An Updated Review on the Treatment Strategy for Spinal Metastasis from the Spine Surgeon’s Perspective
Article information
Abstract
Spinal metastasis is a common issue causing significant pain and disability in cancer patients. A multidisciplinary approach consisting of chemotherapy, radiotherapy, and surgical treatment is used for treating patients with metastatic spinal tumors. Due to recent advancements in medical and radiation oncology, like tumor genetics and stereotactic radiotherapy, this treatment strategy would change inevitably. Therefore, the decision-making systems developed for assisting physicians and surgeons to choose the most appropriate treatment for each patient with spinal metastasis need to evolve. In this review, the recent developments, validations, and modifications of these systems, as well as suggestions for future systems have been discussed. Recently, separation surgery combined with stereotactic radiotherapy (hybrid therapy) has gained popularity. Additionally, the evidence for hybrid therapy presented in the literature has been reviewed.
Introduction
Spinal metastasis is a common problem resulting in adverse effects on the clinical course of patients with spinal metastasis [1]. The incidence of spinal metastasis has been increasing due to the growing number of cancer patients and an increase in their survival rates [2]. Spinal metastasis affects up to 70% of cancer patients, whereas 10%–20% of cases are symptomatic, causing pain, neurological deficits, and quality-of-life deterioration [3].
Spinal metastasis surgery is predominantly palliative and aims at preserving or improving the quality of life by achieving pain controlling and preserving ambulatory function [4]. A multidisciplinary approach consisting of chemotherapy, radiotherapy, and surgical treatment is used for treating metastatic spinal cancers because of variable treatment responses among cancer patients [5]. As a result, several authors established multiple decisionmaking systems for treating metastatic spinal tumors. This could assist physicians and surgeons in determining the most appropriate treatment option for each patient [6]. However, recent advancements in oncology and methodology have demanded a further evolution of these decision-making systems. In the current review, the developments, validations, and modifications in these systems presented in recent studies have been discussed [7].
Growing evidence is available for separation surgery combined with stereotactic radiosurgery (SRS) for treating spinal metastasis. In this study, several techniques and evidence for the efficacy of hybrid therapy for metastatic spinal tumors have been reviewed.
Present and Future of Decision-Making Systems
1. Classification-based prognostic models
Many authors have devised several “classification-based” prognostic models, like the Tomita, Tokuhashi, Bauer, and Katagiri scoring systems, to estimate the survival of patients with metastatic spinal tumors [8-13]. In these scoring systems, a patient’s life expectancy is predicted by the total prognostic score calculated by adding the scores assigned to each prognostic factor. Each decision-making or scoring system has different prognostic factors. However, the factors commonly included in most systems are the histological subtype of primary cancer and the presence of visceral metastasis (Table 1). Spine surgeons and oncologists can use these scoring systems to select patients with a sufficient life expectancy and decide if surgical treatment is needed.
However, in recent studies, these survival prediction systems have shown poor accuracy [14-17]. In a nationwide study done in France, the survival prediction accuracy was 42.8% and 25.6% for the Tokuhashi and Tomita scoring systems, respectively [18]. The inability to reflect prolonged survival due to novel developments in cancer treatments, like molecular targeted therapies, immunotherapy, and hormonal therapies, leads to the occurrence of inaccuracies in these systems.
More recently, the New England Spinal Metastasis Score (NESMS), consisting of a modified Bauer score, serum albumin level, and ambulatory status, was introduced in 2015 [19] (Table 2). The authors developed the scoring system using multi-institutional data and validated the system retrospectively [20,21] and prospectively [22,23] as an accurate and reliable prediction tool for spinal metastasis. In a recently published prospective study, the authors reported that the NESMS differentiated patient survival notably better than the traditional scoring systems (Tokuhashi, Tomita, and Spinal Instability Neoplastic Score [SINS]) [24].
