New Horizons in the Diagnosis of Tuberculosis of the Spine: The Role of Whole Genome Sequencing
Article information
Abstract
Study Design
Prospective study.
Purpose
To evaluate the utility of whole genome sequencing (WGS) in drug resistance testing, lineage of the organisms, and organism-related factors responsible for bacilli settling in the spine.
Overview of Literature
The workstream for the diagnosis of tuberculosis (TB) involves isolation and culture of the organism and drug resistance testing using phenotypic methods. Xpert MTB/RIF Ultra is a genetic-based method that detects for Mycobacterium tuberculosis DNA in the rpoB gene. Meanwhile, WGS is a newer genetic-based method that assesses the whole genome of the bacterium. Very few studies have reported the use of WGS for extrapulmonary TB. Herein, we used WGS to diagnose spinal TB.
Methods
Tissues from 61 patients undergoing surgery for spinal TB underwent histologic examination, Xpert MTB/RIF Ultra, and culture and sensitivity testing. DNA from the cultured bacteria was sent for WGS. The test bacterial genome was compared to a reference strain of pulmonary TB.
Results
Acid-fast bacilli were observed in 9/58 specimens. Meanwhile, histology confirmed TB in all the patients. Bacilli were cultured in 28 patients (48.3%), and the average time to culture was 18.7 days. Xpert MTB/RIF Ultra was positive in 47 patients (85%). WGS was performed in 23 specimens. Overall, 45% of the strains belonged to lineage 2 (East Asian). There was one case of multidrug-resistant TB and two cases of non-tuberculous mycobacteria on WGS. We could not confirm any genomic difference between pulmonary and spinal TB strains.
Conclusions
Xpert MTB/RIF Ultra of tissues or pus is the investigation of choice when diagnosing spinal TB. Meanwhile, WGS can diagnose multidrug-resistant TB and non-tuberculous mycobacteria more accurately. No mutations were identified in spinal and pulmonary TB bacteria.
Introduction
Tuberculosis (TB) is a chronic infectious disease caused by Mycobacterium tuberculosis (Mtb), which infects approximately a third of the worldwide population [1]. TB results in the death of more than one and a half million people each year and is responsible for 8–10 million new cases of active TB [2]. TB is a disease that is associated with poverty, immunosuppressive drugs, and immunodeficiency-inducing diseases, notably human immunodeficiency virus infection [3]. Although it is primarily a pulmonary disease, it can spread to the spine and cause paraplegia, which is the most devastating complication of spinal TB.
Laboratory diagnosis is aimed at isolating the organism and testing for drug susceptibility. Examination on light microscopy reveals classical granulomas and Langhan’s giant cells and acid-fast bacilli on the Ziehl–Nielsen stain. The first-line test when diagnosing TB in South Africa is the Xpert MTB/RIF Ultra assay, which is a nucleic acid gene amplification test that detects the presence of Mtb DNA as well as mutations in the rpoB gene that predicts resistance to rifampicin [4]. The Xpert MTB/RIF Ultra assay is the most rapid and useful diagnostic tool currently available. The specimen is cultured using the mycobacterial growth indicator tubes (MGIT) for a maximum of 6 weeks. Once growth is flagged, line probe assays are used to determine susceptibility to anti-tuberculous drugs.
Whole genome sequencing (WGS) is a novel diagnostic modality that examines the whole genome of the organism for a more accurate diagnosis and identification of other loci that can predict drug resistance. WGS can also identify the lineage of the strain causing the disease. When applied to clinical isolates, WGS may provide comprehensive results more rapidly than the currently available methods [5].
There are few reports on the use of WGS in diagnosing extrapulmonary TB [6,7]. Hence, this study aimed to investigate the role of WGS in the diagnosis and treatment of spinal TB and to investigate if there are genomic factors that lead to the bacilli settling in the spine.
Materials and Methods
The study was approved by the Research and Ethics Committee of the University of Pretoria (Ethics Ref. 73/2019). Documented informed consent was obtained from all individual participants included in the study. Overall, 61 consecutive patients undergoing open decompression and biopsy of the spine were enrolled in the study. Tissue and pus samples were sent for histological and microbiological examination for diagnosis and drug susceptibility testing. WGS was performed on DNA extracts from the culture isolates. Specimens that were insufficient for processing, not suitable for a particular examination, or contaminated were excluded from that specific examination.
Tissues for histology were stained with hematoxylin and eosin and Ziehl–Nielson stain and examined under light microscopy. The TB granulomas were classified into four categories based on the arrangement and types of cells observed during microscopy.
