Effect of Drain Duration and Output on Perioperative Outcomes and Readmissions after Lumbar Spine Surgery
Article information
Abstract
Study Design
Single-center retrospective cohort.
Purpose
To compare surgical outcomes of patients based on lumbar drain variables relating to output and duration.
Overview of Literature
The use of drains following lumbar spine surgery, specifically with respect to hospital readmission, postoperative hematoma, postoperative anemia, and surgical site infections, has been controversial.
Methods
Patients aged ≥18 years who underwent lumbar fusion with a postoperative drain between 2017 and 2020 were included and grouped based on hospital readmission status, last 8-hour drain output (<40 mL cutoff), or drain duration (2 days cutoff). Total output of all drains, total output of the primary drain, drain duration in days, drain output per day, last 8-hour output, penultimate 8-hour output, and last 8-hour delta (last 8-hour output subtracted by penultimate 8-hour output) were collected. Continuous and categorical data were compared between groups. Multivariate logistic regression analysis and receiver operating characteristic (ROC) analysis were performed to determine whether drain variables can predict hospital readmission, postoperative blood transfusions, and postoperative anemia. Alpha was 0.05.
Results
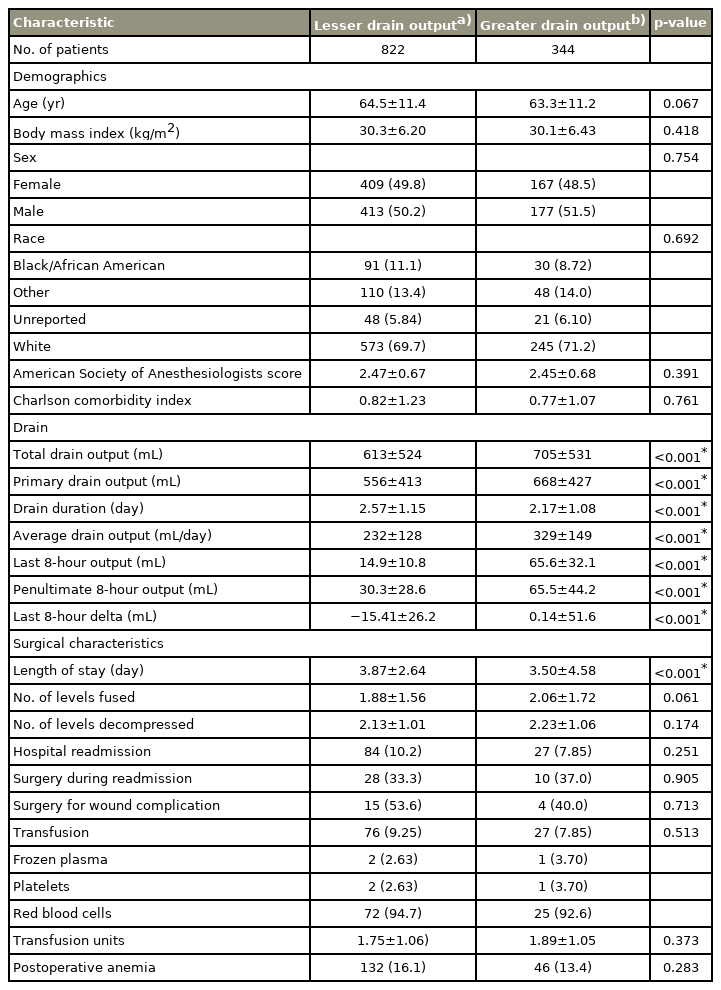
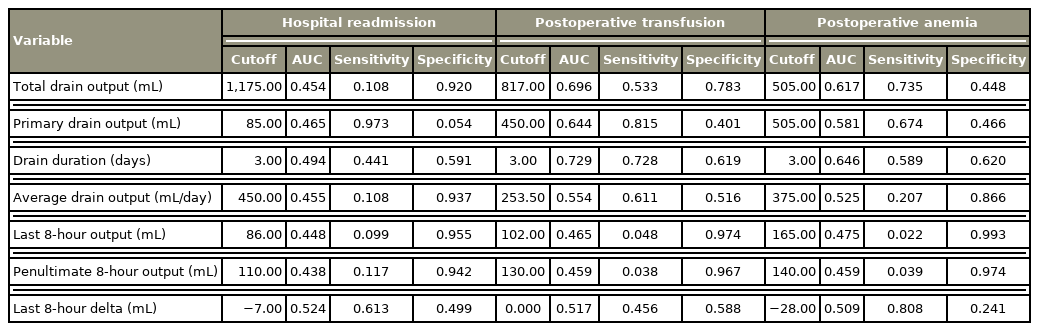
Our cohort consisted of 1,166 patients with 111 (9.5%) hospital readmissions. Results of regression analysis did not identify any of the drain variables as independent predictors of hospital readmission, postoperative blood transfusion, or postoperative anemia. ROC analysis demonstrated the drain variables to be poor predictors of hospital readmission, with the highest area under curve of 0.524 (drain duration), corresponding to a sensitivity of 61.3% and specificity of 49.9%.
