Radiological Characteristics of Low-Grade Lytic Spondylolisthesis: Similarity to Dysplastic Spondylolisthesis
Article information
Abstract
Study Design
Retrospective case-control study.
Purpose
This study aimed to analyze the etiology of low-grade lytic spondylolisthesis based on the radiologic features of the vertebra.
Overview of Literature
According to the Marchetti-Bartolozzi classification scheme, high-grade lytic spondylolisthesis (Meyerding grade 3–5) is classified as dysplastic. However, determination of the etiology for low-grade lytic spondylolisthesis as developmental or traumatic remains controversial.
Methods
Patients admitted and treated for one-level (L4/5 or L5/S1) low-grade spondylolisthesis were included in the study. A total of 135 patients were divided into the degenerative or lytic spondylolisthesis groups according to their condition (81 patients [degenerative group] vs. 54 patients [lytic group]). To assess the level of similarity in the radiological findings between low-grade lytic spondylolisthesis and dysplastic spondylolisthesis, the pedicle diameters and vertebral heights of the L4 and L5 vertebrae were measured on computed tomography images. Measurements were then converted to each vertebra’s ratio to reduce confounding factors among individuals.
Results
The affected vertebra had a smaller sagittal pedicle diameter/transverse pedicle diameter ratio in the low-grade lytic spondylolisthesis group compared to the degenerative group, and the posterior vertebral height/anterior vertebral height ratio of L5 was smaller in the L5/S1 lytic spondylolisthesis group compared to the degenerative spondylolisthesis group.
Conclusions
Low-grade lytic spondylolisthesis and dysplastic spondylolisthesis demonstrated similar radiological findings. Hence, surgeons should be attentive to the morphology of the vertebral body and posterior column during preoperative planning for the treatment of low-grade lytic spondylolisthesis.
Introduction
Spondylolisthesis is characterized by spinal deformity and instability [1]. To date, the Wiltse classification of spondylolisthesis has been the most popular scheme; however, this classification remains controversial because it incorporates developmental and acquired spondylolisthesis in the same isthmic category [2]. To overcome this problem, Marchetti and Bartolozzi classified spondylolisthesis as developmental or acquired. The central concept of this classification scheme is that patients with isthmic spondylolisthesis based on the Wiltse classification are classified as having “developmental” or “traumatic” spondylolisthesis [3]. Some researchers have recently recommended the Marchetti-Bartolozzi classification because it helps spine surgeons to predict the natural course and prognosis of spondylolisthesis [2].
According to the Marchetti-Bartolozzi classification, high-grade lytic spondylolisthesis (Meyerding grades 3–5), which is characterized by distinct radiologic features, such as asymmetry of the vertebral body and posterior column, is classified as dysplastic [4–7]. However, in cases of low-grade lytic spondylolisthesis (Meyerding grades 1 and 2), determination of the etiology as developmental or traumatic remains controversial. As most spondylolistheses requiring surgical treatment are low-grade lytic spondylolisthesis, understanding the underlying etiology of this condition may be useful in developing clinical practice guidelines that will improve preoperative planning for surgical treatment.
To date, many morphological studies of the vertebrae have been conducted [8–12]. However, to the best of our knowledge, there has been no study that analyzes the etiology of low-grade lytic spondylolisthesis based on the radiologic features of the vertebra. In degenerative spondylolisthesis, disk space narrowing, hypertrophic facet degeneration, and endplate sclerosis are common conditions. In contrast, dysplastic spondylolisthesis is caused by dysplasia of the pars, lumbar facets, disks, or vertebral endplates [7]. Thus, characteristic radiological findings of vertebral body and posterior column asymmetry, particularly elongation of the pedicles, are observed in dysplastic spondylolisthesis [4]. According to the authors’ experience, the pedicle in low-grade lytic spondylolisthesis is often small or atypical in shape, similar to that in dysplastic spondylolisthesis, which has caused significant difficulties in pedicle screw fixation or in the determination of fusion level during surgery.