Pus and tissue samples were sent to microbiology. All specimens were processed using the Xpert MTB/RIF Ultra assay (Cepheid, Sunnyvale, CA, USA) and were also cultured for Mtb for a maximum of 42 days in liquid culture. This was followed by drug susceptibility testing.
The genomic material from the cultured isolates was sent for WGS. Care was taken to ensure purity of each isolate. Only a single isolate unique to a patient was included in the testing. DNA was extracted using the Nuclisens easyMAG DNA extraction system (BioMérieux, Marcy-l’Étoile, France) from 1 mL of concentrated positive culture MGIT medium. Sequencing libraries were prepared using the Illumina Nextera DNA Flex Library Preparation kit (Illumina, San Diego, CA, USA) as per the manufacturer’s instructions and sequenced on the NextSeq 500/550 High Output Kit ver. 2.5 (300 cycles). Sequencing analysis was completed using CLC Genomics Workbench ver. 11.0 (Qiagen, Venlo, Netherlands) to detect any variants (single nucleotide polymorphism [SNP], insertions and deletions) against the reference genome of Mtb H37Rv.
Lineages were assigned using the SNP bar coding as described by Coll et al. [8]. Maximum likelihood trees were constructed using the SNP approach.
Results
Ziehl–Nielsen staining was positive in nine patients (15.51%) and negative in 50 patients, while three specimens could not be processed. Hematoxylin and eosin staining confirmed the diagnosis in all 55 specimens.
Xpert MTB/RIF Ultra was performed in 55 specimens; 47 (85.5%) were positive. In cases wherein both pus and tissue specimens (n=23) underwent testing, the result was positive for both specimens in all cases. Four patients had rifampicin mono-resistance. There was only one case of multidrug-resistant TB (MDRTB), and there were no cases of extensively drug resistant TB.
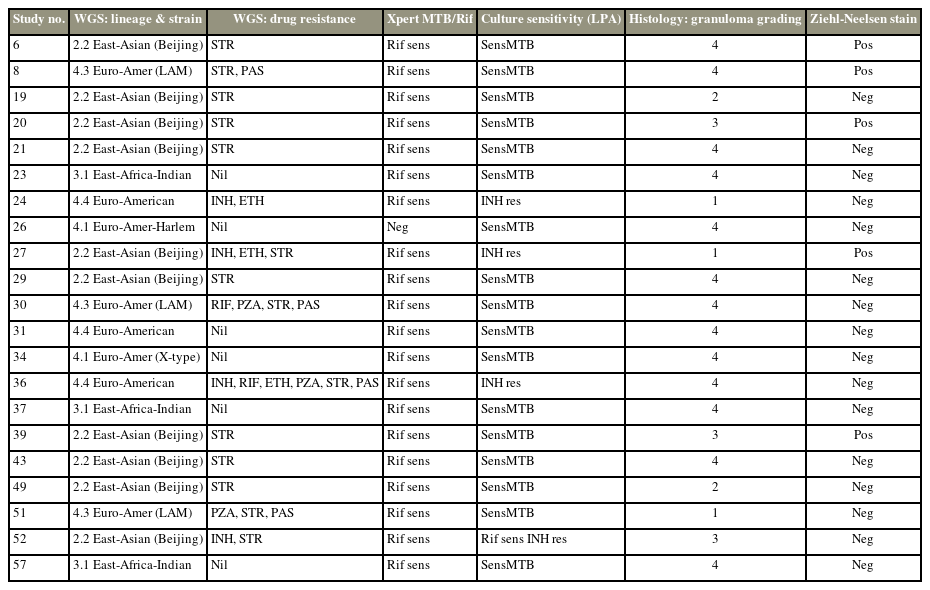
In 30 patients (51.7%), there was no growth after 42 days of incubation. Culture was positive in 28 patients (48.3%). The average time for culture was 18.7 days (range, 11–32 days). WGS was performed on 23 of the 28 isolates that were cultured.
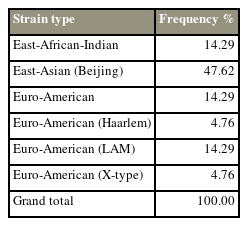
Fig. 1 is a graphic representation of the lineage and phylogenetic relatedness of the 23 isolates. One is the reference strain of Mtb H37Rv, while two (8.6%) are non-tuberculous mycobacteria (NTM). The remaining 21 isolates are under investigation. Ten out of the 21 strains (47.62%) belong to the East Asian (Beijing) strain lineage 2, 4.28% each belonged to East African-Indian, Euro-American, and Euro-American (LAM) strains, and 4.76% each belonged to Euro-American (X-type) and Euro-American (Haarlem), as seen in Table 1.
