 |
 |
- Search
| Asian Spine J > Volume 17(2); 2023 > Article |
|
Abstract
Purpose
Postoperative evaluation of the cross-sectional area of paraspinal muscle and clinical findings in patients who had interlaminar route uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion (EPTLIF) after 2 years.
Overview of Literature
There are limited short-term follow-up studies on efficacy, safety, and physiological changes with a 2-year follow-up. There is no study on paraspinal muscle cross-sectional area change in patients who had undergone uniportal EPTLIF.
Methods
We evaluated patients who underwent EPTLIF with a minimum 24-month follow-up. Clinical parameters of the Visual Analog Scale (VAS) and Oswestry Disability Index (ODI) were measured at the preoperative, 1-week postoperative mark, postoperative 3-month mark, and final follow-up. Preoperative and 1-year postoperative magnetic resonance imaging measurement of preoperative and postoperative Kjaer grade, right and left psoas muscle mass area, and right and left paraspinal muscle mass area was performed.
Results
EPTLIF with a minimum 24-month follow-up of 35 levels was included. The complication rate was 6%, and the mean BridwellŌĆÖs fusion grade was 1.37 (1ŌĆō2). There was statistically significant improvement at 1 week, 3 months, and 2 years in VAS (4.11┬▒1.23, 4.94┬▒1.30, and 5.46┬▒1.29) and in ODI (40.34┬▒10.06, 46.69┬▒9.14, and 49.63┬▒8.68), respectively (p<0.05). Successful operation rate with excellent and good MacNabŌĆÖs criteria at 2 years was 97%. There was an increment of statistically significant bilateral psoas muscle cross-sectional area, right side (70.03┬▒149.1 mm2) and left side (67.59┬▒113.2 mm2) (p<0.05).
The demand for spinal fusion due to the aging population is increasing as lumbar spinal fusion outcomes had shown favorable results [1ŌĆō3]. Minimally invasive spinal fusion had shown benefits of reduced hospital stay, less blood loss, and less perioperative morbidity [4]. Endoscopic spine surgery is gaining traction as a minimally invasive spine surgery option [5]. Lumbar degenerative spinal conditions which require spinal decompression and/or discectomy had been described in the literature [6]. There is a corresponding increase in describing endoscopic fusion [7,8]. There are two routes of endoscopic fusion, Trans-Kambin and posterolateral as described by Heo et al. [8] in their review of endoscopic fusion. The uniportal TransKambin approach is typically performed with a small diameter uniportal endoscope, with or without foraminoplasty. There is foraminal decompression and indirect interlaminar decompression during uniportal Trans-Kambin fusion. The posterolateral approach can be done with a large diameter uniportal stenosis endoscope or unilateral biportal endoscopy with both direct foraminal and interlaminar decompression as part of the procedure [8]. A posterolateral approach was first performed as a biportal endoscopic-assisted posterolateral lumbar interbody fusion [9,10]. Kim et al. [11ŌĆō13] and Wu et al. [14] published several reports on uniportal endoscopic posterolateral transforaminal lumbar interbody fusion (EPTLIF) applications on various degenerative conditions using large diameter stenosis endoscope. Psoas and paraspinal muscle cross-sectional area is correlated to lower back pain [15,16]. EPTLIF is a relatively new technique with only a short-term follow-up study available. There is no literature on the effect of EPTLIF on paraspinal muscle area, fatty infiltration, and psoas muscle cross-sectional area. We performed a retrospective study to evaluate the medium-term results of EPTLIF.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Nanoori HospitalŌĆÖs Ethics Committee (NR-IRB2021-00) and the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients had given their informed consent for photographs, videos, and images for publication.