Conclusions
Drain output or duration did not affect readmission rates following lumbar spine surgery.
Introduction
Postoperative drains have been used by surgeons to minimize complications of lumbar spine surgery in over 10% of patients [1]. However, the use of postoperative drains continues to engender significant debate over their efficacy and impact on patient outcomes. The primary theoretical benefit of drain utilization is minimization of hematoma formation, which may reduce postoperative pain, neurologic compression, and reduce the risk of surgical site infections (SSI) [2,3]. While drains can reduce the saturation rate of wound dressing and the incidence and size of postoperative hematomas, the risk of anemia and allogeneic blood transfusions increase [4–8]. Additionally, clinical outcomes, hematoma formation, or SSI are not significantly different between patients with and without drains [9,10].
Drains continue to be used in spine surgery despite the greater risk of blood loss and the subsequent elevated transfusion rates, as well as retained drain fragments requiring reoperation and cerebrospinal fluid (CSF) leaks [11,12]. The relationship between drain utilization and increased transfusion requirements may increase the risk for SSI due to immunosuppressive response to the allogeneic blood [4,8,13]. Currently, there is conflicting evidence regarding the increased risk of SSI due to drain placement, although most evidence points to no significant differences based on drain use [7,14–16].
At present, a consensus on protocols for the management of postoperative lumbar spine drains is lacking, specifically for determining when they should be used and when they should be discontinued [2]. Therefore, this study was aimed to determine whether specific drain usage variables can predict hospital readmission, postoperative blood transfusion, or postoperative anemia.
Materials and Methods
1. Study design and data acquisition
This study was approved by the Institutional Review Board at Thomas Jefferson University (Control #19D.508), and the requirement for patient informed consent was waived due to the minimal risk to subjects and the study’s retrospective nature. A structured query language (SQL) search of current procedural terminology codes 22612, 63047, 63030, and 63042 were used to identify patients who underwent lumbar fusion or decompression and fusion between 2017 and 2020 at a single academic institution. Inclusion criteria were age of 18 years and placement of a postoperative lumbar suction drain. The exclusion criterion was incomplete drain documentation. A total of 1,166 patients were finally included in the study cohort. The electronic medical record (EMR) was retrospectively reviewed for drain data including the number of drains, date and time of drain removal, total drains and primary drain outputs, output volume over last 8-hour shift, and output volume over the penultimate 8-hour shift. In cases with multiple drains, the output volumes of each drain over the last or penultimate 8-hour shift were totaled if the drains were removed at the same time. In the case of multiple drains, the primary drain was considered the drain with the longest postoperative duration. If the patient only had one postoperative drain, then the primary drain output and total drain output were identical. If removal was sequential, the last or penultimate 8-hour output was recorded. SQL retrieved age, sex, body mass index, and American Society of Anesthesiologists score, length of stay, hospital readmission, and Charlson comorbidity index (CCI). The EMR was reviewed for surgical data including the number of levels fused or decompressed, if a patient required surgery during hospital readmission and if it was due to a wound complication, if a patient required postoperative blood transfusion, number and type of units transfused, and if a patient had a postoperative hemoglobin level below 8 g/dL (representing “postoperative anemia”).
