Association between Age and Trunk Muscle Area and Density in Patients with Spinal Metastases
Article information
Abstract
Study Design
A retrospective study.
Purpose
This study aimed to evaluate the relationship between age and trunk muscle composition and between trunk muscle composition and overall survival in patients with spinal metastasis.
Overview of Literature
A low skeletal muscle mass is associated with a poor overall survival in patients with cancer. However, no previous studies have evaluated the relative effects of age and disease on muscle mass and muscle quality in patients with advanced cancer.
Methods
This study included 539 patients diagnosed with spinal metastasis from February 2009 to July 2018. The patients were categorized into four groups based on age: <59, 60–69, 70–79, and ≥80 years. Differences in trunk muscle composition among age groups and among groups were classified on the basis of survival (< or >3 months after spinal metastasis diagnosis) were evaluated.
Results
In total, 515 patients (273 men, 242 women; mean age, 67.8 years) with complete medical records were included in the analysis. No significant differences were observed in the area of the psoas and paravertebral muscles among age groups in either sex. A significant trend toward a low muscle density with the increase in age was found for both sexes. Patients who survived less than 3 months had significantly smaller trunk muscle area than those who survived for more than 3 months in both sexes.
Conclusions
The results suggest that the reduction in muscle density is associated with advanced age, whereas a decreased muscle area is associated with pathology. Additionally, a small trunk muscle area was associated with a short overall survival. Further studies are needed to elucidate the underlying mechanisms of age- versus cancer-related changes in the muscle area and their influence on overall survival.
Introduction
Bone is the third most common site of metastasis after lung and liver [1], whereas the spine is the most common site for bone metastasis [2,3]. Recently, morphometric analyses of the trunk muscles have attracted attention because of the relationship between morphometric findings and overall survival or surgical outcomes in patients with cancer. A meta-analysis and systematic review reported that a low skeletal muscle mass was associated with a short overall survival among patients with cancer [4].
Advanced cancer often causes rapid weight loss, including deterioration in muscle mass. Muscle loss in patients with advanced cancer has been attributed to two distinct mechanisms: sarcopenia, which is defined as the age-related decrease in muscle mass and muscle function [5], and/or cachexia, which is defined as the weight loss resulting from an underlying illness [6]. A review article that investigated muscle loss among patients with cancer recorded that 15%–50% were sarcopenic, and 25%–80% were cachectic [7]. Malnutrition affects the muscle composition and can increase the risk of surgical site infection following spinal surgery, which then can directly influence the survival of patients.
The effect of aging on muscle size and quality has been reported in patients with lumbar disease and in healthy volunteers. Previous studies of non-cancer patients revealed that the trunk muscle area is age dependent [8–11] and the presence of age-dependent progressive fatty infiltration [9–13]. Identification of the etiology of loss of muscle mass and quality in patients with cancer is important because the treatments for sarcopenia and cachexia differ; however, no previous studies have analyzed the differential effects of age and cancer on muscle composition in patients with spinal metastasis. This study aimed to investigate the association between age and trunk muscle composition in patients with spinal metastasis. We also analyzed the relationship between the trunk muscle composition and overall survival in this population.
Materials and Methods
1. Study population
The data from 539 patients who were diagnosed with spinal metastases at our institution from February 2009 to July 2018 were retrospectively reviewed. Spinal metastases were radiologically diagnosed with computed tomography (CT), magnetic resonance imaging, or positron emission tomography. The primary site was categorized as having slow, moderate, or rapid growth, in accordance with previous reports [14]. The minimum follow-up period after diagnosis of spinal metastasis was 3 months, except in cases of death before 3 months. We excluded 24 patients who had incomplete clinical or radiological data. Given the anonymous nature of the data, the requirement for informed consent was waived. This study was approved by the Institutional Review Board of Yodogawa Christian Hospital (approval no., 2019-035). The requirement for informed consent from individual patients was omitted because of the retrospective design of this study.