A prospectively collected data of patients who underwent single-level EPTLIF was evaluated. We included symptomatic patients who suffered lumbar claudication and back pain after a minimum of 6 weeks of failed conservative treatment with the clinical diagnosis of grade 2 and below spondylolisthesis and spinal stenosis with segmental instability. We excluded patients who had spinal fusion surgery due to trauma, revision surgery, tumor, infection, pseudoarthrosis, congenital spinal deformity, sagittal malalignment, and coronal deformity with more than 20┬░ of the coronal curve. Patient recruitment was from June 2018 to October 2019. The authors performed all one-level lumbar interbody fusions using an endoscopic approach. No open lumbar interbody fusion was performed during the study period. We evaluated clinical outcomes of the Visual Analog Scale (VAS) and Oswestry Disability Index (ODI) at preoperative, 1 week postoperative, 3 months postoperative, and final follow-up at 2 years [17]. MacNabŌĆÖs criteria evaluation was performed at the final follow-up. Postoperative complications were documented. We defined it as the number of patients with good to excellent MacNab criteria/total number of patients at final follow-up and used this as the percentage of successful operations. Preoperative and postoperative 1-year mid disk axial cut magnetic resonance imaging (MRI) evaluation of Kjaer grade, psoas, and paraspinal muscle cross-sectional area was measured with INFINITT PACS M6 version (INFINITT Healthcare Corp., Seoul, Korea) (Fig. 1). The cross-sectional area measurements were in mm2. Computed tomography (CT) lumbar spine evaluation was done on the final follow-up to evaluate the fusion grade using Bridwell grade.
Kjaer grading system was used to evaluate the percentage of fat infiltration [16]. Kjaer grade 0 denotes there is normal condition (0%ŌĆō9%); grade 1, slight fat infiltration (10%ŌĆō50%); and grade 2, severe fat infiltration (>50%) in the lumbar multifidus muscle. All readings were manually measured by I.L. and J.S.P. Independent manual assessment was performed using INFINITT PACS M6 version software.
EPTLIF is an alternative posterolateral approach to the traditional Trans-Kambin approach in uniportal endoscopic fusion and was used in this cohort study. It was previously described by Kim et al. [12,13,18] and Wu et al. [14,19]. Surgery was performed on a Wilson Frame over a radiolucent table, and the patient was under general anesthesia. An endoscopic portal skin incision and docking were placed at the ipsilateral upper mid pedicle for the level intended for fusion on the anteroposterior view of intraoperative fluoroscopy under fluoroscopic guidance (left L4 mid pedicle for left L4/5 EPTLIF for example). Skin marking is typically 3ŌĆō4 cm from the midline and a 1ŌĆō1.5 cm long incision is made on the skin with the fascia incised, sequential dilation with a final endoscope working channel of outer diameter 13.7 mm. The author used a 15┬░ viewing angle endoscope, an outer diameter of 10 mm, a working channel diameter of 6 mm, and a working length of 125 mm. The working retractor target was placed on the ipsilateral facet through the Wiltse muscle splitting approach between multifidus and longissimus muscles. Soft tissue was dissected with a radiofrequency ablator to expose pars interarticularis and inferior articular processes. Isthmus bone and inferior articular process were drilled to perform inferior articular facetectomy and harvested as bone grafts [13]. After inferior articular facetectomy, the superior articular facet was drilled under endoscopic guidance and harvested as a bone graft. Ligamentum flavum decompression was performed exposing the ipsilateral disk space and adjacent segment traversing the exiting nerve root. The working channel is rotated with a bevel facing lateral and inferior direction protecting the exiting and traversing nerve root [12]. Epidural vessel hemostasis is performed over the disk space. Disk and endplate preparation was performed with a radiofrequency