Effects of Epothilone Administration on Locomotion Recovery after Spinal Cord Injury: A Systematic Review of Animal Studies
Article information
Abstract
This is a systematic review and meta-analysis of existing evidence regarding the possible effects of epothilones on spinal cord injury (SCI). This study aimed to investigate the possible effects of epothilone administration on locomotion recovery in animal models of SCI. Despite increasing rates of SCI and its burden on populations, no consensus has been reached about the possible treatment modality for SCI. Meanwhile, low-dose epothilones have been reported to have positive effects on SCI outcomes. Electronic databases of Web of Science, Scopus, Embase, and Medline were searched using keywords related to epothilones and SCI until the end of 2020. Two researchers screened the articles, and extracted data were analyzed using STATA ver. 14.0. Final results are reported as a standardized mean difference (SMD) with a 95% confidence interval (CI). After the screening, five studies were included in the analysis. Rats were used in all the studies. Two types of epothilones were used via intraperitoneal injection and were shown to have positive effects on the motor outcomes of samples with SCI (SMD, 0.87; 95% CI, 0.51 to 1.23; p=0.71). Although a slightly better effect was observed when using epothilone B, the difference was not significant (coefficient, −0.50; 95% CI, −1.52 to 0.52; p=0.246). The results of this study suggest that epothilones have positive effects on the improvement of motor function in rats, when administered intraperitoneally until a maximum of 1 day after SCI. However, current evidence regarding the matter is still scarce.
Introduction
Owing to the increase in the rates of spinal cord injury (SCI) and its burden, especially on young populations [1], extensive research is conducted in various fields to find a suitable solution and thus improve the complications of SCI. Pathogenic mechanisms in SCI lead to lasting lesions that greatly impair the movement and sensation of patients [2]. These mechanisms include axonal destruction, scar formation, proteoglycan barrier formation, secretion of inhibitory mediators for axonal regeneration, etc. All of the mentioned mechanisms, along with the inability of the central nervous system to regenerate (proliferate and differentiate) lost cells, have challenged the treatment of SCI dramatically [3].
From the initial days of understanding SCI mechanisms, it has been hypothesized that if these pathogenic mechanisms in spinal injuries are inhibited or even reversed, positive effects might be observed as improvements in patient’s sensation and movement. Therefore, the effectiveness of various treatments such as cell therapy, gene therapy, and molecular and pharmaceutical strategies has been investigated [4-6].
Retrograde degeneration in SCI is one the most important mechanisms prohibiting the formation of functional synapses [7]. Therefore, the use of microtubule-stabilizing drugs may help in the formation of new synapses. Epothilones are one of the microtubule-stabilizing drugs that were reported to have positive effects on reducing lesion size, inhibiting scar formation in the injury site, and reactivating axonal regeneration [8]. Although epothilones are known anticancer drugs, in lower dosages, their axonalstabilizing effects inhibit spinal cord pathogenic mechanisms.
Even though tissue changes are usually accompanied by the improvement of movement and sensation, clinically, behavior improvement (sensation and movement) is more important than tissue changes. Overall, there are major inconsistencies in studies about the efficacy of epothilones in movement recovery following SCI. For example, Zhao et al. [9] showed that epothilones cause movement recovery in animals, whereas Sandner et al. [10] believe that epothilones have no effects on movement recovery in animals following SCI. In addition, Mao et al. [11] showed that epothilones have negative effects on animal movements. These inconsistencies have made it impossible to have a decisive opinion about the efficacy of epothilones in SCI.
Therefore, this systematic review, by summarizing the existing preclinical evidence, aimed to shed light on the efficacy of epothilones in improving movement in animals with SCI.
Methods
1. Study design
In this systematic review, data from animal studies that have investigated the effect of epothilones on movement recovery were collected. The researchers of this study have conducted various systematic analyses previously, some of which have been presented in the references [5,12-24]. In the present study, the PICO format was followed, which is defined as follows: SCI in animal studies (P), use of epothilone (I), comparison with a group of SCI animals receiving no treatment (C), and movement performance of animals based on standard tests (O).