In this study, we aimed to determine whether low-grade lytic spondylolisthesis has radiological findings similar to those of dysplastic spondylolisthesis by analyzing the vertebral pedicle and body size, to help clarify the etiology of low-grade lytic spondylolisthesis.
Materials and Methods
1. Study design and participants
This retrospective study was approved by the institutional review board of Ilsan Paik Hospital (no., 2022-02-010) and conducted in accordance with the Declaration of Helsinki. Patients who were admitted to our hospital between January 2010 and December 2018 and treated for low-grade spondylolisthesis were included. We excluded patients with high-grade spondylolisthesis because their characteristic deformities could be classified as dysplastic without controversy. In addition, the prevalence rate of high-grade spondylolisthesis is very low. Therefore, the authors focused on patients with low-grade spondylolisthesis. Informed consent was obtained from all patients for being included in the study.
Patients were divided into two groups based on their computed tomography (CT) images. Patients with and without a pars interarticularis defect were classified into the lytic and degenerative spondylolisthesis groups, respectively.
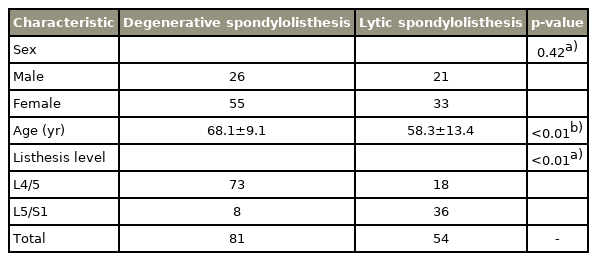
The authors included the two most frequently reported levels of spondylolisthesis. Degenerative spondylolisthesis occurs most frequently at L4/5, whereas lytic spondylolisthesis most often occurs at L5/S1 [13–15]. Thus, patients who were treated for one-level spondylolisthesis (L4/5 or L5/S1) were included in the study. Naturally, there were cases of spondylolisthesis at other levels; however, we only included the L4/5 and L5/S1 levels to reduce confounding factors when comparing different levels of the vertebrae.
The inclusion criteria were as follows: (1) patients who were admitted and treated for one-level (L4/5 or L5/S1) spondylolisthesis; (2) patients with low-grade spondylolisthesis (Meyerding grade 1); and (3) patients who had undergone CT within 1 year from the date of admission.
Patients were excluded if they had (1) high-grade spondylolisthesis, (2) multi-level spondylolisthesis, (3) spondylolisthesis at levels other than L4/5 or L5/S1, (4) vertebrae with metastatic changes, traumatic pars fractures, previous spine surgery, and other significant deformities, such as degenerative scoliosis or kyphosis on radiographs, and (5) CT scans performed at other hospitals.
Finally, a total of 135 patients were enrolled in this study.
2. Computed tomography measurements
Measurements of the pedicle size by CT are well correlated with actual cortical measurements using calipers [16]. Therefore, we performed CT (320 CT, Aquilion One; Toshiba Medical System, Tokyo, Japan) to measure the pedicle diameter and vertebral height, using axial, sagittal, and coronal slices at 3-mm intervals. Since slice interval varies between hospitals, patients who had undergone CT at other hospitals were excluded from this study. Characteristic radiological findings of vertebral body and the posterior column asymmetry, particularly elongation of the pedicles, are observed in dysplastic spondylolisthesis [4,7]. Therefore, we measured the L4 and L5 vertebral body and pedicle sizes to determine whether lytic spondylolisthesis shows similar radiological findings to those of dysplastic spondylolisthesis. The S1 vertebra was excluded from the measurements, since it does not show morphological changes in the pedicles and is already fused to the sacrum. Furthermore, it is more reasonable to perform a comparison of the lumbar vertebrae compared to including the sacral segment. Additionally, S1 is tilted relative to the CT cut, making it difficult to measure its size accurately. We measured the following dimensions at the L4 and L5 vertebra: anterior vertebral height (AVH) and posterior vertebral height (PVH), transverse pedicle diameter (TPD), and sagittal pedicle diameter (SPD).