ablator, forceps, and endoscopic drill under direct endoscopic vision without damaging the endplate. After a trial of an appropriate-sized cage, a three-dimensional (3D) printed cage was inserted under a working cannula of size 13.7-mm outer diameter. Autologus graft mixed with allograft is tamped into the disk space under image fluoroscopy guidance. A single large 3D printed cage with demineralized bone matrix was inserted as oblique as possible, and the final cage position was checked under both fluoroscopy as well as endoscopy, ensuring that the cage is in a satisfactory position and neural elements are decompressed. The surgical drain was inserted. The endoscope and working cannula were withdrawn with skin closure in layers (Fig. 2).
Data was analyzed with PASW SPSS ver. 18.0 statistical analysis software (SPSS Inc., Chicago, IL, USA). The continuous variables were expressed as mean and standard deviation. Periodic outcomes of MRI and clinical data using the paired t-test were compared. A value of (p<0.05) was considered significant.
From June 2018 to October 2019, a total of 35 patients underwent single-level EPTLIF. Their mean ages were 64.2 years (range, 39ŌĆō78 years) with a mean follow-up of 26.1 months (range, 24ŌĆō34 months). The majority of the patients were female (25 patients, 71.4%). The majority of the preoperative diagnosis was spondylolisthesis (31, 88.6%) with the remaining patients having spinal stenosis with segmental instability. Most of the operating levels were L4/5. The complication rate was 6% with one case of incidental durotomy repaired by endoscopic patch blocking repair technique [20]. The patient had no neurological deficit or subsequent revision. There was one case of a retained drain tip that required removal in the operating theater under local anesthesia. There were no neurological complications, no revision, or adjacent segment disease during the 2-year follow-up (Table 1). The Bridwell grade at postoperative year one CT scan was 1.37, with 22 grade 1 and 13 grade 2 fusion, there were no clinical or radiological signs of pseudoarthrosis. Preoperative and 1-year postoperative MRI were performed for all EPTLIF patients. The mean preoperative Kjaer grade was 0.69 (0ŌĆō1) and the postoperative Kjaer grade was 0.77 (0ŌĆō1). The cross-sectional areas of paraspinal and psoas muscles were measured as shown in Table 2.
In the EPTLIF cohort, at postoperative 1 week, 3 months, and final follow-up, there was a statistically significant improvement in VAS score (4.11┬▒1.23, 4.94┬▒1.23, and 5.46┬▒1.29) and ODI score (40.34┬▒10.06, 46.69┬▒9.14, and 49.63┬▒8.68), respectively (p<0.05). In terms of MacNabŌĆÖs criteria, there were 13 excellent outcomes, 21 good outcomes, and one fair or poor result making 97% good the excellent clinical outcome (Table 3).
The reliability of MRI readings between I.L. and J.S.P. is measured through the interclass correlation coefficient (ICC). A value of more than 0.75 is considered reliable. It is found that there ICC ranges from 0.96 to 0.99 in all parameters of MRI paraspinal muscle measurement.
Compared to the preoperative measure, a 1 year postoperative MRI measurement showed statistically significant change in the mid-disc axial cut cross-sectional area of the right psoas (70.03┬▒149 mm2) and left psoas (67.59┬▒113.2 mm2) as well as in Kjaer grade (0.09┬▒0.28), respectively (p<0.05). However, there was no significant difference in the right and left paraspinal muscle cross-sectional area (Table 4 and Figs. 3, 4).