2. Outcome measures
The primary outcome was the relationship between trunk muscle composition and age in patients with spinal metastasis. The psoas major, paravertebral muscle area, and density were measured as indicators of the trunk muscle composition. Age was categorized into four groups: <59, 60–69, 70–79, and ≥80 years. The secondary outcome was the association between trunk muscle composition and overall survival in patients with spinal metastasis. Poor overall survival was defined as the survival of less than 3 months after the diagnosis of spinal metastasis.
3. Measurements
The bilateral psoas major and paravertebral muscles (multifidus and erector muscles) were measured by aggregating the cross-sectional area (mm2) at the L3 level on CT images captured at the time closest to the diagnosis of spinal metastasis. The L3 level was used as a standard landmark because its correlates best with whole-body muscle mass [15,16]. The muscle area was normalized for patient height (m2) to calculate the muscle indexes for the psoas muscle in mm2/m2. The muscle density was evaluated based on the muscle radiation attenuation rate in Hounsfield units (HU). Adipose tissue and skeletal tissue areas were defined in accordance with standard HU ranges (−190 to −30 HU for adipose tissue and −29 to 150 HU for skeletal muscle) [17].
4. Statistical analysis
The significance of differences in demographic characteristics and composition of trunk muscles among age groups was tested by one-way analysis of variance, with post hoc testing performed with the Bonferroni method for continuous variables and with chi-square tests for categorical data. The Jonckheere-Terpstra trend test was used to identify trends in the relationship among age, trunk muscle area, and density. The analysis of covariance adjusted for age was performed to examine the trunk muscle area and density among patients who survived more than 3 months versus those who survived less than 3 months.
Survival was estimated with the Kaplan-Meier method, and survival estimates were compared with the log-rank test. Data were censored on September 30, 2019. Patients who were lost to follow-up were censored at the date of last contact/follow-up. Patients who were alive on September 30, 2019 were censored for overall survival analysis. Overall survival was calculated from the date of diagnosis of spinal metastasis to the date of death. IBM SPSS Statistics ver. 19.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses; p<0.05 was considered statistically significant.