Many authors have also utilized novel methodologies, including machine-learning algorithms, to develop decision-making systems. The Skeletal Oncology Research Group (SORG) conducted a study comparing the estimated survival of 649 patients predicted by classic, nomogram, and boosting algorithms [25]. In their study, the authors suggested that the nomogram was intuitive and accurate. More recently, the SORG created a novel prognostic prediction model for spinal metastasis using a machine-learning algorithm [26], which is referred to as one of the “second-generation models” [27]. Subsequent studies have externally validated these models [28-30].
2. Principle-based systems
Principle-based decision-making systems could provide more specific treatment suggestions for each patient with spinal metastasis based on their oncologic, systemic, and functional status in contrast to prognostic models, which only predict patients’ life expectancy. Additionally, these systems could reflect advancements in systemic, radiation, and surgical treatments, like molecular target therapy, SRS, and separation surgery, better than classificationbased prognostic models (Table 3).
The NOMS framework was first introduced in 2006 as a principle-based decision-making system [31]. In this framework, the neurologic (N) component was assessed using the Bilsky grade [32]. The oncologic (O) component is determined based on the expected response to available treatments, primarily radiotherapy, whereas the mechanical (M) component is determined by assessing the spinal column stability using the SINS. This could guide surgeons in deciding whether to perform surgical stabilization regardless of other components [33]. Finally, the systemic (S) component determines whether the patient can tolerate the suggested treatment. The most appropriate treatment for each spinal metastasis patient is suggested based on these four components, including novel treatment modalities, like SRS and separation surgery [31].
Paton et al. [34] introduced the “LMNOP” system as a modification of the NOMS framework. Two additional components were added: the number and location of metastatic spinal lesions (L) and the response to previous treatment (P). The response to previous systemic therapy or radiotherapy was considered to be an important factor in selecting an appropriate treatment option for each patient. For example, a newly diagnosed cancer patient with symptomatic spinal metastasis (synchronous metastasis), having several residual cancer treatment options, is expected to have longer survival compared to those with spinal metastasis diagnosed during the course of cancer treatment (metachronous metastasis) [35].
3. Current trends and future directions
The prognostic models or decision-making systems for metastatic spinal tumors need to evolve due to recent advancements in cancer genetics and the introduction of novel treatment techniques. The suggestions for future spinal metastasis decision-making systems based on the trends evident in the recent literature are as follows: (1) development and validation using a multi-institutional or multinational database; (2) consideration of tumor genetics; (3) utilization of novel methodologies, like artificial intelligence; and (4) integration of prognostic models and principle-based decision-making systems.
The sample size is crucial for the performance of predictive algorithms and decision-making systems. Studies with a large sample size from a multi-institutional or multinational cohort should be conducted for developing an accurate and reliable prognostic model. Therefore, recently introduced predictive algorithms have been developed and validated using multi-institutional databases [19,25,36]. Additionally, a large database from multicenter and multinational tumor registries should be a prerequisite in developing future prognostic models for spinal metastasis.
The evolution of biological treatments, like targeted molecular therapy and immunotherapy, has brought a paradigm shift in cancer treatment. Determining the genetic subtypes of primary malignancy provides a guide for these biological treatments. Hence, its importance has been increasing [37]. Examples of target mutations include epidermal growth factor receptor (EGFR) in nonsmall cell lung cancer, v-raf murine sarcoma viral oncogene homolog B1 in melanoma, and hormonal receptors in breast and prostate cancers. Recently, Kim et al. [38] discovered that the addition of EGFR mutation positivity to the NESMS system improves its discrimination ability. Their results emphasize the importance of genetic profiles while establishing treatment strategies for spinal metastasis.
Novel computational methodologies are being utilized for developing prognostic models for metastatic spinal tumors. Traditionally, scoring systems have been created based on regression analysis (logistic or proportional hazard). However, the SORG used multiple machinelearning algorithms, like gradient boosting, decision trees, random forests, and neural networks, for developing their prognostic models [25,39]. More recently, Karhade et al. [36] developed and introduced predictive algorithms for 6-week mortality in patients with spinal metastasis using five different machine-learning algorithms. These algorithms could assist surgeons in identifying patients for whom surgery could do more harm than good [36]. These evolving computational methodologies would be extensively utilized in predicting the prognosis of spinal metastatic tumors.