Minimally invasive lumbar spinal fusion through microscopic tubular and retractor-based approaches had been well-established in literature to show benefits in perioperative pain, blood loss, and length of stay with comparable long-term results compared to open lumbar spinal fusion surgery [21]. There were limited studies on medium-term results for endoscopic lumbar interbody fusion. Heo et al. [8] described in their meta-analysis of endoscopic fusion techniques in literature that there were two main approaches, namely Trans-Kambin and posterolateral. Uniportal endoscopic fusion studies have shown that the majority was performed through the Trans-Kambin approach using a smaller diameter transforaminal endoscope and had shown good short-term results [8,22]. There were limited studies on the posterolateral route of endoscopic fusion using a larger diameter uniportal stenosis endoscope. There was no fusion data and muscle cross-sectional area evaluation in these studies [12ŌĆō14]. We found that there was sustained improvement in pain and function with VAS, ODI, and MacNabŌĆÖs criteria in this study while the complications were minor with no implant-related revision or adjacent segment disease at 2 years after index operation.
Correlation studies showed fatty infiltration and decreased paraspinal muscle cross-sectional area associated with lower back pain [23,24]. Radicular pain on the affected side decreased the psoas muscle cross-sectional area [25]. More literature on these parameters after surgical intervention would be helpful in understanding muscle physiology as a surrogate dynamic radiological marker to assess patientsŌĆÖ function.
Cho et al. [26] found that in his cohort of 40 patients who underwent open posterior lumbar interbody fusion at L4/5. There was no significant change in the psoas muscle [26]. Ortega-Porcayo et al. [27] found that when they performed minimally invasive transforaminal lumbar interbody fusion on one side with unilateral pedicle screw instrumentation, there was no significant difference between the surgical and the non-surgical sides. Our finding echoes the study by Ortega-Porcayo et al. [27].
There is some evidence that perhaps minimally invasive decompression increased while open decompression decreased paraspinal muscle cross-sectional area [28,29]. The authors mainly attributed this to resection and retraction of paraspinal muscle leading to muscle atrophy. We found in our studies that after EPTLIF there were statistically significant increments in bilateral psoas cross-sectional sectional area. While there was a trend of increment in bilateral paraspinal muscle cross-sectional area, it did not reach statistical significance. These findings contradict the previous open lumbar interbody fusion evaluation when there is a general reduction in the paraspinal muscle parameters [28,29]. It is interesting to find that there was less fatty infiltration measured by Kjaer grade after EPTLIF in postoperative 1-year MRI evaluation. The authors felt that EPTLIF had several potential advantages to paraspinal and psoas muscle which could potentially explain these radiological findings. First, EPTLIF docked directly on the bony surface of the lamina and facet of the affected level. There was limited soft tissue dissection required. Second, the working channel in a large diameter endoscope allowed endoscopic instruments and drills to bypass the soft tissues to reach the target site. This will limit the repeated soft tissue trauma when equipment is introduced through soft tissues. Third, radiofrequency ablation was used which provided a relatively lower energy method of soft tissue coagulation as compared to diathermy. Finally, in our practice, before we prepare the endplate for EPTLIF, we would perform radiofrequency ablation to the epidural neovascularization site with adhesion to the nerve root. This practice has been shown to relieve reflex inhibitory mechanisms of paraspinal muscle in radiofrequency ablation surgery in the high-intensity zone [30]. We felt this observation in radiofrequency ablation surgery would be extended to our EPTLIF practice and might explain the increment of paraspinal muscle and psoas muscle cross-sectional area. This is a theoretical proposition, and more studies are required to verify this finding. All these technical modifications might explain the difference in muscle physiology 1 year after index operation.