2. Search strategy
Electronic databases including Web of Science, Scopus, Embase, and Medline were searched from their inception until the end of 2020. Keywords related to epothilones and SCI, as well as synonymous words, were selected, and a search was made using appropriate combinations and standard tags of the respective databases. References of acquired articles were also looked into so that no articles were missed. A manual search was also made in Google and Google Scholar. The search strategy for this study is accessible in Appendix 1.
3. Selection criteria
The study included original animal studies that investigated the efficacy of epothilones on SCI by evaluating the motor outcomes of the samples. Review articles, studies without a control group, studies that did not evaluate our intended outcome, studies that did not provide details on the dosage and method of epothilone administration, retracted articles, and duplicate studies were excluded.
4. Data collection
The records were saved in EndNote (Clarivate, Philadelphia, PA, USA). Duplicate studies were removed, and after importing the records into a Word file (Microsoft Corp., Redmond, WA, USA), two researchers independently screened and selected the articles in two steps. First, each researcher selected possibly related articles by title and abstract screening. Second, the full texts of the selected articles were screened, and related articles were included according to the selection criteria. The included articles were summarized into a checklist and designed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement [25]. Any disagreements were solved by discussions with a third researcher.
Assessed variables were credentials of the first author, year of publication, country of study, demographics of the studied samples (age, sex, and mechanism of SCI), sample size, epothilone type, dosage, method of epothilone administration, time between injury and drug administration, and final outcome. The intended outcome was motor function of the animals. In this study, the latest follow-up was taken into consideration. Data presented as figures were extracted by Plot Digitizer software (https://plotdigitizer.sourceforge.net/).
5. Quality assessment
The quality of the articles was assessed using the guideline provided by Hassannejad et al. [26]. Any disagreements were solved by discussions with a third researcher.
6. Statistical analysis
Data were entered into the statistical software as mean and standard deviation (along with the sample size of each group), and a standardized mean difference (SMD) with a 95% confidence interval (CI) was defined for each study. Then, these values were added to get an overall SMD.
Heterogeneity was investigated using the I2 test, and in case of heterogeneity, if the source of heterogeneity could not be identified through subgroup analysis, a random-effect model was used for analysis. A fixed-effect model was used to report the findings of homogenous studies. Moreover, publication bias was investigated using Egger’s tests [27]. All analyses were performed in STATA ver. 14.0 (Stata Corp., College Station, TX, USA) using “metan” command.
Results
1. Characteristics of the included articles
A total of 1,042 non-duplicate articles were screened, and 15 candidate articles were selected for more extensive screening, from which 10 articles were excluded. The reasons for exclusion were the failure to report motor recovery (three studies), non-related articles (three studies), duplicate reports (two articles), review article (one article), and not having a control group without treatment (one article). Finally, data of five studies were included in the present meta-analysis [8-10,28,29] (Fig. 1).
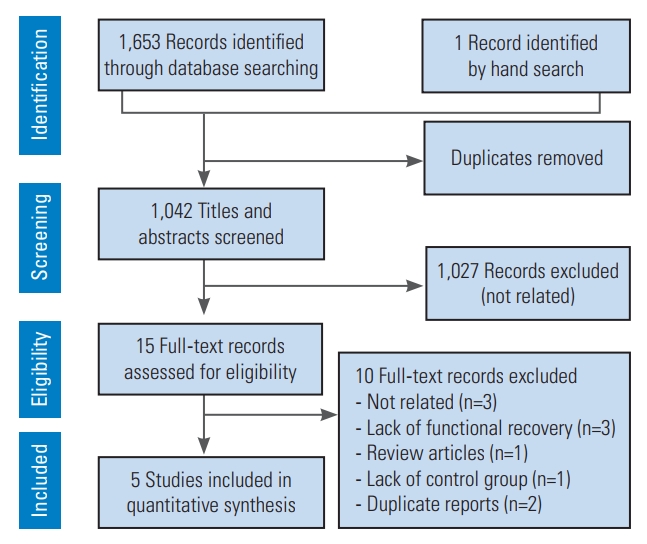
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow diagram of present studies.