2. Statistical analysis
Drain variables included total output of all drains, total output of the primary drain, drain duration in days, drain output per day, last 8-hour output, penultimate 8-hour output, and last 8-hour delta (last 8-hour output subtracted by penultimate 8-hour output). Descriptive statistical analysis was conducted to determine the mean and standard deviation for patient demographic, surgical, and readmission data. Patients were grouped based on readmission status, and last 8-hour drain output total drain duration. Those with final 8-hour drain output ≥40 mL were included in the greater drain output (GDO) group, while those with <40 mL were included in the lower drain output (LDO) group; grouping based on drain duration used a cutoff timepoint of 2 days. Independent t-tests were utilized for continuous data to compare differences between two groups. For non-parametric data, Mann-Whitney U tests were used. Pearson’s chi-square test was used for any categorical data. Logistic regression was performed to identify factors associated with hospital readmission, postoperative blood transfusion, and postoperative anemia using each drain variable separately in order to limit the effect of collinearity. Receiver operating characteristic (ROC) curves were generated using drain variables in an attempt to identify variables predictive of hospital readmission, postoperative blood transfusion, or postoperative anemia based on area under curve (AUC), and cutoffs that maximized sensitivity and specificity were reported with their corresponding values. Data were imported to R Studio ver. 4.0.2 (RStudio, Boston, MA, USA) for statistical analysis. All p-values <0.05 were considered statistically significant.
Results
Of the 1,166 total patients included in the cohort, there were 111 (9.5%) hospital readmissions, of which 44 were classified as surgical complications and 38 required reoperation. Of those with a reoperation, five were for symptomatic hematomas and 16 were for SSI. The remaining reoperations were unrelated to drain placement and included persistent CSF leaks that occurred during the surgical procedure and recurrent stenosis. Of the remaining hospital readmissions not deemed surgical complications, none required blood transfusions or required supportive measures due to perceived hypotension potentially caused by the surgery or drain placement. Patients who were readmitted had a higher CCI (1.16 versus 0.77, p<0.001) as well as decreased penultimate 8-hour drain output (39.4 mL versus 40.8 mL, p=0.030). There were no other significant differences among groups (Table 1).