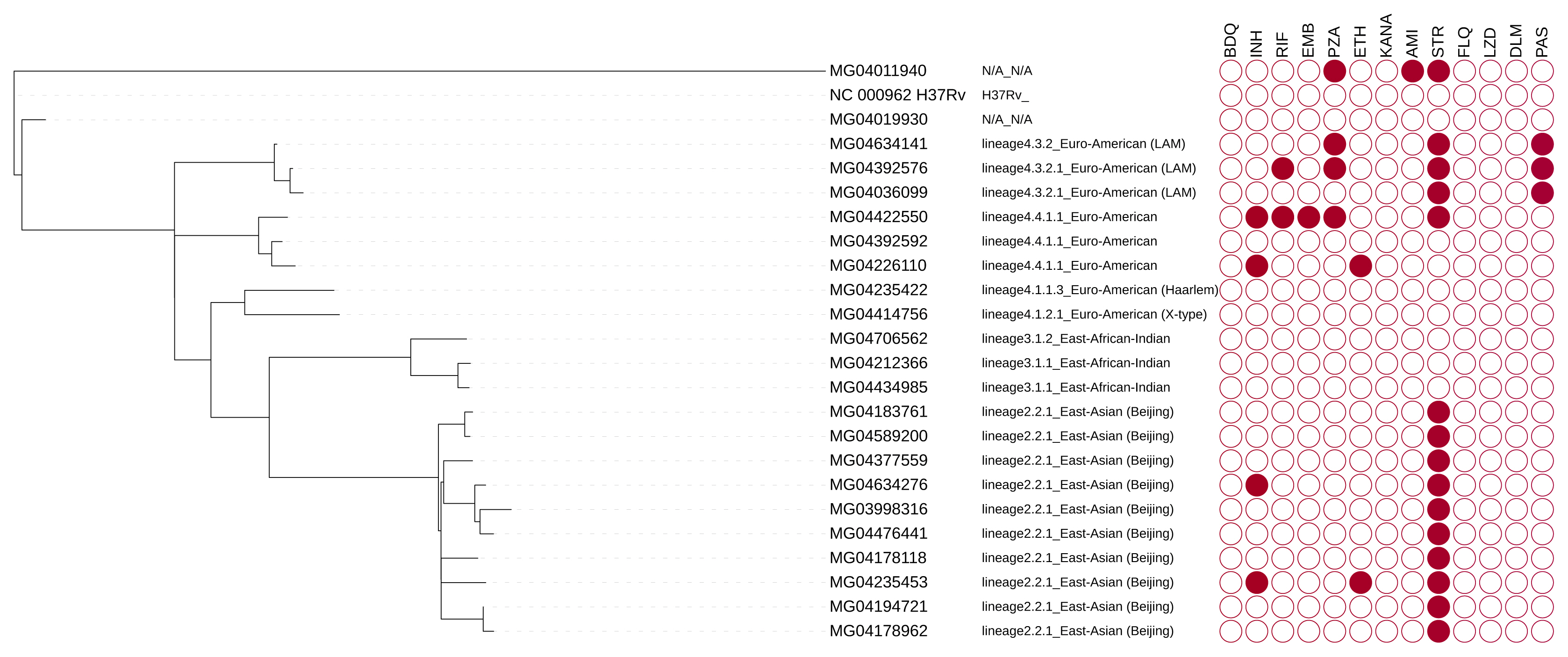
Tree of isolates showing lineage and genotypic resistance profile. Filled circles indicate resistance to the drug tested. BDQ, bedaquiline; DLM, delamanid; FLQ, fluoroquinolones; LZD, linezolid; INH, isoniazid; RIF, rifampicin; EMB, ethambutol; PZA, pyrazinamide; ETH, ethionamide; KANA, kanamycin; AMI, amikacin; STR, streptomycin; PAS, para-aminosalicylic acid.
There is a high frequency of resistance to streptomycin. One isolate (MG04422550) was resistant to multiple first-line anti-TB drugs.