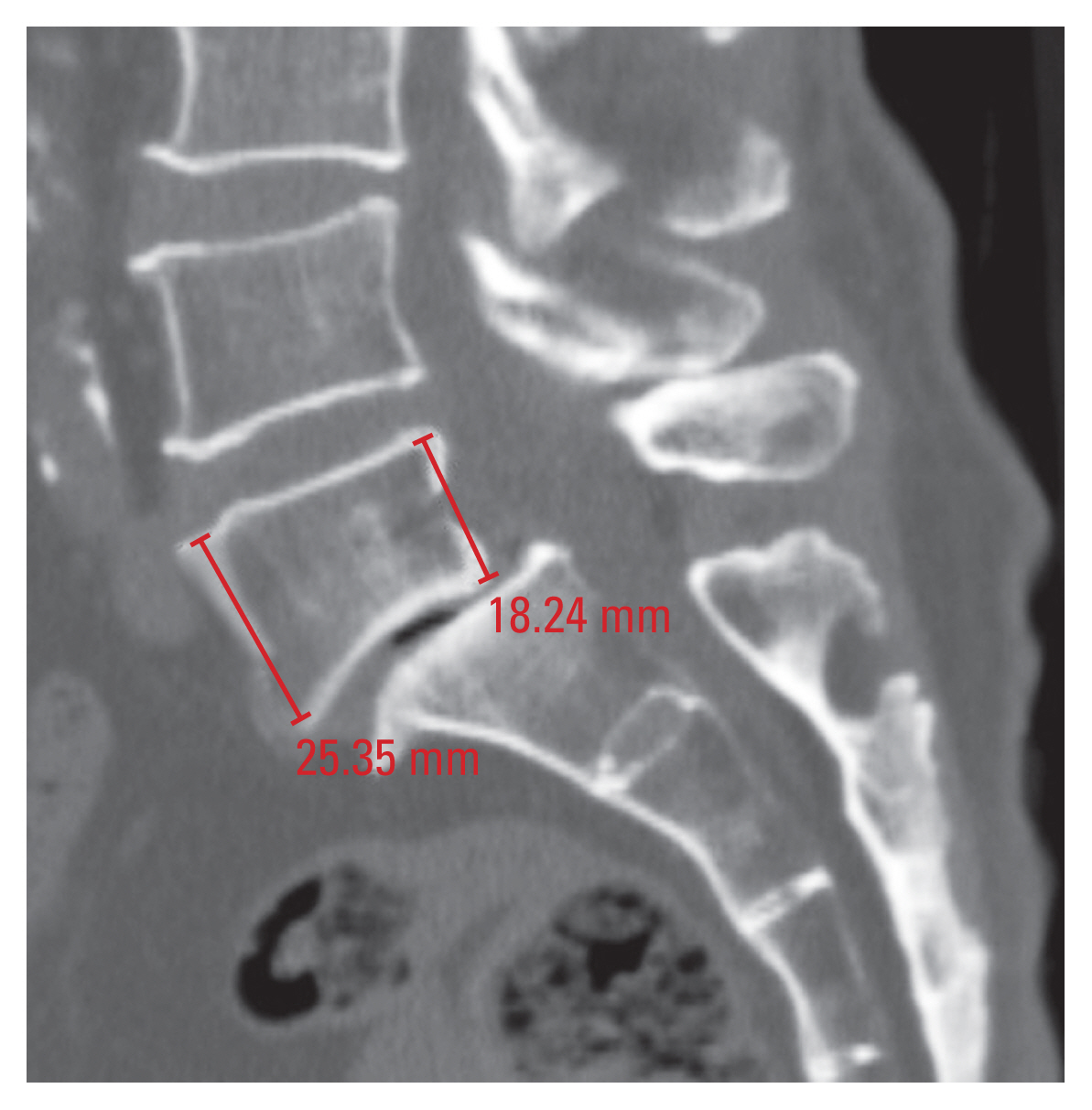
1) Vertebral height
Vertebral height (AVH and PVH) was measured in the mid-sagittal plane of the vertebral body, and it was defined as the distance between its superior and inferior cortices (Fig. 1).