Despite the minimally invasive nature and potential benefit to soft tissue in EPTLIF, there are some potential pitfalls in EPTLIF. Firstly, there is a steep learning curve in EPTLIF, as described by Wu et al. [7], EPTLIF is a quaternary level of endoscopic surgery which requires a surgeon to be familiar with endoscopic decompression and usage of an endoscopic drill. Performance bias can be a confounding factor in this study. EPTLIF as a technique is new and more long-term studies are required to validate its result. There is also a limitation in large sagittal and coronal deformity correction.
Overall, in the 2-year follow-up patient cohort, EPTLIF appears to be an efficacious procedure with 100% good to excellent functional outcome measured by MacNabŌĆÖs criteria, statistically significant VAS and ODI improvement with minor complications, and good fusion result with no loosening of implants. Minimally invasive benefits of EPTLIF are inferred with increments of psoas bulk and decreased fat infiltration with no reduction in muscle mass of the paraspinal muscle.
This retrospective evaluation prospective data collection with patients who have undergone EPTLIF. The study could have inherent selection and performance bias in the study. Preoperative data such as comorbidities, Charlson Morrison Index, and length of operations time were not collected which might introduce confounders in the study. We limited these confounding factors by having the same team of anesthetists and surgeons for all the operations performed in the data set. A follow-up of 2 years in EPTLIF was short, and we continued to follow up on these patients with a view to evaluating the long-term results in the future. A prospective study and randomized controlled trial would be more ideal to eliminate these biases.
Acknowledgments
We would like to acknowledge scientific team members, Ms. Seonghee Park and Mr. Kyeong Rae Kim for providing assistance in statistical support, acquiring full-text articles, and managing digital works.
Notes
Author Contributions
Conception: HSK, PHW; methodology: HSK, PHW; software: HSK, JWA, MK, IL, JSP, JHL, SK, JK, YYJHL, JHP, JHL, IYJ, PHW; validation: HSK, PHW; formal analysis: HSK, PHW; investigation: HSK, PHW; resources: HSK, PHW, ITJ; data curation: JWA, MK, IL, JSP, JHL, SK, JK, YY, JHL, JHP, JHL, IYJ; writingŌĆöoriginal draft preparation: HSK, PHW; writingŌĆöreview and editing: HSK, PHW, ITJ; visualization: HSK, PHW; supervision: HSK, ITJ; project administration: HSK, PHW; funding acquisition: ITJ; all authors have read and agreed to the published version of this manuscript.
Fig.┬Ā1
Preoperative and postoperative 1-year measurement of paraspinal and psoas muscle parameters in a 53-year-old male patient who had spondylolisthesis L5/S1 and underwent uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion (EPTLIF) L5/S1. (A) Preoperative measurement of paraspinal and psoas muscle parameters. Preoperative mid-disc axial cut magnetic resonance imaging (MRI) of a 53-year-old patient who has spondylolisthesis planned for right EPTLIF L5ŌĆōS1. (B) Postoperative 1 year measurement of paraspinal and psoas muscle parameters. Postoperative 1-year mid-disc axial cut MRI of a 53-year-old patient who had undergone right uniportal full EPTLIF L5ŌĆōS1. SD, standard deviation.
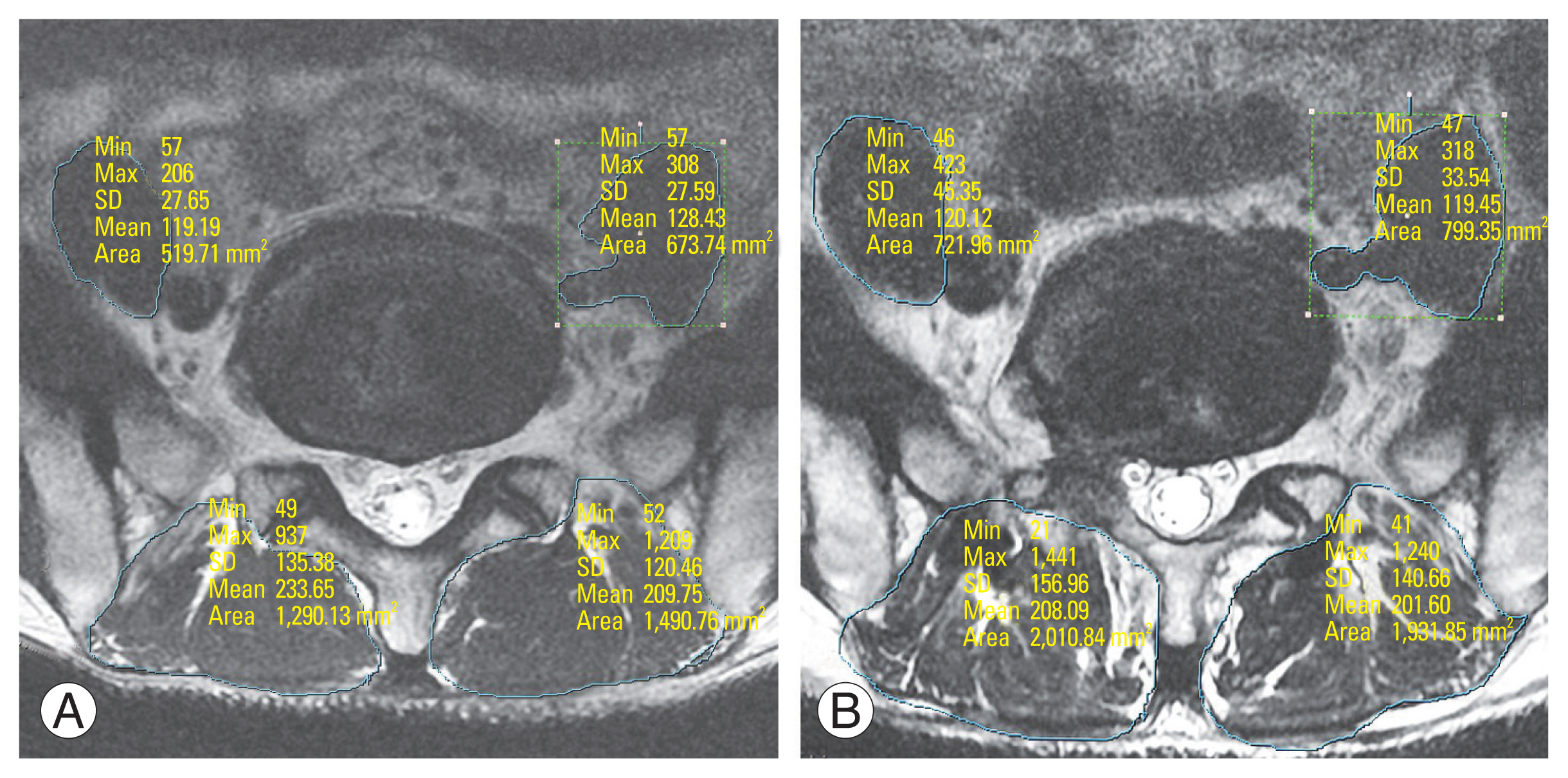
Fig.┬Ā2
Fluoroscopic images of uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion (EPTLIF) of left L4/5. (A) Intraoperative anteroposterior fluoroscopic image demonstrates the placement of endoscopic working retractor over the right L4/5 facet joint with the bevelled opening facing away from traversing and exiting nerve root. (B) Intraoperative lateral fluoroscopic image demonstrates a three-dimensional printed titanium cage inserted through endoscopic working retractor. (C, D) Final intraoperative fluoroscopic anteroposterior (C) and lateral (D) image of uniportal full EPTLIF of left L4/5.