Patient characteristics, drain data, and surgical characteristics based on hospital readmission status
When stratifying by the final 8-hour drain output, 344 patients (29.5%) were placed into the GDO group, while the remaining 822 (70.5%) were in the LDO group. By definition, the final 8-hour drain output was larger in the GDO group (65.6 mL versus 14.9 mL, p<0.001) than the LDO group. However, demographic characteristics, hospital readmissions (GDO [7.85%] versus LDO [10.2%], p=0.251), postoperative blood transfusions (GDO 7.85% versus LDO 9.25%, p=0.513), or postoperative anemia (GDO 13.4% versus LDO 16.1%, p=0.283) were not significantly different (Table 2).
When recategorizing patients by drain duration, 480 patients (41.2%) had a drain duration longer than 2 days, while 686 (58.8%) had a drain duration of 2 days or less. The >2-day drain duration group was significantly older (65.1 years versus 63.4 years, p=0.029) and had a higher proportion of women (54.2% versus 46.1%, p=0.008), significantly longer length of hospital stay (4.86 versus 2.99, p<0.001), more levels fused (2.31 versus 1.57, p<0.001) and decompressed (2.33 versus 2.05, p<0.001), and higher rate of postoperative blood transfusions (15.6% versus 4.08%, p<0.001) and postoperative anemia (21.9% versus 10.6%, p<0.001). Meanwhile, readmission rate was not significantly different between groups (>2 days [10.2%] versus ≤2 days [9.04%], p=0.569) (Table 3).
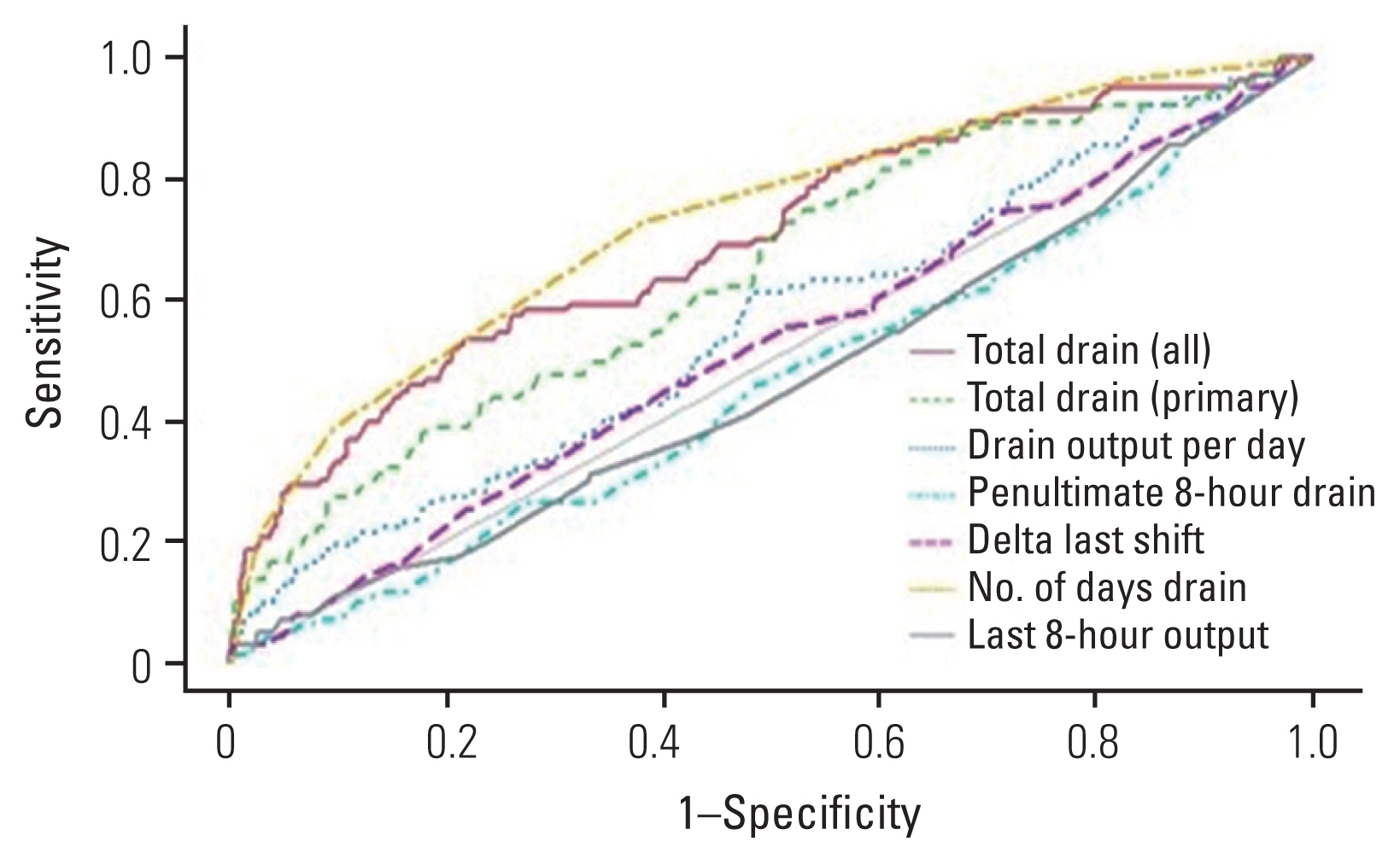
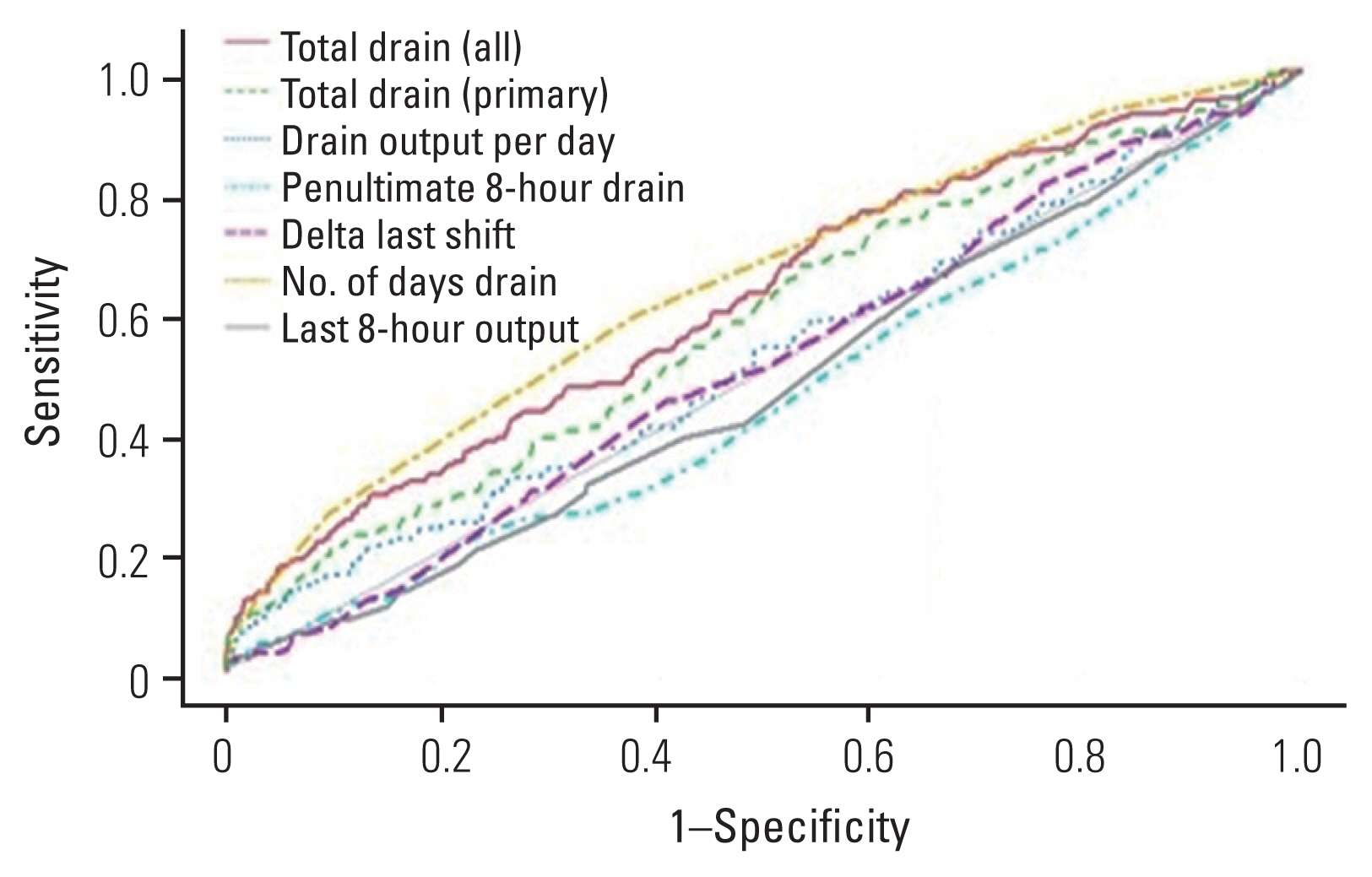
Results of multivariate logistic regression analysis revealed that none of the drain variables were independent predictors of hospital readmission, postoperative blood transfusion, or postoperative anemia (Table 4). An ROC curve was generated for each drain variable with the goal of predicting hospital readmission. The last 8-hour drain output had the highest AUC with a value of 0.524 (sensitivity, 61.3%; specificity, 49.9%) (Fig. 1). ROC curve analysis aimed at identifying risk factors for postoperative blood transfusions found drain duration to have the highest AUC, with a value of 0.729 (sensitivity, 72.8%; specificity, 61.9%) (Fig. 2). Similarly, ROC curve analysis targeted at identifying the greatest predictor for postoperative anemia found drain duration to have the highest AUC, with a value of 0.646 (sensitivity, 58.9%; specificity, 62.0%) (Fig. 3, Table 5).