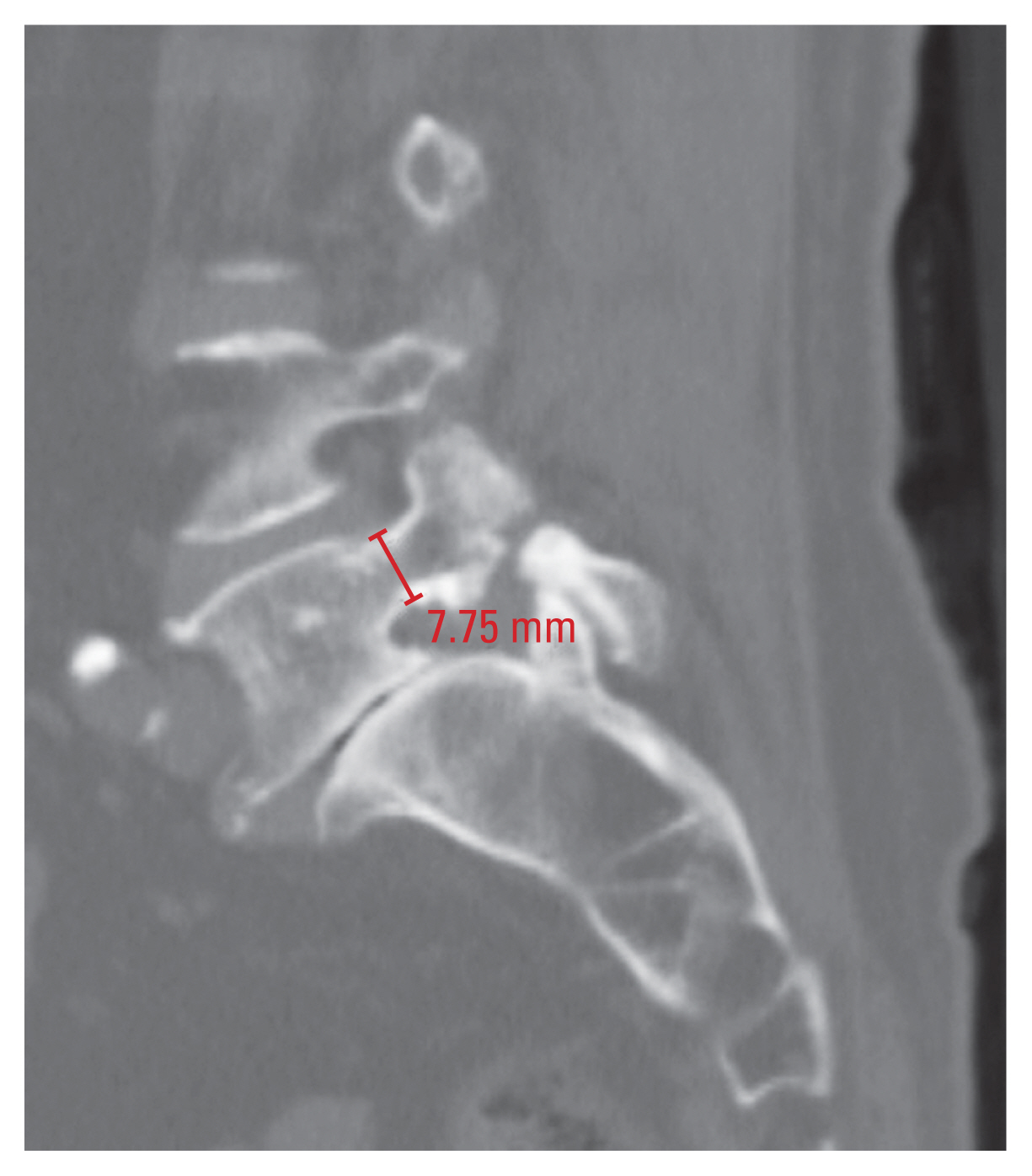
2) Pedicle diameter
Measurements of the TPD and SPD were performed in accordance with a previous study [17]. The TPD was measured in the axial plane at the middle of the pedicle height, and it was defined as the distance between the medial and lateral cortices of the isthmus (Fig. 2). In addition, the SPD was measured in the sagittal plane at the middle of the pedicle width, and it was defined as the distance between the superior and inferior cortices of the isthmus (Fig. 3). TPD and SPD were both measured at the bilateral pedicles.