Fig.┬Ā3
Case example of a 67-year-old female with grade one spondylolisthesis who underwent uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion of L4/5. (A, B) Preoperative anteroposterior and lateral view X-ray showing grade 1 spondylisthesis. (C, D) Preoperative sagittal and axial mid disc cutline magnetic resonance imaging (MRI) showing grade 1 spondylisthesis with severe spinal stenosis. (E, F) One-week postoperative anteroposterior and lateral view X-ray showing reduction of grade 1 spondylisthesis with three-dimensional printed titanium cage and screws. (G, H) One-week postoperative sagittal and axial mid disc cutline MRI showing decompression of spinal canal.
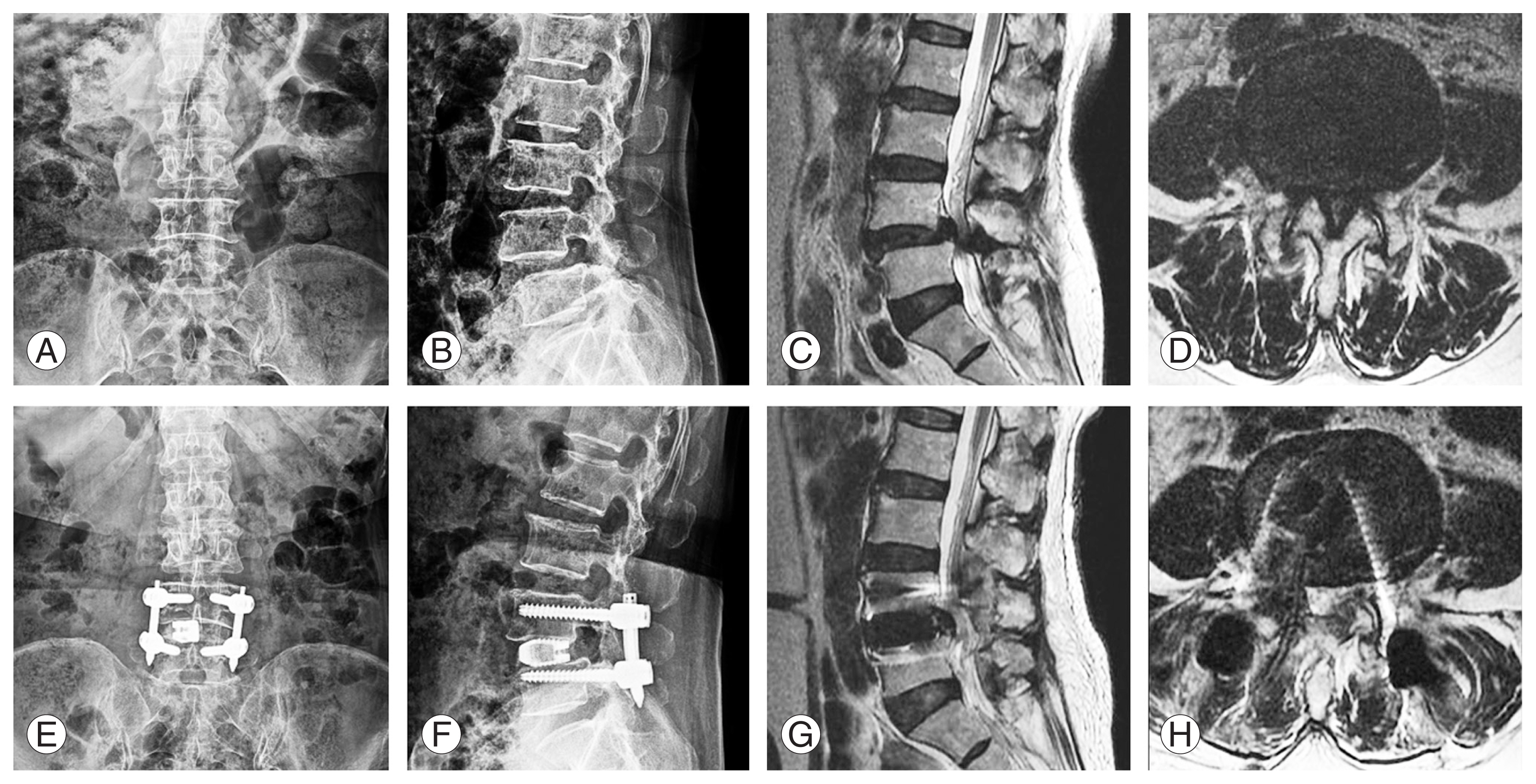
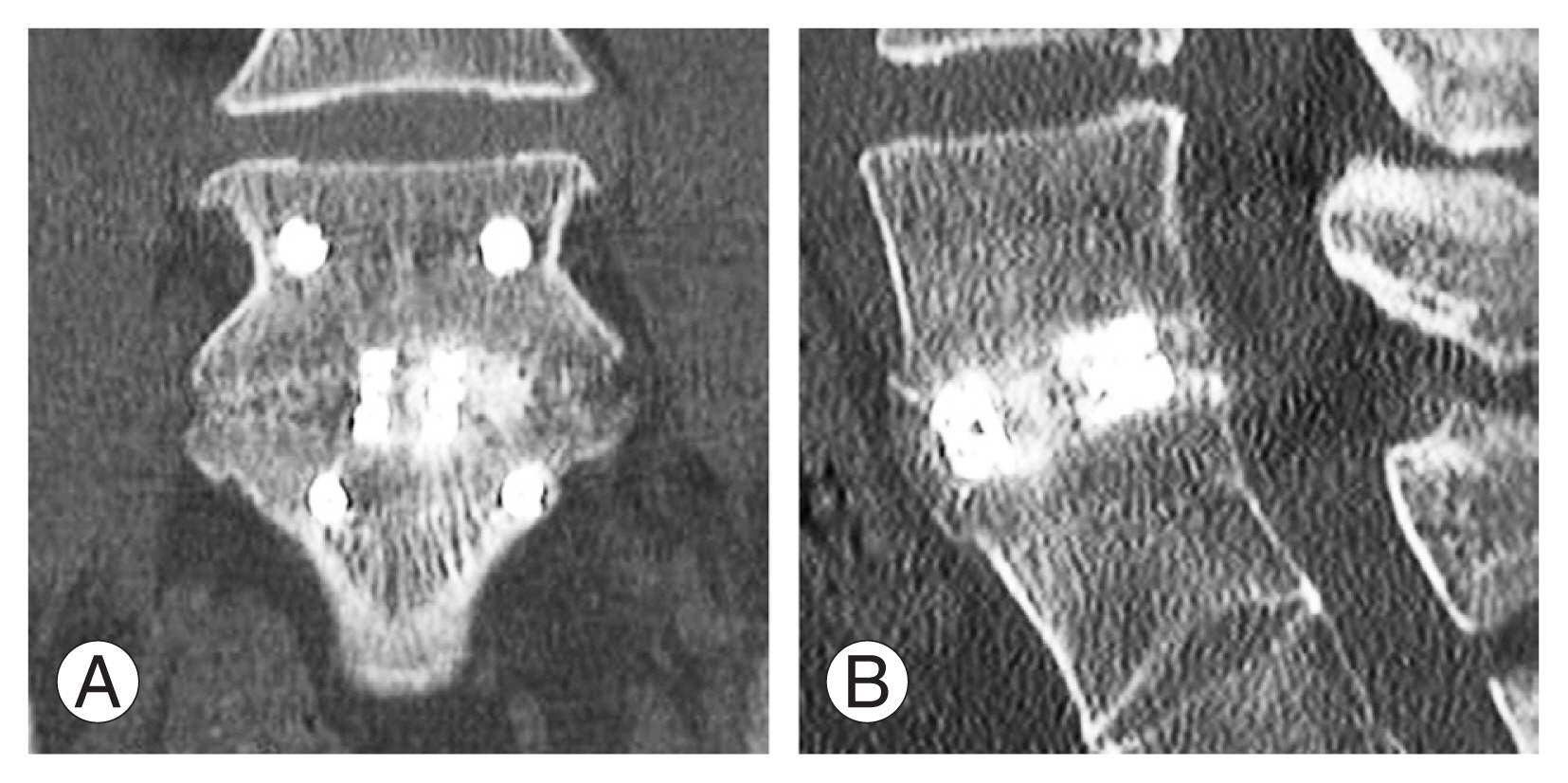
Fig.┬Ā4
Postoperative computed tomography scan 1 year of a 67-year-old female who underwent uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion of L4/5 showing grade 1 Bridwell fusion grade in both coronal (A) and sagittal cut (B).
Table┬Ā1
Baseline demographics data and clinical parameters of endoscopic posterolateral transforaminal lumbar interbody fusion
Table┬Ā2
Baseline radiographic data of endoscopic posterolateral transforaminal lumbar interbody fusion
Table┬Ā3