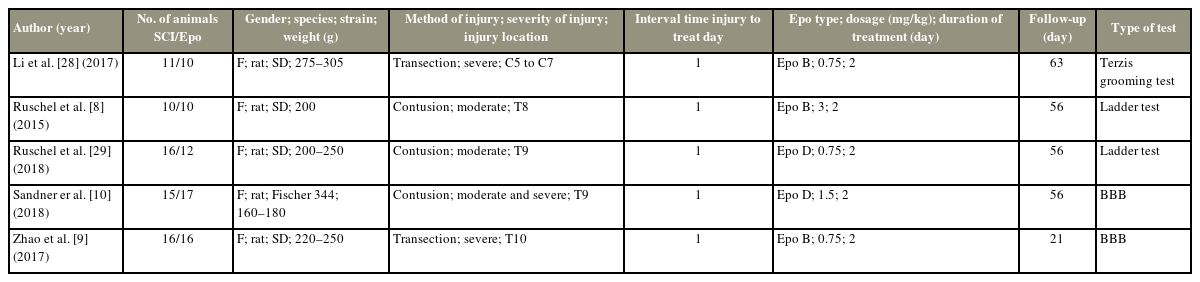
Rat species were used in all of the studies. Two studies used the transection method, and the other three used the contusion method to induce SCI. The injury was moderate in two and severe in the other two studies. Moreover, in one of the studies, the effect of epothilone was investigated separately in moderate and severe injuries. The location of injury was cervical in one and thoracic in the other four studies. The maximum time between injury and treatment was 1 day in all of the studies. Epothilone B was used in three and epothilone D was used in the other two studies. The method of administration was intraperitoneal in all the studies. Follow-up time varied from 21 up to 63 days (56 days in three studies). For the evaluation of motor recovery, the Basso, Beattie, and Bresnahan test was used in two, the ladder test in two, and the Terzis grooming test in one of the studies (Table 1).
2. Quality assessment and publication bias
The SYRCLEs tool (SYRCLE, Nijmegen, The Netherlands) was used to assess the quality of the articles. The quality status of the articles in random housing, random outcome assessment, and selective outcome reporting was unknown in all five studies. Sequence generation, outcome assessor blinding, and incomplete outcome data were at low risk in two studies and unknown in three studies. All five studies graded low risk in baseline characteristics. The quality of the articles in allocation concealment was low risk in one study and unknown in the other four. Moreover, the quality of the articles regarding caregivers’ and/or investigators’ blinding was high risk in one, low risk in one, and unknown in the three remaining studies (Table 2). No publication bias was found in the included studies (p=0.422) (Fig. 2).
3. Meta-analysis on epothilone effect on motor improvement following spinal cord injury
Overall, epothilones had positive effects on motor improvement in animals with SCI (SMD, 0.87; 95% CI, 0.51–1.23; p=0.71). Evidence shows that intraperitoneal injection of epothilone B (SMD, 1.11; 95% CI, 0.61–1.60; p=0.868) has better effects than epothilone D (SMD, 0.61; 95% CI, 0.08–1.13; p=0.675) in movement recovery following SCI (Fig. 3). However, meta-regression showed that this difference was not significant (coefficient, −0.50; 95% CI, −1.52 to 0.52; p=0.246).
Discussion
This systematic review and meta-analysis showed that epothilones had positive effects on the improvement of motor function in rats, and intraperitoneal injection of epothilones until a maximum of 1 day after SCI is associated with motor improvements. Although intraperitoneal injection of epothilone B demonstrated better effects than epothilone D, this difference was not significant. Therefore, a more accurate conclusion about the superiority of any of these compounds over the other requires more prospective studies.
Epothilones inhibit mitosis and cell division by attachment to cellular microtubules; thus, Epothilones are used as anticancer drugs that inhibit the division of cancer cells [8]. In SCI, the release of inflammatory cytokines and the inability of neural cells to divide and regenerate lost tissues trigger a cascade of events, which result in the replacement of the lost neural tissue with scar tissue. Thus, with the use of epothilones, this replacement could be suppressed to some extent, and neural tissue could have the time to regenerate. On the contrary, axonal retrograde degeneration is one of the most important mechanisms that inhibit neural tissue repair [30]. Therefore, with microtubular stabilization of these axons and the prevention of the mentioned phenomenon, epothilones can be effective in improving nerve tissue repair, opening a window for the formation of new synapses.