Measurement of the transverse pedicle diameter (TPD) at L4 vertebra. Right TPD of L4 is 12.90 mm. Left TPD of L4 is 12.38 mm.
Measurements were performed by two orthopedic spine surgeons, and the average values were analyzed to minimize errors. The measured pedicle size was converted to each vertebra’s SPD/TPD ratio, and the measured AVH and PVH values were converted to a PVH/AVH ratio to maximally reduce potential confounding factors among individuals.
3. Statistical analysis
To determine the appropriate sample size, the authors compared five lytic spondylolisthesis patients with five degenerative spondylolisthesis patients in a pilot study. The results of the pilot study showed that the PVH/AVH ratios were 0.80 and 0.88 in the lytic and degenerative spondylolisthesis groups, respectively, and the standard deviation was 0.05. In accordance with these results, the appropriate number of samples was determined to be six per group when the power and significance levels were set at 80% and 0.05, respectively. Therefore, inclusion of a total of 135 patients (L4/5: 73, degenerative spondylolisthesis and 18, lytic spondylolisthesis; L5/S1: 8, degenerative spondylolisthesis and 36, lytic spondylolisthesis) in the current study was thought to be adequate sample size.
Parametric statistics were used for the normally distributed variables of the two groups. Otherwise, non-parametric statistics were used. Comparisons of continuous variables between each group were performed using the independent samples t-test. For nominal variables, the Fisher’s exact test or the chi-square test was performed. A p-value of <0.05 was considered statistically significant.
Results
A total of 135 low-grade spondylolisthesis patients were analyzed (81, degenerative spondylolisthesis; 54, lytic spondylolisthesis), comprising 47 males and 88 females. The mean age of the patients with degenerative spondylolisthesis at the time of admission was greater compared to patients with lytic spondylolisthesis (68.1 years versus 58.3 years, respectively). L4/5 spondylolisthesis was more common in the degenerative spondylolisthesis group (73 patients versus 18 patients in the lytic spondylolisthesis group), while L5/S1 spondylolisthesis was more common in the lytic spondylolisthesis group (36 patients versus eight patients in the degenerative spondylolisthesis group). The patient data and corresponding p-values are shown in Table 1.
1. Mean sagittal/transverse pedicle diameter of L4 and L5
The results of the SPD/TPD measurements of the two groups are summarized in Table 2. Depending on the level of lysis, there was a significant difference in the SPD/TPD ratio between the lytic and degenerative spondylolisthesis groups, with the ratio being smaller in patients with low-grade lytic spondylolisthesis.
2. Mean posterior/anterior vertebral height of L4 and L5
The results of the PVH/AVH measurements in the two groups are summarized in Table 3. Patients with L5/S1 spondylolisthesis showed a significant difference in the PVH/AVH ratio of L5 between the degenerative and lytic spondylolisthesis groups (0.86 and 0.78, respectively; p<0.001). These results suggest that for patients with L5/S1 spondylolisthesis, L5 had a more wedged shape in lytic than in degenerative cases.