Finally, classification- and principle-based systems should be integrated into future decision-making systems. Classification-based systems or prognostic models, estimate the patient’s remaining survival, whereas principlebased systems estimate the most appropriate treatment option based on these survival estimations. Generally, two separate systems are used by oncologists and spine surgeons while choosing an appropriate decision-making process for patients with spinal metastasis. Therefore, novel decision-making systems should combine these two systems and estimate the remaining survival and the most appropriate management option, simultaneously.
Radiotherapy for Spinal Metastasis
1. Stereotactic radiosurgery bringing a paradigm shift
Stereotactic radiotherapy is effective in local tumor control with relatively low complication rates in patients with spinal metastasis [7]. In the treatment of spinal metastasis, SRS has become a game-changer because of recent technical improvements, like radiation delivery systems (imageguided, intensity-modulated) and software [40]. Previous studies have revealed that SRS locally controls spinal metastatic tumors for an extended period, which is not influenced by the tumor histology or radiosensitivity of primary cancer [41]. The effectiveness of SRS, independent of tumor histology, has expanded the role of radiotherapy in managing patients with spinal metastases.
The clinical practice of surgeons treating patients with spinal metastases has been reshaped by SRS by reducing the invasiveness of surgical treatment. For example, in single metastatic lesions not accompanied by spinal cord compression, SRS could be applied as a definitive treatment [42]. Curative surgical procedures with high complication rates, like total en-bloc spondylectomy (TES), have been replaced by SRS, which demonstrated a local control rate of 84%–88% in single metastatic lesions [43,44]. In patients with high-grade metastatic epidural spinal cord compression (MESCC), the effectiveness of adjuvant SRS reduces the need for aggressive debulking surgery and makes separation surgery, in which tumor resection is limited to decompressing the spinal cord.
2. Vertebral compression fracture following stereotactic radiosurgery
An increased risk of vertebral compression fractures (VCF) after radiotherapy is a pitfall of SRS. VCF is reported after SRS in up to 36% of patients, compared to 5% after conventional radiotherapy [45]. VCF is dosedependent and occurs more frequently when over 20 Gy per fraction of radiation dose is given to patients having risk factors [46]. The risk factors for VCF after SRS are as follows: (1) older age, (2) lytic lesions, and (3) spinal malalignment. In patients with these risk factors, a radiation dose of less than 16–18 Gy per fraction is recommended [47]. Additionally, preventive stabilization surgery could be considered before performing SRS. SINS could be applied to assess the mechanical instability of the metastatic lesion and predict further vertebral collapse after SRS. This could assist in deciding whether to perform surgical stabilization before SRS or not [33,48].
3. The interval between surgery and radiotherapy
In the literature, controversy exists on the adequate timing of adjuvant radiotherapy following surgical treatment. This could be associated with wound complications. Based on recent systemic reviews and expert opinions, it is recommended to keep an interval of 2 weeks (minimum of 7 days) between surgery and radiotherapy [49,50]. A few authors believe that SRS could shorten the interval compared to conventional radiotherapy. In a previous study, Versteeg et al. [51] reported that no increase in wound complications was seen at 90 days after surgery, despite administering SRS within 24 hours of surgery. The findings of previous studies suggest that SRS was associated with fewer wound complications than conventional radiotherapy when administered at a similar time interval between surgeries [52-54]. However, due to the lack of highlevel evidence, the extent to which the interval between surgery and adjuvant SRS could be reduced remains debatable. It should also be noted that minimally invasive surgical techniques, discussed later in this review, could reduce the interval between surgery and radiotherapy.
Surgery for Spinal Metastasis
1. Surgical indications
The purpose of surgical treatment for patients with metastatic spinal tumors is pain relief and improving neurological deficits through nerve decompression and stabilization. Surgical indications for spinal metastasis generally include uncontrolled pain and neurological deficits, like motor weakness in the extremities [55]. As suggested by the decision-making systems, radioresistant tumors with high-grade epidural spinal cord compression are a typical indication for surgical treatment [31,34].