By contrast, the administration of epothilones is accompanied by unexpected complications on healthy cells and immune cells at the injury site. In addition, the interference of inflammatory factors in the presence of epothilones should be investigated. Some authors believe that the use of epothilones alone has more side effects than positive effects. Mao et al. [11] showed that injection of epothilone B, despite releasing neutrophilic factors, causes the release of microglia and a cascade of cytokines by activating the macrophage colony-stimulating factor (M-CSF). The placement of neural tissue adjacent to the M-CSF next to epothilone B causes difficulties in the repair of the injured site; eventually, motor improvement may not occur with epothilone B injection. Although the injection of a mixture of cytokine-inhibiting antibodies improves the effects of epothilone B administration [11].
Despite the results of this study regarding the overall positive effects of epothilones on motor functionality following SCI, considering the low number of existing studies and the reports of significant side effects in some studies, more studies are needed to determine the role of epothilones in SCI. Other limitations of the present study are as follows: differences in the administered dose of epothilones, type of induced injury, and weight of the studied samples. Finally, to advance studies toward human studies, in addition to further investigations of the side effects of epothilones in histological studies, studies with a larger sample size and more control over confounding factors and methods of motor evaluations are warranted.
Conclusions
This systematic review and meta-analysis showed that the intraperitoneal administration of epothilones has a positive effect on the improvement of motor function in SCI rats, being administered until a maximum of 1 day after the injury. Moreover, despite slight differences, epothilones A and D are not significantly different in promoting functional recovery. To advance animal studies toward human studies, in addition to further investigations on the side effects of epothilones in histological studies, studies with a larger sample size and more control over confounding factors and method of motor evaluation are necessary.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding
Iran University of Medical Sciences has supported this research (Grant number: 98-4-99-16778).
Author Contributions
MY and MH designed the study. MY, AMN, and MJT gathered the data. MH analyzed the data. All authors interpreted the findings. MY and AMN wrote the first draft and other authors critically revised the manuscript.
References
Appendices
Appendix 1.
Search strategy
Medline (via PubMed)
1. “Epothilones”[mesh term] OR “epothilone B” [all field] OR “Tubulin Modulators” [mesh term] OR “Microtubules” [mesh term] OR Epothilones [tiab] OR epothilone B [tiab] OR Tubulin Modulators [tiab] OR Microtubules [tiab] OR Modulators, Tubulin [tiab] OR Microtubule Modulators [tiab] OR Modulators, Microtubule [tiab] OR Tubulin Promoters[tiab] OR Promoters, Tubulin [tiab] OR Tubulin Polymerization Promoters [tiab] OR Polymerization Promoters, Tubulin[tiab] OR Promoters, Tubulin Polymerization [tiab] OR Tubulin Inhibitors [tiab] OR Inhibitors, Tubulin [tiab] OR Tubulin Polymerization Inhibitors [tiab] OR Inhibitors, Tubulin Polymerization [tiab] OR Polymerization Inhibitors, Tubulin [tiab] OR Epothilone [tiab] OR Epothilon [tiab] OR epothilone D [tiab] OR Microtubule [tiab].
2. (“spinal” [All Fields] AND “cord” [All Fields]) AND (Contusion) OR injury) OR trauma OR Transection) OR “Spinal Cord Injuries” [Mesh]).
3. #1 AND #2.
Embase
1. ‘tubulin modulator’/exp OR ‘tubulin modulator’ OR ‘epothilone derivative’/exp OR ‘epothilone derivative’ OR ‘epothilone b’/exp OR ‘epothilone b’ OR ‘microtubule’/exp OR ‘microtubule’.
2. ‘spinal cord injury’/exp OR ‘spinal cord contusion’/exp OR ‘spinal cord hemisection’/exp OR ‘spinal cord transsection’/exp OR ‘cervical spine injury’/exp OR ‘spinal compression’: ab, ti OR ‘spinal cord trauma’: ab, ti OR ‘trauma, spinal cord’: ab, ti OR ‘injured spinal cord’: ab, ti OR ‘spinal cord injured’:ab, ti OR ‘spinal cord injuries’: ab, ti OR ‘nerve transection’: ab, ti.