3. sagittal/transverse pedicle diameter, posterior/anterior vertebral height, and age
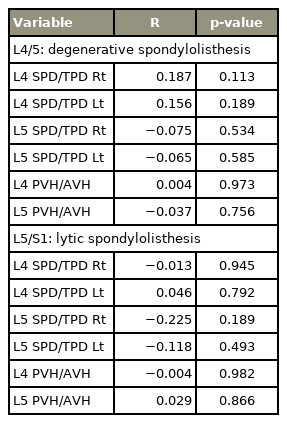
Since this study showed a significant difference between the mean ages of the degenerative (68.1 years) and lytic (58.3 years) spondylolisthesis groups, we further analyzed whether the SPD/TPD and PVH/AVH ratios were associated with age in spondylolisthesis patients. However, our results revealed that there was no significant correlation based on Pearson’s correlation test (Table 4).
Discussion
Our study shows that the radiological features of lytic spondylolisthesis were different from those of degenerative spondylolisthesis. The SPD/TPD ratio of the affected vertebra was lower in patients with low-grade lytic spondylolisthesis. Furthermore, in cases of L5/S1 low-grade lytic spondylolisthesis, the L5 had a considerably asymmetrical vertebral body. These features are similar to those of vertebra in dysplastic spondylolisthesis. Therefore, this study suggests that low-grade lytic spondylolisthesis has a dysplastic etiology, and it would be reasonable to classify such cases as dysplastic rather than traumatic in adults.
Wedging of the L5 vertebra is a typical feature of L5/S1 dysplastic spondylolisthesis. In dysplastic spondylolisthesis, the constant reparative metabolic process predisposes more toward bone resorption than bone formation, leading to a wedge-shaped vertebra. Furthermore, several studies have reported that both dysplastic and lytic spondylolisthesis show a wedge-shaped L5 vertebra [5,6,18,19]. Previous studies have also shown that L5 spondylolysis causes alterations in the biomechanical properties of the lumbar spine with increased pressure on L5, resulting in a wedge shape. Rosenberg [18] found that in a sample of 200 patients with degenerative spondylolisthesis, the anterior margin of L5 was on average 2 mm greater than the posterior height, while in 61 patients with isthmic spondylolisthesis, the anterior height of L5 was on average 12 mm greater than the posterior height. These results are similar to the findings in our study, which demonstrated more severe L5 vertebral wedging in lytic spondylolisthesis than in degenerative spondylolisthesis. However, the research by Rosenberg [18] focused on isthmic spondylolisthesis, which is a broader category compared to lytic spondylolisthesis. Additionally, the author calculated the difference between AVH and PVH [18]. In contrast, considering the differences in individual vertebral body size, we calculated the ratio of PVH to AVH, which could be regarded as a more meaningful parameter to measure compared to the measurements performed in the previous study. In fact, and since low-grade lytic spondylolisthesis was characterized by a more wedge-shaped L5 vertebra than degenerative spondylolisthesis, a dysplastic etiology could be considered contributory to some extent in low-grade lytic spondylolisthesis.
Furthermore, several studies have measured the pedicle size in spondylolisthesis [10–12,17]. For instance, Choi et al. [12] measured the length, width, and height in L5/S1 lytic spondylolisthesis patients to investigate the anatomical characteristics of the L5 pedicles. The authors found that the pedicle was longer in patients with L5/S1 lytic spondylolisthesis than in patients without spondylolysis, whereas the pedicle width was not significantly different between the two groups [12]. Assuming that the volume of the pedicle is constant, our SPD/TPD ratio thus indicates the relative height of the pedicle, and a low value can be considered indicative of pedicle elongation, a common characteristic of dysplastic spondylolisthesis [4]. To our knowledge, this is the first study to measure the SPD/TPD ratio. Considering the etiology of lytic spondylolisthesis, the SPD/TPD ratio is more important than the exact size of the pedicle, as this ratio can correct for differences in individual pedicle size. Therefore, considering the small SPD/TPD ratio in the affected vertebra along with the wedging of the L5 vertebra in L5/S1 lytic spondylolisthesis, a dysplastic etiology may contribute to low-grade lytic spondylolisthesis.