Mechanical instability is considered an independent indication for stabilization, as suggested in the NOMS and LMNOP systems [31,34]. SINS is an independent tool developed to guide surgeons in determining the need for surgical stabilization in each patient based on clinical and radiological findings [56] (Table 4). A score of ≥13 is evidence of spinal instability warranting surgical stabilization. For patients with impending instability (intermediate SINS score of 7–12), the decision of performing surgical stabilization is often difficult. Lenschow et al. [57] reviewed 331 spinal metastasis patients with intermediate SINS and found no significant differences in neurological outcomes between instrumented and noninstrumented patients. However, an increase in surgical complications was noted in the instrumented group [57]. Kim et al. [58] suggested that surgical stabilization should be performed in patients having >50% vertebral body collapse with intermediate SINS.
Estimated life expectancy is considered the most important deciding factor in performing surgery for metastatic spinal tumors. Based on the findings of previous studies, more than 3 months of estimated survival is a prerequisite for proceeding with surgical treatment [59,60]. Several prognostic models have been utilized for predicting the survival period of patients with spinal metastases. Additionally, the performance status of the patient should be good enough to have sufficient tolerance for the surgical treatment. Surgical treatment for spinal metastasis can be performed on patients with any previously mentioned surgical indications (e.g., mechanical instability or MESCC) on meeting these conditions.
2. Separation surgery and stereotactic radiosurgery (hybrid therapy)
As mentioned previously, the improved local control ability of SRS, regardless of tumor histology, further reduces the need for invasive surgical procedures in patients with spinal metastases [61] (Fig. 1). The role of decompressive surgery in MESCC can be refined to secure the spinal cord in the era of SRS [62]. “Separation surgery” is defined as a surgical procedure in which the tumor resection is limited to decompressing the spinal cord and creating a gap between the spinal cord and the tumor to provide a safe target for SRS [63]. Circumferential decompression (360°) was performed to ensure a complete re-expansion of the dural sac with at least a 2–3 mm gap between the tumor and spinal cord. Among several surgical approaches, the posterolateral transpedicular approach described by Bilsky et al. [64] is a safe, effective, and versatile method for circumferential spinal cord decompression.
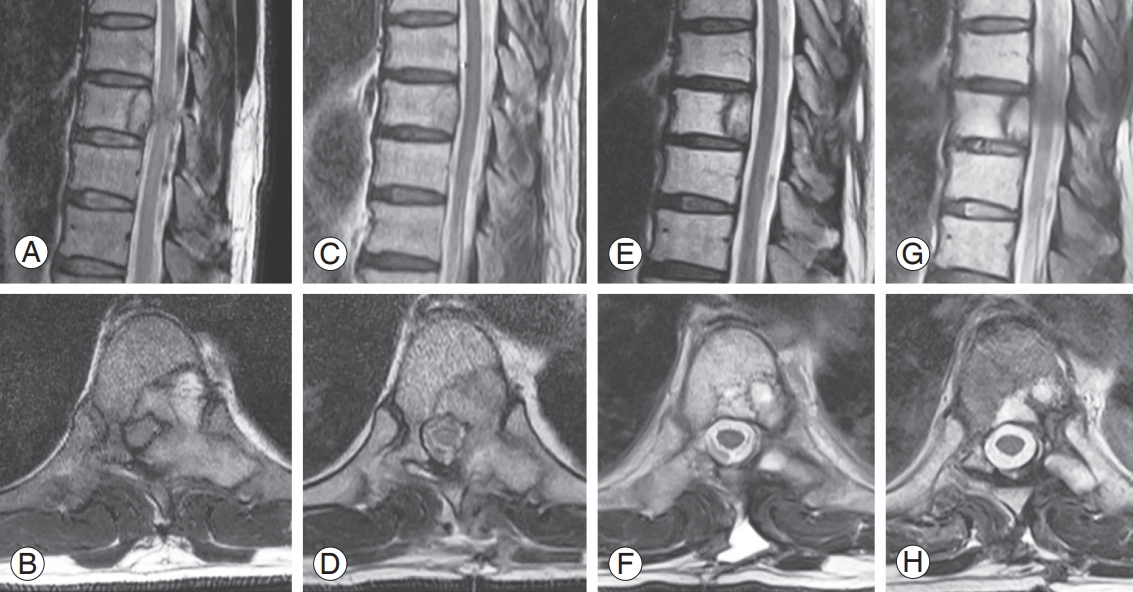
A case of metastatic epidural spinal cord compression treated by hybrid therapy (separation surgery and stereotactic radiosurgery [SRS]). (A, B) Preoperative magnetic resonance imaging (MRI) shows severe spinal cord compression at T9. (C, D) MRI at postoperative 2 weeks after separation surgery shows a gap created between the spinal cord and residual tumor. (E, F) Following a single-fraction SRS, which was performed 3 weeks after the separation surgery, the residual tumor was completely ablated. (G, H) At postoperative 2 years, there is no evidence of tumor in the follow-up MRI.