3. #1 AND #2.
Scopus
1. (TITLE-ABS-KEY (“Epothilones”) OR TITLE-ABS-KEY (“epothilone B”) OR TITLE-ABS-KEY (“Tubulin Modulators”) OR TITLE-ABS-KEY (“Microtubules”) OR TITLE-ABS-KEY (“Modulators, Tubulin”) OR TITLE-ABS-KEY (“Microtubule Modulators”) OR TITLE-ABS-KEY (“Modulators, Microtubule”) OR TITLE ABS-KEY (“Tubulin Promoters”) OR TITLE-ABS-KEY (“Promoters, Tubulin”) OR TITLE-ABS-KEY (“Tubulin Polymerization Promoters”) OR TITLE-ABS-KEY (“Polymerization Promoters, Tubulin”) OR TITLE-ABS-KEY (“Promoters, Tubulin Polymerization”) OR TITLE-ABS-KEY (“Tubulin Inhibitors”) OR TITLE-ABS-KEY (“Inhibitors, Tubulin”) OR TITLE-ABS-KEY (“Tubulin Polymerization Inhibitors”) OR TITLE-ABS-KEY (“Inhibitors, Tubulin Polymerization”) OR TITLE-ABS-KEY (“Polymerization Inhibitors, Tubulin”) OR TITLE-ABS-KEY (“Epothilone”) OR TITLE-ABS-KEY (“Epothilon”) OR TITLE-ABS-KEY (“epothilone D”) OR TITLE-ABS-KEY (“Microtubule”).
2. (TITLE-ABS-KEY (“spinal cord injury”) OR TITLE-ABS-KEY (“spinal cord contusion”) OR TITLE-ABS-KEY (“spinal cord hemisection”) OR TITLE-ABS-KEY (“spinal cord transection”) OR TITLE-ABS-KEY (“cervical spine injury”) OR TITLE-ABS-KEY (“spinal cord injury”) OR TITLE-ABS-KEY (“spinal cord contusion”) OR TITLE-ABS-KEY (“spinal cord hemisection”) OR TITLE-ABS-KEY (“spinal cord transection”) OR TITLE-ABS-KEY (“cervical spine injury”) OR TITLE-ABS-KEY (“Spinal compression”) OR TITLE-ABS-KEY (“spinal cord trauma”) OR TITLE-ABS-KEY (“trauma, spinal cord”) OR TITLE-ABS-KEY (“injured spinal cord”) OR TITLE-ABS-KEY (“spinal cord injured”) OR TITLE-ABS-KEY (“spinal cord injuries”) OR TITLE-ABS-KEY (“nerve transection”).
3. #1 AND #2.
Web of Science
1. TS=(“Epothilones” OR “epothilone B” OR “Tubulin Modulators” OR “Microtubules” OR “Modulators, Tubulin” OR “Microtubule Modulators” OR “Modulators, Microtubule” OR “Tubulin Promoters” OR “Promoters, Tubulin” OR “Tubulin Polymerization Promoters” OR “Polymerization Promoters, Tubulin” OR “Promoters, Tubulin Polymerization” OR “Tubulin Inhibitors” OR “Inhibitors, Tubulin” OR “Tubulin Polymerization Inhibitors” OR “Inhibitors, Tubulin Polymerization” OR “Polymerization Inhibitors, Tubulin” OR “Epothilone” OR “Epothilon” OR “epothilone D” OR “Microtubule”).
2. TS=(“spinal cord injury” OR “spinal cord contusion” OR “spinal cord hemisection" OR “spinal cord transection” OR “cervical spine injury" OR “spinal cord injury” OR “spinal cord contusion” OR “spinal cord hemisection” OR “spinal cord transection” OR “cervical spine injury” OR “Spinal compression” OR “spinal cord trauma" OR “trauma, spinal cord” OR “injured spinal cord” OR “spinal cord injured” OR “spinal cord injuries” OR “nerve transection”).
3. #1 AND #2.