Logistic regressions for hospital readmission, postoperative blood transfusion, and postoperative anemia
Discussion
Drains have historically been used after spine surgery to reduce postoperative epidural hematomas that can potentially compress the spinal cord or nerve roots. These complications are associated with significant patient morbidity and can lead to increased health care costs and resource utilization [17]. However, the utility of postoperative drains in preventing wound complications and readmissions in spinal surgery has been controversial [14,18]. Our data suggest there may not be a benefit to drain usage in decreasing readmission rates, and the majority of drain variables were more accurate in predicting postoperative blood transfusions or anemia rather than hospital readmission.
In 2005, a retrospective database study of 14,932 patients undergoing spinal surgery found that drains were not associated with a decreased risk of postoperative spinal epidural hematoma [19]. Instead, multiple operative levels, anemia, and excessive blood loss were predictive of epidural hematoma formation, the results of which have been corroborated by a National Surgical Quality Improvement Program study of 75,878 lumbar decompression procedures [20]. A multicenter study has also questioned the degree to which drains reduce development of epidural hematomas, citing that symptomatic hematomas still occurred in the majority of patients managed with postoperative drains [21]. Accordingly, patient selection and perioperative factors such as medical management of bleeding and surgical hemostasis may play a more significant role than drains in reducing SSI and hematoma formation. While a prospective randomized study demonstrated that both the incidence and size of postoperative hematomas decreased after a drain was inserted following lumbar disc surgery and that late clinical outcomes were not significantly different between patients with and without a drain, the incidence of symptomatic hematomas or SSI was not determined [6]. Therefore, postoperative drains do function to reduce the size of postoperative hematomas, but their use does not appear to have clinical relevance in reducing postoperative complications from the hematoma formation.