Table 2 shows the results of WGS and clinical testing performed on the culture-positive specimens. Although specimen #26 was Xpert MTB/RIF Ultra negative, culture and WGS showed that the specimen was positive for TB. Additionally, although specimen #26 only showed resistance to isoniazid, culture and WGS showed that it was a multidrug resistant isolate. The Ziehl–Nielsen-negative specimens were all positive on culture and WGS.
Comparing the results of whole genome sequencing to the tests currently done in clinical assessment of tuberculosis spine
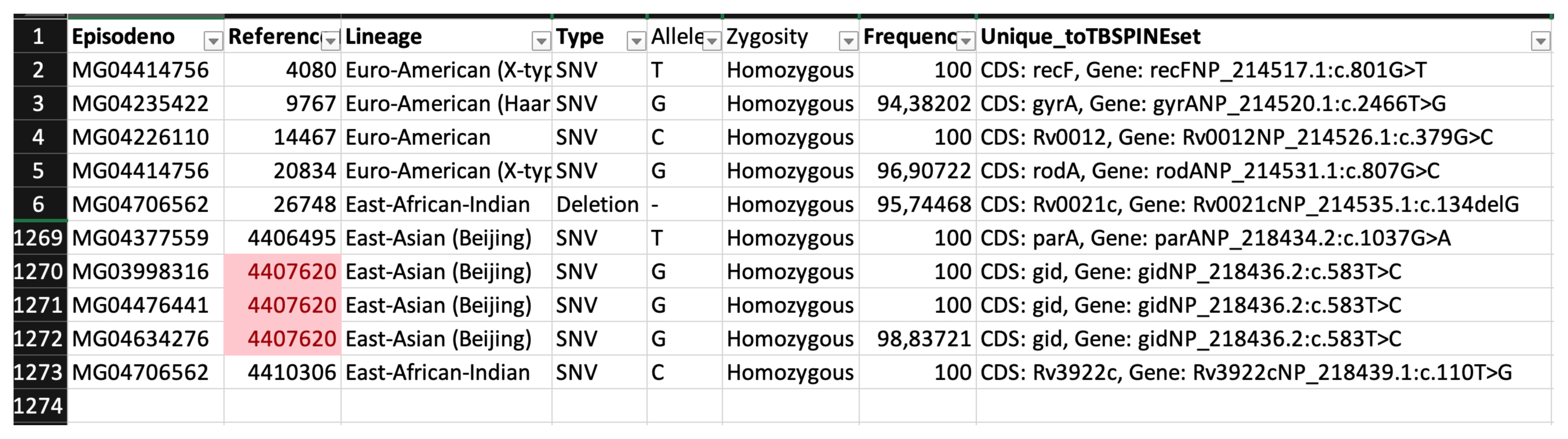
In identifying unique genetic markers that may be associated with spinal TB, we found 28,551 mutations across all 21 spinal TB genomes. After removing all other mutations as stated in the methods, we were left with 1,272 mutations that were uniquely associated with spinal TB isolates (Fig. 2).
When the relationships of the known pulmonary samples and spinal TB samples were analyzed at the nucleotide level, we could not find any unique mutation across all lineages that were specific to spinal TB samples. The most frequent unique mutation was found in the East Asian (Beijing) lineage, with a frequency of 50% among the Beijing lineages. Additionally, there were 864 unique mutations by lineage.
With analysis at gene level, we could not observe a pattern related to a specific gene across all lineages. There were 590 unique genes in which mutations were found. We compared variants within the set and against the pulmonary sample variants to identify any variant exclusive to the study samples. However, no variant was found to be unique to these spine isolates.