Results
A total of 515 patients were included in this study. The mean patient age was 67.8 years (range, 21–93 years); 53% of patients were male. The most frequent primary site was the lung (187 patients, 36%), followed by breast (92 patients, 18%) and prostate (57 patients, 11%) (Table 1). A total of 49 patients (10%) underwent surgery for spinal metastasis, 229 patients (44%) received radiation therapy for spinal metastasis, and 342 patients received bone-modifying agents (66%). Table 2 shows the patients’ demographic and clinical data by age group. Among women, the oldest age group (>80 years) had a higher proportion of patients with rapid growth and poor performance status than other age groups.
1. Survival
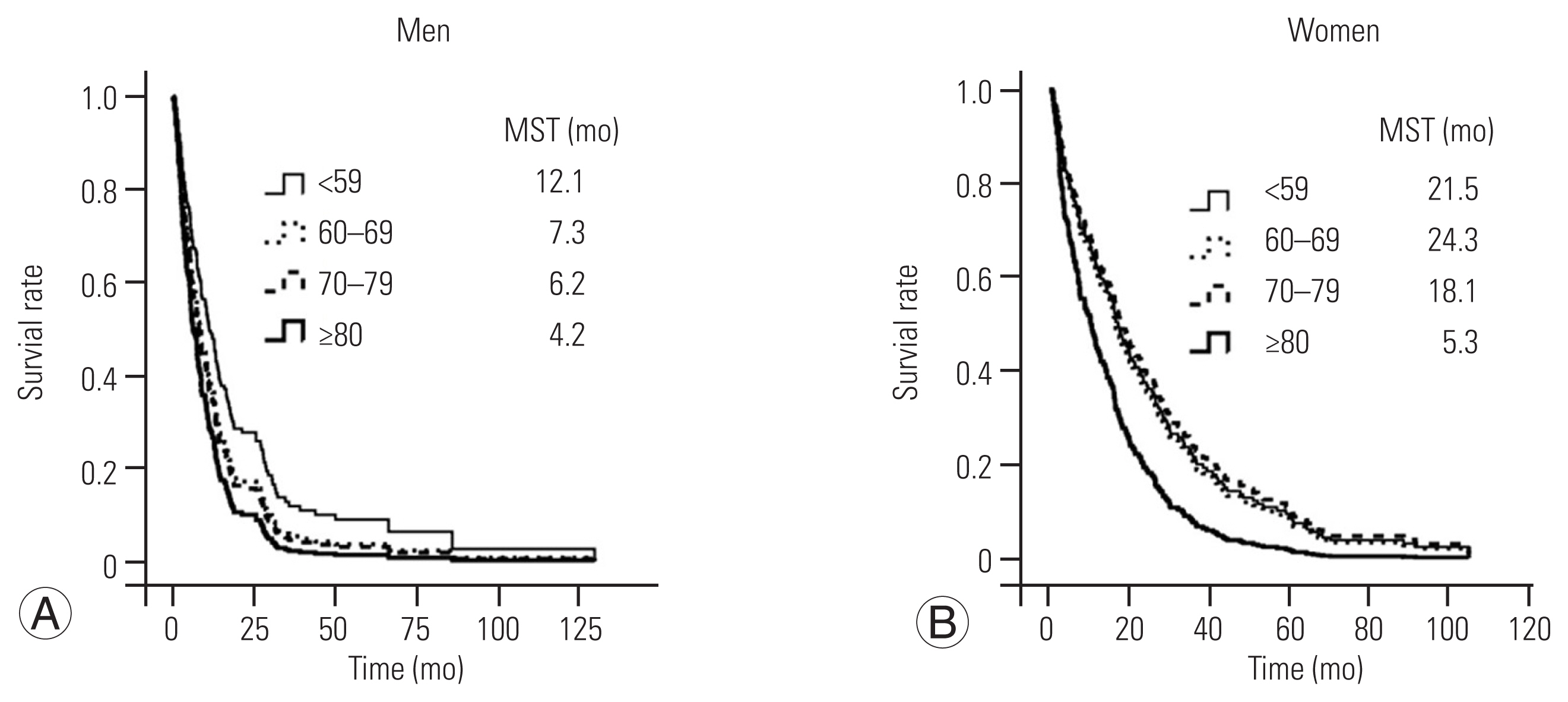
The overall median survival time for all patients was 10.9 months. The overall median survival time among women was significantly longer than that among men (18.3 versus 7.3 months, respectively; p<0.001). Survival curves were significantly different in patients ≥80 years versus those <59 years in both sexes (Fig. 1).
2. Muscle area and density
The area of the psoas and paravertebral muscles was not significantly different among the age groups for either sex (Fig. 2). A statistically significant trend toward the low density of the psoas and paravertebral muscles with high age groups was observed for both sexes (Fig. 3). Among men, muscle density in patients aged over 80 years was significantly lower than that in patients aged less than 79 years. Among women, muscle density in patients aged over 70 years was significantly lower than that in patients aged less than 59 years. Table 3 shows the differences in demographic characteristics, trunk muscle area, and density among patients who survived for more than 3 months versus those who survived less than 3 months. The patients who survived for less than 3 months were significantly older than those who survived more than 3 months in both sexes. The analysis of covariance adjusted for age showed that psoas major and paravertebral muscle areas were significantly lower in patients who survived for less than 3 months than those who survived for more than 3 months.

Area of psoas major and paravertebral muscles in men (A) and women (B) according to age group. ANOVA, analysis of variance.