Baseline clinical data of endoscopic posterolateral transforaminal lumbar interbody fusion
Table┬Ā4
Clinical and radiographic parameters of uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion
| Variable | Mean┬▒SD | p-valuea) |
|---|---|---|
| VAS improvement at 1 week | 4.11┬▒1.23 | <0.001 |
| VAS improvement at 3 months | 4.94┬▒1.30 | <0.001 |
| VAS improvement at final follow-up | 5.46┬▒1.29 | <0.001 |
| ODI improvement at 1 week | 40.34┬▒10.06 | <0.001 |
| ODI improvement at 3 months | 46.69┬▒9.14 | <0.001 |
| ODI improvement at final follow-up | 49.63┬▒8.68 | <0.001 |
| Change in Kjaer grade after operation at 1year | 0.09┬▒0.28 | 0.043 |
| Ch_ange in MRI axial cut spinal canal area of right psoas muscle at mid disc level (mm2) | 70.03┬▒149.1 | 0.009 |
| Ch_ange in MRI axial cut spinal canal area of left psoas muscle at mid disc level (mm2) | 67.59┬▒113.2 | 0.001 |
| Ch_ange in MRI axial cut spinal canal area of right paraspinal muscle at mid disc level (mm2) | 40.03┬▒311.7 | 0.453 |
| Ch_ange in MRI axial cut spinal canal area of left paraspinal muscle at mid disc level (mm2) | 74.39┬▒286.0 | 0.133 |
References
1. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990ŌĆō2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163ŌĆō96.