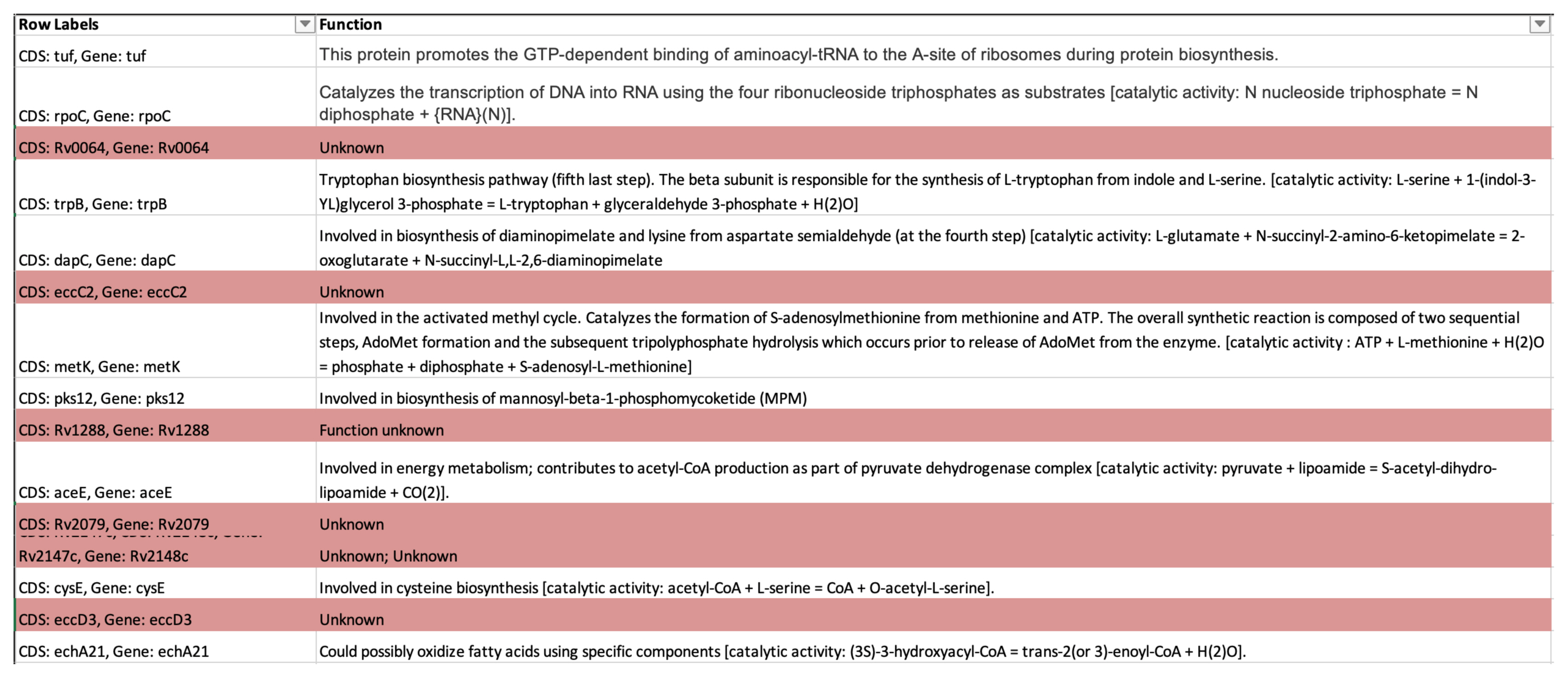
Meanwhile, 504 genes occurred in less than 10% of the isolates, with genes having unknown function (Fig. 3). It is not certain if these isolates are those that may be responsible for certain functions that will enable the bacteria to survive in the spine. In a future study, the remaining 86 genes will be reviewed to determine their function and whether they are possibly related to the spinal TB samples.
Discussion
WGS is a novel molecular technique that comprehensively assesses the whole genome of the organism, enabling drug sensitivity determination and lineage identification and revealing relationships between strains, making it a useful method during disease outbreaks. In this study, WGS was performed to determine whether the genomic material of the TB bacilli causing spinal disease had a common genomic marker compared to the strains causing pulmonary disease.
Smear microscopy was positive in 15.51% of the specimens. In other extrapulmonary sites (pus, ascites fluid, urine, cerebrospinal fluid) smear microscopy was found to be accurate in 68.4% [9]. Because of the pauci-bacillary nature of spinal TB, smear microscopy is not an appropriate examination for spinal TB. Even in pulmonary TB, smear microscopy has been superseded by Xpert MTB/RIF Ultra [10]. Xpert MTB/RIF Ultra was positive in 85% of the specimens. The specificity and sensitivity for spinal TB was reported to be 96.2% and 95.6%, respectively [11]. Currently, Xpert MTB/RIF Ultra is the most optimal test for diagnosing spinal TB as it is more sensitive and the results are available within 24 hours compared to a mean of 18.7 for TB culture.
Of the 23 isolates, WGS confirmed 21 isolates as being positive for Mtb, while the remaining two isolates had NTM. Coinfection with NTM is not uncommon in endemic countries, while it was as high as 17% in some series. In this series, the NTM rate was 8.6%. Accurately diagnosing NTM is important for correct and effective treatment. Meanwhile, poor response to TB treatment may be erroneously ascribed to drug resistance if these co-infecting mycobacteria have not been diagnosed [12].