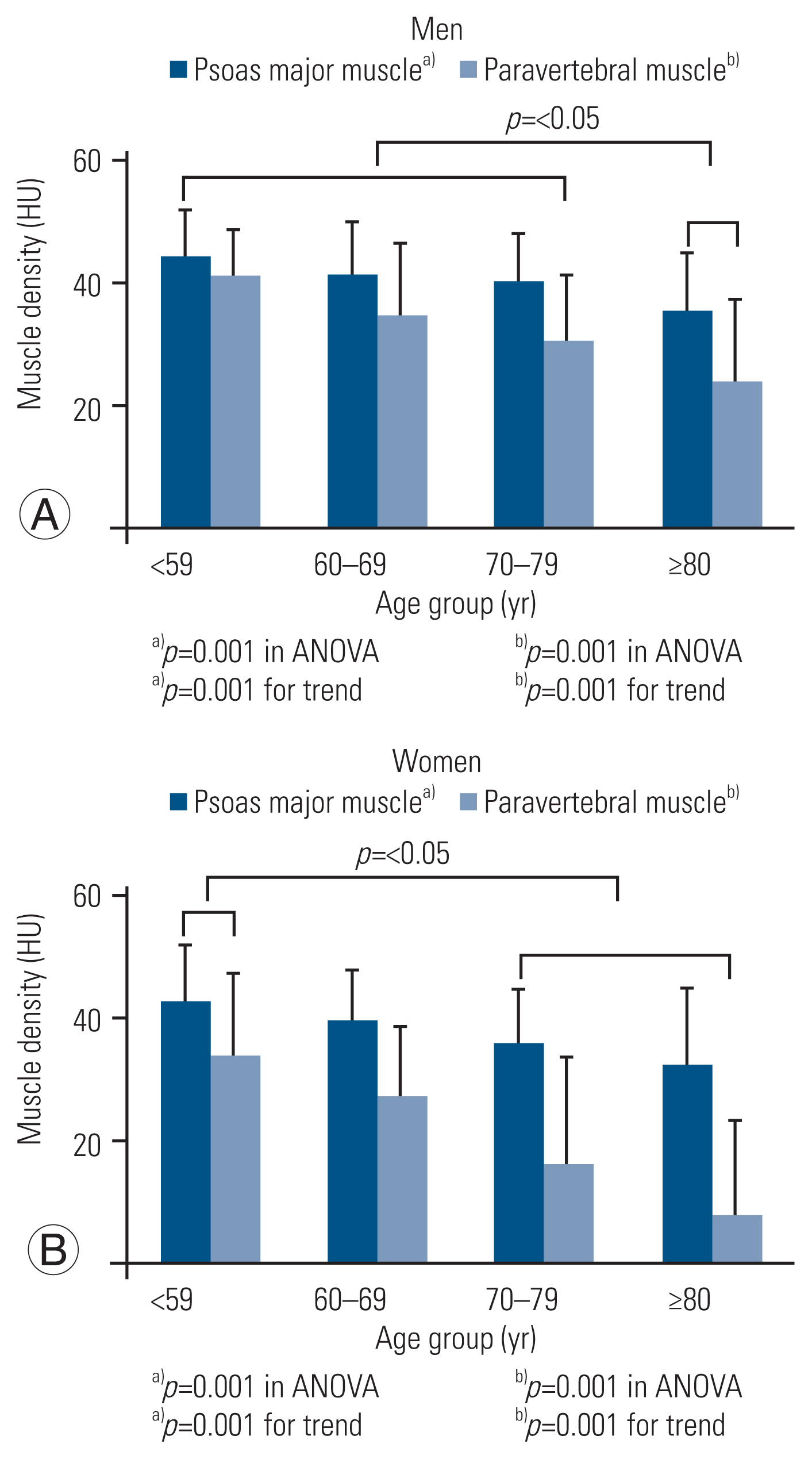
Density of psoas major and paravertebral muscles in men (A) and women (B) according to age group. ANOVA, analysis of variance.