Despite the aforementioned evidence, many surgeons continue to postoperatively use drains. Therefore, evidence-based guidelines should be established to determine how long drains need to remain in place when used postoperatively. A retrospective case-control analysis of SSIs following posterior spine fusion found that the risk for SSI increased for every 1.6 days that a drain was present [15]. Another retrospective case-control study of both laminectomy and fusion procedures identified drain duration of greater than 3 days as a risk factor for SSI [22]. Our study found that using a 2-day threshold for drain removal was not associated with an increase readmission rate after surgery. Additionally, multivariate regression and ROC analysis utilizing drain duration as a continuous variable demonstrated that removing a drain after 2 postoperative days was not an effective predictor of hospital readmission (AUC: 0.494), but it was a strong predictor of requiring postoperative blood transfusion (AUC: 0.729). This has major implications for clinical practice, especially for the patient who are medically cleared for discharge but remain in the hospital due to the presence of a drain. These findings also suggest that drains in lumbar spine surgery can be removed within the first 2 days regardless of drain output without increasing complication rates including readmission. Further, our results demonstrate that patients in the GDO group had drains in place for a significantly shorter period of time and had a shorter hospital stay, while patients with drains maintained for greater than 2 days had significantly longer lengths of stay. A retrospective cohort study of multilevel anterior cervical discectomy and fusion in 321 patients similarly observed an almost two-fold longer length of stay for patients with postoperative drain placement. Moreover, the incidence of postoperative hematomas or SSI were not significantly different in patients with and without drains [23].
While many studies have compared the absence or presence of a drain, none have proposed recommended thresholds for removal based on drain output volumes. A retrospective case-control study found that postoperative drain output was a risk factor for symptomatic epidural hematomas (SEH) after lumbar decompression. However, the mean drain output for patients that developed SEH was actually less than patients that did not develop SEH [24]. While the exact reason for this finding is unclear, it could be possible that those who developed SEH may have had technical issues with their drains. Regardless, it complicates the interpretation of drain outputs in the postoperative spine patient. Drains are susceptible to many technical issues once placed, including loss of suction, clotting of the drain, or unintended premature removal. An additional retrospective case-control study found that the most important tool for early diagnosis of postoperative epidural hematomas was the clinical evaluation, not drain output [19]. In our study, we collected data on the drain output over the last 8-hour shift prior to drain removal and the penultimate 8-hour period and found that the GDO group did not have a higher incidence of readmissions, readmissions due to wound complications, or surgery if they were readmitted. Furthermore, multivariate regression and ROC analysis demonstrated that drain output over the last 8-hour shift was not an effective predictor of readmission. The findings of the present study are consistent with those of other studies that have demonstrated that drains do not significantly influence rates of symptomatic hematoma formation, neurologic deficit, or SSI, in that the maximum drain output or minimum drain duration are not necessary clinical parameters to determine when to pull drains [25,26]. If surgeons are inclined to place a postoperative drain, the drain may be pulled on the first postoperative day without concern for increasing postoperative complications. Further, early drain removal may minimize blood loss, while reducing the hospital length of stay.
The study had some limitations, including those inherent to retrospective study design. Wound drainage that did not require a revision surgery and the preoperative use of anticoagulants were not evaluated. While general comorbidity indexes were compared, specific factors such as tobacco use, diabetes, malignancy, history of spine trauma, and prior spine surgery have been identified as potential risk factors for increased postoperative bleeding and wound complications and these were not specifically evaluated in our analysis [15,19,20,27,28]. Further, on ROC analysis, surgical characteristics, such as the number of levels fused or decompressed, were not adjusted, which might have introduced bias to our findings. Therefore, while this study may be generalizable to short construct lumbar fusion, our data should be cautiously interpreted for longer construct lumbar fusions or thoracolumbar fusions, especially for adult deformity procedures.
Conclusions
Drain output and drain duration are not accurate predictors for postoperative complications or hospital readmission due to hematoma formation. Surgeons should consider prompt removal of drains, especially in short construct lumbar fusions, to minimize blood loss and hospital length of stay.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: BK, GT, ML; data curation: PK, GT, JM, RN, SA, FS, NK, NS; formal analysis: GT; funding acquisition: none; methodology: BK, GT, ML; project administration: none; visualization: BK; writing–original draft: BK, PK, GT, ML; writing–review and editing: JC, AH, CK, AV, GS; and final approval of the manuscript: all authors.