With respect to age-related effects on vertebral morphology, and despite the significant difference between the mean ages of the degenerative and lytic spondylolisthesis groups, our results showed that there was no significant correlation between the SPD/TPD and PVH/AVH ratios and age. Thus, the morphological differences between the two groups for each level of spondylolisthesis should be considered as being due to etiology and not age.
Given that the radiological findings of low-grade lytic spondylolisthesis were similar to those of dysplastic spondylolisthesis, careful attention would be required during preoperative planning. Posterior decompression and reduction using pedicle screw fixation is commonly used for spondylolisthesis, but in cases of lytic spondylolisthesis, the method of pedicle screw insertion would require modifications due to the different anatomical characteristics of the L5 vertebra [20–22]. Since the angle of the pedicle is wide in patients with L5/S1 lytic spondylolisthesis, Choi et al. [12] have recommended that the L5 pedicle screw should be inserted more medially in such cases. Furthermore, Don and Robertson [23] revealed that patients with L5/S1 lytic spondylolisthesis have a more coronal orientation of the facet joints at the L3/4 and L4/5 levels. This means that an L5 pedicle screw could violate the L4/5 facet joint and influence adjacent segment degeneration [24]. Therefore, extensive muscle dissection for larger pedicle screw convergence or percutaneous screw fixation could be considered for L5/S1 lytic spondylolisthesis. Elongation of the pedicle is also reported to be prominent in low-grade lytic spondylolisthesis, which is consistent with the findings in this study [12]. Therefore, it is important to measure the pedicle size of the affected vertebra preoperatively and choose an adequate pedicle screw size to prevent nerve injury. Furthermore, in cases of L5/S1 dysplastic spondylolisthesis, a dysmorphic pedicle with a very small diameter will render screw insertion difficult. Instrumentation and fusion up to the L4 level would therefore be recommended to ensure stability [25]. In addition, the sacrum’s dome shape complicates the interbody fusion approach and cage placement. Consequently, the shape of the endplates at the involved level should be clearly defined on preoperative imaging, and cage placement and positioning should be considered in the preoperative planning stages. In spondylolisthesis with posterior dysplasia, the L5 transverse process may be small [26]. This reduces the space available for bone grafting, resulting in a high rate of pseudoarthrosis for posterior in situ fusion. In such cases, active use of graft materials, such as bone morphogenic proteins, would be recommended. Finally, recent studies have shown that L5 spondylolysis is associated with increased pelvic incidence [27]. Therefore, whole spine scanography should be performed prior to surgery for lytic spondylolisthesis, and the appropriate lordosis and fusion level should be determined based on the pelvic parameters.
The limitations of our study are as follows: First, relative to measuring the SPD, the plane of the axial CT scan was not precisely parallel to the TPD. Therefore, fine-cut CT scan or measurements of the pedicle of cadavers may be needed in the future. Second, only L4/5 and L5/S1 spondylolisthesis were included, and other levels were not analyzed. However, L4/5 and L5/S1 were the most common sites of degenerative and lytic spondylolisthesis, and spondylolisthesis is extremely rare at other levels. Multi-center analysis is necessary in the future to analyze the morphologic characteristics of spondylolisthesis at other levels. Finally, although an appropriate sample size was determined in our pilot study, future studies with larger sample sizes are required.
Conclusions
This study showed that low-grade lytic spondylolisthesis and dysplastic spondylolisthesis had similar radiological findings, indicating that the etiology of this spinal condition may also be dysplastic. Surgeons should therefore be attentive to the morphology of the vertebral body and posterior column, as well as the pelvic parameters, during preoperative planning for patients with low-grade lytic spondylolisthesis.
Acknowledgments
Thank you for Ji Yeon Lee in the medical record office for helping to collect patient data.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Study concept and design, analysis and interpretation of data and drafting of the manuscript: Sung Tan Cho and Jin Hwan Kim; acquisition and analysis of data: Sung Tan Cho; and all authors read and approved the final manuscript.