; p<0.05). Specifically posterior spinal fusion in the thoracolumbar region had a greater risk of complications than (OR, 4.9; 95% CI, 2.3–10.5; p<0.001) an anterior approach (OR, 3.6; 95% CI, 1.5–8.9; p<0.001). Patients in the CM cohort undergoing surgery for a Chiari malformation had a significant risk of complications when an osteotomy was performed as part of their surgery (OR, 2.9; 95% CI, 1.4–6.0; p<0.05) and if a spinal fusion was concurrently performed (OR, 1.8; 95% CI, 1.2–2.9; all p<0.05). Patients in the CMS cohort who had a Chiari malformation with concomitant scoliosis were significantly likely to develop postoperative complications if they underwent a spinal fusion from both an anterior (OR, 2.5; 95% CI, 1.3–5; p<0.05) and a posterior approach (OR, 2.7; 95% CI, 1.5–4.7; p<0.001). The OR for complications were highest within CMS cohort for patients who underwent a posterior spinal fusion in the thoracolumbar region (OR, 2.1; 95% CI, 1.2–3.7; p<0.05) and those who underwent an anterior lumbar fusion (OR, 6.8; 95% CI, 1.3–3.6; all p<0.001).
6. Sub-analysis on Charlson comorbidity index with associated complications
ROC curves were used to identify CCI cut-off values for each patient group for procedures that increased the rate of postoperative complications. For patients in the Sc cohort undergoing fusions, the CCI cut-off value for which this procedure should be performed to minimize the complications was 1.5 and was not affected by the fusion location nor approach. CM patients undergoing a fusion surgery with 9+ levels fused and osteotomies had a CCI cut-off value of 0.5. Patients that were concomitantly treated for their scoliosis and CM (CMS) with a fusion in the thoracolumbar region had a CCI cut-off of 1.5, but if the surgery was performed in the lumbar spine only via an anterior approach the CCI cut-off was 1. Postoperative complications independently associated with CMS patients were acute respiratory distress (OR, 2.1; 95% CI, 3.4–1.3; p<0.05) and anemia (OR, 0.6; 95% CI, 0.85–0.36; p<0.05).
Discussion
In this retrospective cohort study, we utilized a large publicly available pediatric database to investigate the risks and complications of surgery in patients with Chiari malformation and scoliosis when compared to patients with scoliosis alone or Chiari malformation alone. From a sample size of 90,707 total patients, we discovered that compared to the CM and CMS groups, patients in the Sc only group were significantly older with more medical comorbidities and a higher CCI. They also had significantly more invasive procedures performed than the other two groups. Patients in the Sc group also had a higher rate of fusion and osteotomies than the other two groups. This can be attributed to the fact that deformity correction surgeries often necessitate the need for osteotomies and extensive fusion constructs. The significantly higher percentage of 2+ levels fused in this group is also explained by this.
Patients in the CMS group had a significantly higher surgical rate as well as rate of surgical decompression than the other two groups. This can be attributed to the fact that this group contained patients with a Chiari malformation and scoliosis. The higher surgical rate and higher decompression rate are likely due to the need to address both issues.
The overall complication rate was higher in the Sc group as was the rate of infection related complications. This can be attributed to the extensive incisions that are often necessary for deformity correction surgery. Cardiovascular, respiratory, and dysphagia related complications were greater in the CMS group. This is likely due to the fact that the brainstem and cranial nerves are at risk during posterior fossa decompression surgeries for a Chiari malformation. Delays in surgery, though more common in CM cohort, lead to an increase in complications for all groups. The cause of such delays is itself difficult to assess, though previous analyses have demonstrated that in patients with CM, post-admission sepsis complications have been associated with mean operative delays of at least 3 days, and respiratory complications and anemia have been associated with delays of 8 to 14 days [18].
When controlling for age and invasiveness posterior thoracolumbar fusion had a greater OR for complications in the Sc group. For the CM group had higher complication rates if osteotomies or spinal fusion procedures were added to their decompression surgeries. The increase in complications with the addition of such extensive procedures is expected. Similarly, the increase in postoperative complications in the CMS group when spinal fusion was performed is also understandable. Furthermore, we identified that patients’ CCI is associated with complications and for all three groups higher CCI values were associated with increased OR for complications.