Discussion
This study investigated the relationship between the trunk muscle composition and age in patients with spinal metastasis. The bilateral psoas major and paravertebral muscles were measured by aggregating the cross-sectional area at the L3 level on CT images. The results revealed that the area of the trunk muscles did not significantly differ among the age groups; however, a significant trend toward the low density of the trunk muscles was observed with older age. Additionally, a low trunk muscle area was associated with poor overall survival.
1. Relationship between age and trunk muscle area
We observed that the trunk muscle area did not differ among age groups of men or women. Previous studies of patients without cancer have shown mixed results on the association between age and trunk muscle area, partly because of the differences in pathology among studies. Several studies have revealed that trunk muscle area was age independent [12,18], but this result conflicted with that of several other studies [8–11].
Among patients with advanced cancer, muscle loss can result from sarcopenia, which is defined as the age-related decrease in muscle mass associated with changes in muscle synthesis signaling pathways [5], and/or cachexia, which is defined as the cytokine-mediated degradation of muscle and adipose depots [6]. We noted that the trunk muscle area did not differ among age strata. Thus, age had little effect on trunk muscle area. A possible explanation for this finding is that the presence of advanced cancer dramatically increases muscle loss, especially in young patients. This finding suggests that reduction in muscle area is more affected by pathology than advanced age.
2. Relationship between age and trunk muscle density
This study showed that the density of the psoas major and paravertebral muscles decreases with age in patients with spinal metastasis. A low muscle density indicates a high amount of fatty infiltration in the muscle. Several studies have analyzed fatty infiltration by using magnetic resonance imaging [10–12] or CT [9,13] in patients with and without lumbar disease; these reports showed the presence of age-dependent progressive fatty infiltration and agree with this study of patients with spinal metastasis. In contrast to muscle mass, trunk muscle density is not greatly affected by advanced cancer. Although why muscle density is more related to aging than advanced cancer remains unclear, this parameter is not likely related to the effect of advanced cancer on muscle fat infiltration.
This study also showed that in elderly patients, fatty degeneration was more prominent in paravertebral muscles than in the psoas muscle (Fig. 3), a finding similar to the results of a study in patients without cancer [13]. Age seemed to affect degeneration in the paravertebral muscles more than in the psoas muscle. This difference might be related to muscle type-specific degeneration [13].
3. Association between trunk muscle area and overall survival
This study showed that trunk muscle area was significantly smaller in patients who survived for less than 3 months than in those who survived for more than 3 months. Several recent studies have revealed a relationship between skeletal muscle mass and outcomes in patients with cancer. A meta-analyses and systematic review reported that a low skeletal muscle mass is associated with a short overall survival [4]. Similarly, this study showed an association between the low trunk muscle area and poor overall survival in patients with spinal metastasis.
Patients with spinal metastasis typically present with back pain, neurological symptoms, and/or signs of mechanical instability. Additionally, the malignant disease itself causes the loss of muscle mass and low muscle function. A retrospective study showed that rehabilitation improved functional outcomes in patients with spinal metastasis. Furthermore, those who achieved a high functional gain after rehabilitation had longer survival [19]. Therefore, rehabilitation is important to regain functional ability, regardless of age.
Several limitations of this study should be acknowledged. First, its retrospective nature was a limitation; the treatment of spinal metastases and systemic treatments for primary cancer were not uniform. Second, the analyses remain subject to biases resulting from unobserved confounding factors, such as the interval after the diagnosis of primary cancer or spinal metastasis. Further studies with a larger sample size and propensity score matching to reduce bias should be conducted to confirm the present results.
Conclusions
This study examined the relationship between age and trunk muscle area and density in patients with spinal metastasis. The results suggest that the reduction in muscle density is associated with advanced age, whereas a decreased muscle area is associated with pathology. Additionally, a low trunk muscle area was associated with poor overall survival. Further studies are needed to elucidate the underlying mechanisms of age- versus cancer-related changes in the muscle area and their influence on overall survival.
Acknowledgments
We thank Rebecca Tollefson, DVM, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript and helping to draft the abstract.
Notes
No potential conflict of interest relevant to this article was reported.