MDRTB is defined as resistance of Mtb to rifampicin and isoniazid, which are the two primary first-line anti-TB drugs [13]. One of the 21 isolates (4.7%) was identified as MDRTB on WGS; the rate is within the reported range for spinal TB in the literature [14]. As WGS was performed late in the study, we could only diagnose this MDRTB isolate 12 months after treatment had commenced. Had WGS already been in clinical use, diagnosis and treatment of MDRTB would have been initiated earlier.
As WGS relies on a sufficient amount and quality of DNA to provide adequate depth of coverage, the method still requires that bacteria be cultured as it ensures sufficient bacterial DNA for analysis. However, WGS using samples directly from the sputum is now possible with targeted sputum enrichment [15,16], which provides rapid detection of drug resistance within a clinically short time. Thus, it is only a matter of time before targeted enrichment of extrapulmonary specimens, such as pus and bone fragments in spinal TB, become possible.
There are few reports regarding drug resistance in spinal TB [17], with some from centers that perform genotypic resistance testing [18,19]. In spinal TB, drug resistance is caused by several factors, including monotherapy, suboptimal drug intake, and inadequate duration of treatment [20]. Most patients with spinal TB have poor socioeconomic backgrounds and usually reside far from treatment centers [21]. As such, these patients often do not complete the course treatment. In some parts of South Africa, the rate of MDRTB of the spine is as high as 4% [22]. Hence, it is particularly important that TB is diagnosed early so treatment may also be initiated immediately [23]. The mortality for MDRTB is reported to be as high as TB in the pre-antibiotic era [20].
This study is one of the few that utilized WGS to further assess drug resistance and lineage identification from an extrapulmonary site. In their work on 5 extrapulmonary isolates, Sharma et al. [6] could not find any common genetic mutations in the isolates and recommended that more work be done to map the genetic diversity of Mtb.
Sarkar et al. [7] postulated that the development of extrapulmonary TB depends on the affinity of the pathogen to cells other than those of pulmonary tissue. They found that different strains have different capacities to invade different cell types, and that some strains showed a propensity to invade osteoblasts. Li et al. [24] searched for genetic differences that may be responsible for the development of pulmonary or spinal TB, revealing that some genotypes were significantly associated with pulmonary TB.
We also searched for pathogen-related factors that cause the disease to spread from the lungs to the spine. Our results that compared the genome of the 21 samples with that of Mtb H37Rv showed that no mutations were specific to spinal Mtb. Spinal TB isolates had a mixed lineage, just as in pulmonary TB, with most (45%) being the East Asian (Beijing) type. The distribution of the lineages of these bacteria are similar to those found in pulmonary TB [25, 26]. At the genome level, we identified genes encoding unknown protein functions. Hence, additional studies are warranted to determine whether these genes alter the metabolic function of Mtb and enable it to adjust to the hypoxic environment of the spine.
WGS is causing a paradigm shift in diagnostic microbiology. Currently, we multiple polymerase chain reaction assays (PCR) are used to determine susceptibility of organisms to drugs. However, current PCR methods will only identify known mutations to the drugs being tested. Meanwhile, WGS also identifies novel mutations to various drugs, which may potentially predict resistance to several drugs, and significantly reduces the time needed to obtain results.
As TB is mostly a disease in poor communities, the cost of WGS (US $137) is currently prohibitive when compared to Xpert MTB/RIF Ultra (US $10), MGIT liquid culture (US $7.05), drug susceptibility testing (US $25), and histology (US $35). However, with increasing use of this method, the infrastructure and associated costs are expected to decrease.
WGS will gradually be applied in clinical practice. Hence, clinicians, especially those who treat patients with TB, must familiarize themselves with its use.
Conclusions
In this study, WGS revealed no mutations that enable Mtb isolates to be more viable in spinal tissues. However, further research is warranted to determine the function of the unknown genes as they could be responsible for Mtb settling in spinal tissues. The introduction of WGS in clinical practice will assist in the early detection of MDRTB and may prevent the debilitating complications of spinal TB. Smear microscopy is not useful for the diagnosis of spinal TB as this is a pauci-bacillary condition. Additionally, the test serves no purpose as deep-seated spinal tissue does not transmit disease unlike sputum. Currently, Xpert MTB/RIF Ultra should be the test of choice to diagnose spinal TB as it can provide results in 24 hours.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: MN; formal analysis: SVO; methodology: SVO, MS, MB; data curation: SVO; Funding acquisition: MN; writing–original draft: MN; writing–review & editing: SVO, MS, MB; and final approval of the manuscript: all authors.