In separation surgery, ventral decompression is the most challenging and crucial surgical step along with intraoperative confirmation of sufficient decompression. In previous studies, sufficient ventral decompression has been associated with improved long-term local control [65]. For adequate ventral decompression, the removal of 20% of the posterior vertebral body after posterior longitudinal ligament resection is generally required [63]. Intraoperative confirmation of ventral decompression can be performed with a stereotactic navigation system or ultrasonography [66] (Fig. 2).

Adequate ventral decompression confirmed by the stereotactic spinal navigation system during a separation surgery using a transpedicular approach. (A–C) Before ventral decompression, the navigation probe is located on the posterior surface of the vertebral body. (D–F) After ventral decompression is achieved by removing the posterior vertebral body, the navigation probe is located within the vertebral body.
Interdisciplinary decision-making systems, like the NOMS framework, include separation surgery as the treatment of choice for patients with high-grade spinal cord compression of radioresistant tumors when combined with SRS [31]. Additionally, several previous studies have reported favorable outcomes of separation surgery followed by SRS (hybrid therapy) [67-69]. A recent metaanalysis by Kang et al. [70] reported that the pooled local progression rate at 1 year following hybrid therapy was 10.2%. Additionally, their study revealed that factors like low doses per fraction, previous radiotherapy, and colorectal cancer were significantly associated with local tumor progression [70]. Favorable results of hybrid therapy limit spine surgeons from performing en-bloc resections in patients with spinal metastasis carrying significant surgical morbidity.
3. Minimally invasive or minimal-access surgery
Minimally invasive surgery (MIS) or minimal-access surgery for spinal metastasis could reduce surgical morbidities and facilitate quick recovery after surgery. This would allow cancer patients to restart their oncological treatment promptly. Previous studies have introduced tubular retractor systems and thoracoscopic assistance for anterior surgeries [71,72], minimally invasive approaches for decompression, and corpectomy for posterior surgeries [73,74]. Other MIS techniques, like percutaneous pedicle screw fixations and image-guided stereotactic navigation systems, are also effective treatment modalities in spinal metastasis surgery [68,75].
In previous studies, it was revealed that compared to open surgery, MIS displays equivalent surgical outcomes and has fewer complications at the surgical site [76-80]. Additionally, a recent systematic review comprising of 26 studies summarized that MIS could potentially reduce surgical site infections (SSIs), hospital stay, and blood loss in patients with spinal metastasis without decreasing instrument accuracy and overall patient outcomes [81].
4. Role of curative surgery (en-bloc resection)
Surgical treatment for spinal metastasis is performed for palliative purposes and the role of curative tumor resection, including TES, is limited. En-bloc resection of the metastatic spinal tumor should only be considered in isolated metastatic lesions of slow-growing malignancy, with an expected survival of more than 2 years and an acceptable performance status because of the high surgical morbidity [82]. En-bloc resection for spinal metastasis could also be considered in conditions where SRS is not available [83].