2. Wu PH, Kim HS, Jang IT. Intervertebral disc diseases part 2: a review of the current diagnostic and treatment strategies for intervertebral disc disease. Int J Mol Sci 2020;21:2135.



3. Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis: four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 2009;91:1295ŌĆō304.


4. Hammad A, Wirries A, Ardeshiri A, Nikiforov O, Geiger F. Open versus minimally invasive TLIF: literature review and meta-analysis. J Orthop Surg Res 2019;14:229.




6. Wu PH, Kim HS, Jang IT. How I do it?: uniportal full endoscopic contralateral approach for lumbar foraminal stenosis with double crush syndrome. Acta Neurochir (Wien) 2020;162:305ŌĆō10.




7. Wu PH, Kim HS, Choi DJ, Gamaliel YH. Overview of tips in overcoming learning curve in uniportal and biportal endoscopic spine surgery. J Minim Invasive Spine Surg Tech 2021;6(Suppl 1): S84ŌĆō96.


8. Heo DH, Lee DC, Kim HS, Park CK, Chung H. Clinical results and complications of endoscopic lumbar interbody fusion for lumbar degenerative disease: a meta-analysis. World Neurosurg 2021;145:396ŌĆō404.


9. Heo DH, Son SK, Eum JH, Park CK. Fully endoscopic lumbar interbody fusion using a percutaneous unilateral biportal endoscopic technique: technical note and preliminary clinical results. Neurosurg Focus 2017;43:E8.



10. Ahn Y, Youn MS, Heo DH. Endoscopic transforaminal lumbar interbody fusion: a comprehensive review. Expert Rev Med Devices 2019;16:373ŌĆō80.


11. Kim HS, Wu PH, Jang IT. Technical note on uniportal full endoscopic posterolateral approach transforaminal lumbar interbody fusion with reduction for grade 2 spondylolisthesis. Interdiscip Neurosurg 2020;21:100712.

12. Kim HS, Wu PH, Lee YJ, Kim DH, Jang IT. Technical considerations of uniportal endoscopic posterolateral lumbar interbody fusion: a review of its early clinical results in application in adult degenerative scoliosis. World Neurosurg 2021;145:682ŌĆō92.


13. Kim HS, Wu PH, An JW, et al. Evaluation of two methods (inside-out/outside-in) inferior articular process resection for uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion: technical note. Brain Sci 2021;11:1169.



14. Wu PH, Kim HS, Lee YJ, et al. Uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion with endoscopic disc drilling preparation technique for symptomatic foraminal stenosis secondary to severe collapsed disc space: a clinical and computer tomographic study with technical note. Brain Sci 2020;10:373.



15. Shin JJ, Kim B, Kang J, et al. Clinical, radiographic, and genetic analyses in a population-based cohort of adult spinal deformity in the older population. Neurospine 2021;18:608ŌĆō17.




16. Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf-Yde C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med 2007;5:2.




17. Charalampidis A, Canizares M, Kalsi PS, et al. Differentiation of pain-related functional limitations in surgical patients with lumbar spinal stenosis (LSS) using the Oswestry Disability Index: a Canadian Spine Outcomes and Research Network (CSORN) study. Spine J 2022;22:578ŌĆō86.


18. Kim HS, Raorane HD, Wu PH, et al. Feasibility of endoscopic transforaminal lumbar interbody fusion (eTLIF) through the posterior paraspinal approach: technical note and preliminary result. J Minim Invasive Spine Surg Tech 2021;6:35ŌĆō41.


19. Wu PH, Kim HS, Jang IT. Uniportal endoscopic lateral to medial direction transforaminal lumbar interbody fusion: a case report and technical guide for navigating through landmarks in left lumbar 4/5 post laminotomy revision lumbar fusion surgery. J Minim Invasive Spine Surg Tech 2021;6:66ŌĆō73.


20. Kim HS, Raorane HD, Wu PH, Heo DH, Sharma SB, Jang IT. Incidental durotomy during endoscopic stenosis lumbar decompression: incidence, classification, and proposed management strategies. World Neurosurg 2020;139:e13ŌĆō22.


21. Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res 2014;472:1727ŌĆō37.



22. Morgenstern C, Yue JJ, Morgenstern R. Full percutaneous transforaminal lumbar interbody fusion using the facet-sparing, trans-Kambin approach. Clin Spine Surg 2020;33:40ŌĆō5.


23. Lee HJ, Lim WH, Park JW, et al. The relationship between cross sectional area and strength of back muscles in patients with chronic low back pain. Ann Rehabil Med 2012;36:173ŌĆō81.



24. Ranger TA, Cicuttini FM, Jensen TS, Heritier S, Urquhart DM. Paraspinal muscle cross-sectional area predicts low back disability but not pain intensity. Spine J 2019;19:862ŌĆō8.


25. Dangaria TR, Naesh O. Changes in cross-sectional area of psoas major muscle in unilateral sciatica caused by disc herniation. Spine (Phila Pa 1976) 1998;23:928ŌĆō31.


26. Cho SM, Kim SH, Ha SK, et al. Paraspinal muscle changes after single-level posterior lumbar fusion: volumetric analyses and literature review. BMC Musculoskelet Disord 2020;21:73.




27. Ortega-Porcayo LA, Leal-Lopez A, Soriano-Lopez ME, et al. Assessment of paraspinal muscle atrophy percentage after minimally invasive transforaminal lumbar interbody fusion and unilateral instrumentation using a novel contralateral intact muscle-controlled model. Asian Spine J 2018;12:256ŌĆō62.




28. Bresnahan LE, Smith JS, Ogden AT, et al. Assessment of paraspinal muscle cross-sectional area after lumbar decompression: minimally invasive versus open approaches. Clin Spine Surg 2017;30:E162ŌĆō8.

- TOOLS