There is a paucity of literature comparing risks and complications after surgery in patients with Chiari malformation with scoliosis, Chiari malformation alone, or scoliosis alone. In one of the few studies published on this, Godzik et al. [19] compared the risks and outcomes of spinal deformity surgery in patients with CM-I associated scoliosis and those with adolescent idiopathic scoliosis. In their single institution study, they identified 36 patients with CMS who underwent instrumented fusion and matched them to 36 patients with AIS [19]. They found similar deformity correction and outcome scores between the groups [19]. While there were no differences in overall complication rate, they reported higher rates of neurological complications and neuromonitoring difficulties in the CMS group [19].
Numerous studies have reported on curve and deformity progression after suboccipital decompression for Chiari malformation associated scoliosis, and few have reported on risks and complications. In their retrospective study of 936 patients who underwent surgical decompression for CM-I, Greenberg et al. [20] identified a 12.5% postoperative surgical complication rate with hydrocephalus as the only comorbidity independently associated with increased surgical risk. In their study of 21 children with CMS who underwent hindbrain decompression, Attenello et al identified thoracolumbar junction scoliosis and failure of syrinx to improve as risk factors for postoperative deformity progression [21]. They also identified large preoperative scoliotic curves as another risk factor [21]. Similarly, Chotai et al. [22] reported a 41.2% improvement in scoliosis associated with CMN-I after posterior fossa decompression and identified preoperative curves >35° and adolescent-onset of scoliosis as risk factors for curve progression and need for corrective surgery. These findings were corroborated by Mackel et al. [23] in their study of 44 patients with CMS that underwent posterior fossa decompression. However, Ozerdemoglu et al. [24] reports that in their series of 105 patients with scoliosis and syringomyelia, syrinx shunting alone did not improve scoliotic deformity in any of the subjects studied. Numerous other studies have also reported curve progression rates ranging from 30%–70% in patients with CMS who underwent hindbrain decompression, and have identified double scoliosis curves and later age of decompression as additional risk factors [9,16,25].
This study is one of few that compares postoperative complications in patients with CMS to CM alone and scoliosis alone. It fills a knowledge gap that currently exists in literature. Specifically, though previous studies have focused primarily on the epidemiologic risk factors of Chiari malformation and concurrent scoliosis, this study uniquely focuses on the operative risks of corrective surgery. Furthermore, the utilization of a high-volume database in conjunction with novel multivariate analysis increases the power of resultant models, thus allowing for more accurate assessments of the aforementioned operative risk of corrective surgery. In their review of existing literature, Kelly et al. [26] reported that decompression surgery for the treatment of CM may itself result in resolution of abnormal spinal curvature, yet noted that many studies have suffered from relatively small sample sizes and concerns of underpowered findings. The present study suggests that due to the increased risk of operative complications in patients with concomitant CM and scoliosis, surgeons should consider primary decompression of CM prior to deformity correction. To our knowledge, our review of national data provides the first large-scale evidence that such prioritization may decrease postoperative complications overall and potentially reduce the rates of complications due to surgical delays in the treatment of concomitant CM and scoliosis in the pediatric population.
However, the present study has both strengths and weaknesses. As this study utilized a nationally available public database of pediatric inpatients it allowed for a large number of patients and their extensively collected data to be analyzed. A limitation of such a database is the inability to obtain additional information from patients and to prospectively plan studies. While large-volume datasets such as KID provide the ability to create high-power analyses, we acknowledge that captured encounters represent hospitalization records and not individual patients [27]. Furthermore, due to inherent limitations of national datasets, we are unable to comment on the ideal timing of scoliosis correction after CM decompression, indicating the need for more granular future studies. The retrospective nature of this study, may also introduce elements of reporting and selection bias. Future investigations on the risks, complications, and outcomes of surgery in patients with Chiari malformations and scoliosis is warranted to fully understand their disease processes.
Conclusions
This study demonstrates that surgery for Chiari malformation-associated scoliosis is associated with increased risk of cardiovascular, respiratory, and dysphagia-related postoperative complications. While the overall complication rates are higher in scoliosis alone patients, patient comorbidities increase the operative risk for when thoracolumbar spinal fusion surgeries are performed. Uniquely, this study also demonstrated that having concurrent scoliosis and Chiari malformation increases operative risk for fusion surgeries despite approach. Being independently inflicted with Chiari malformation also leads to an increased complication rate when fusions are paired with osteotomies.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: SN, WA, KP, PGP; data curation: SN, WA, KP; Formal analysis: SN, PT; funding acquisition: PGP; methodology: SN, PT, PGP; project administration: PGP; visualization: SN, PGP; writing–original draft: SN, PT; writing–review & editing: SN, PT; and final approval of the manuscript: all authors.