Previous studies have reported improved survival and function following spinal metastasectomy [84-86]. Recently, Kato et al. [87] reported medium-to-long-term clinical outcomes of spinal metastasis, in which the 3-year and 5-year survival rates for 124 patients were 70% and 60%, respectively. Contrastingly, some authors recently reported that spinal metastasectomy, including TES, for spinal metastasis did not affect oncological outcomes [88]. These conflicting results and recent reports of improved efficacy of novel radiotherapies suggest that a more careful patient selection through a multidisciplinary team approach is required for determining whether curative surgery should be performed for spinal metastasis.
5. Prevention of perioperative complications
The reported overall complication rate ranges from 10% to 66.7% after surgical treatment for metastatic spinal tumors [89]. Minimizing all possible perioperative complications is crucial in patients with spinal metastasis because it has been reported that 30-day postoperative complications are associated with worse patient survival [90]. In this review, the following common perioperative complications occurring after surgery in metastatic spinal tumors have been discussed: (1) SSI, (2) failure of instrumentation, and (3) intraoperative bleeding.
SSI is experienced by up to 30% of patients treated surgically for spinal metastasis patients. SSI is more prevalent than in patients undergoing other spinal surgeries [89]. Moreover, SSI is the most common reason for reoperation after surgical treatment of metastatic spinal tumors [91,92]. Following spinal metastasis surgery, the proposed risk factors for SSI are poor nutrition, diabetes mellitus, smoking, obesity, and adjuvant therapies, like radiotherapy and systemic treatment [93-95]. Therefore, to minimize the incidence of SSI, all possible preventive measures (e.g., nutritional therapy and an adequate interval between surgery and radiotherapy) should be administered promptly by a multidisciplinary team. Recently, a web-based calculator that can predict the occurrence of SSI and the associated risk of reoperation has been developed to help minimize SSI [96].
Instrumentation failure is the second reason for reoperation after surgery for metastatic spinal tumors [91]. The risk factors suggested for instrumentation failure are longlevel surgery, combined chest wall resection, and higher preoperative SINS [97-99]. However, it is controversial if adjuvant radiotherapy should be considered to be a risk factor for instrumentation failure [100]. Prolonged survival of patients with spinal metastasis is also associated with increased instrumentation failures [101]. Currently, limited evidence is available on the role of additional fusion procedures during surgical stabilization in reducing instrumentation failure [93,102,103]. This study suggests that the two types of instrumentation failure, early and late, should be distinguished in future studies because the mechanisms of these two failures are different [104]. Early failure occurs due to insufficient fixation strength or stability of the construct, whereas late failure occurs due to deformity progression due to tumor progression or lack of fusion.
Intraoperative bleeding can often be extensive in spinal metastasis surgery, leading to serious cardiovascular or cerebral complications [105]. Therefore, before surgery for a metastatic spinal tumor, preoperative embolization has been recommended for hypervascular tumors, like kidney and thyroid cancers. A recent meta-analysis reported significantly less intraoperative blood loss, fewer blood transfusions, and shorter surgical duration in the embolization group for hypervascular tumors [106]. However, in this analysis, scanty evidence was available to support the routine administration of preoperative embolization for nonhypervascular spinal metastases [106]. Nevertheless, when aggressive debulking surgery, like piecemeal corpectomy, is planned, preoperative embolization can help in reducing intraoperative bleeding [107]. To maintain the effect of preoperative embolization and avoid increased bleeding due to reperfusion, the delay between embolization and surgery should be minimized and not exceed 24 hours [108].
Conclusions
The decision-making process for patients with metastatic spinal tumors is challenging and requires a multidisciplinary approach. Recent advancements in radiotherapy and surgical techniques have markedly improved clinical outcomes in patients with spinal metastases. Therefore, the ongoing evolution of decision-making systems should also play a crucial role in state-of-art care for metastatic spinal tumors.
Notes
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: SHH, SYC; data curation: SHH, DHK, SYC; formal analysis: HK, DHK; methodology: BSC, SYC; project administration: BSC, HK; visualization: SHH, DHK; writing–original draft: SHH, BSC, HK, SYC; writing–review & editing: SHH, BSC,HK, DHK, SYC.