Diagnostic Modality in Spine Disease: A Review
Article information
Abstract
Spine diseases are common and exhibit several causes, including degeneration, trauma, congenital issues, and other specific factors. Most people experience a variety of symptoms of spine diseases during their lifetime that are occasionally managed with conservative or surgical treatments. Accurate diagnosis of the spine pathology is essential for the appropriate management of spine disease, and various imaging modalities can be used for the diagnosis, including radiography, computed tomography (CT), magnetic resonance imaging (MRI), and other studies such as EOS, bone scan, single photon emission CT/CT, and electrophysiologic test. Patient (or case)-specific selection of the diagnostic modality is crucial; thus, we should be aware of basic information and approaches of the diagnostic modalities. In this review, we discuss in detail, about diagnostic modalities (radiography, CT, MRI, electrophysiologic study, and others) that are widely used for spine disease.
Introduction
With advances in technology, various imaging modalities have been developed for diagnosing spine pathologies. Radiography, the first-line imaging modality, is based on X-rays and allows the visualization of the bony structure of the spine, estimation of the state of the spinal canal, and identification of specific pathologies, such as ossification of the posterior longitudinal ligament (OPLL) and osteoarthritis. However, the spinal cord and nerve roots cannot be detailed; therefore, radiography has not been recognized as the gold standard for the diagnostic workup of the spine. Computed tomography (CT) and magnetic resonance imaging (MRI) are the second-line imaging modalities for achieving a better understanding of the pathologies of the spine and establish the direction for management. CT is a valuable imaging modality for both, confirmation of diagnosis and elimination of differential diagnosis. CT is fast, non-invasive, and highly accurate; however, it involves certain drawbacks. CT cannot properly detect certain spinal cord lesions, disk pathologies, and minor lesions. Owing to the previously mentioned limitations of radiography and CT, MRI has been considered the gold standard diagnostic modality for spinal pathologies. Additionally, most spine-related diseases impact the neural structures, including the spinal cord and nerve roots, resulting in several neurological symptoms such as radiating pain, numbness, or paralysis of the affected extremity. For accurate diagnosis of the nerve pathologies in such conditions, the electrophysiological test can also be very useful.
Here, we summarized the use of several commonly used diagnostic modalities, including radiography, CT, MRI, and electrophysiological tests.
Diagnostic Modalities
1. Plain radiography (X-ray)
After the discovery of X-rays by Wilhelm Conrad Rontgen in 1895, radiography has been considered the first-line imaging modality for diagnosing spine pathologies. With advances in the techniques for radiography over time, the diagnostic value of radiographs also improved, and it provides important information for most spinal diseases, from trauma assessment, determination of spinal deformity and degeneration, identification of spinal instability, and the identification of abnormal lesions suggestive of malignancy.
Currently, conventional radiography using a hard-copy X-ray film has been replaced by digital radiography (DR) that uses a digital detector and imaging processors in clinical practice. The advantages of DR include improved image quality, speed, accessibility, less radiation exposure, availability of post-imaging processing, quality optimization, and convenient image storage and retrieval. Further, the use of DR enabled more precise and accurate measurement of the spinal alignment status and various spinal parameters [1].
Initial evaluation of plain radiography often begins with the anteroposterior and lateral views of the spinal segment at the area of interest. The need for additional studies, such as oblique, flexion, or extension views, is determined based on the clinical situation. Plain radiography demonstrates the inherent advantages of assessing the structural status in a functional position, especially with the patient in an erect and weight-bearing position. Furthermore, it provides a relatively effective assessment of the spinal instability, with flexion and extension (dynamic) lateral radiography [2]. Moreover, dynamic radiography is helpful for evaluating postoperative stability or radiographic fusion as well as detecting the presence of significant motion or evidence of hardware failure or loosening of the instrumented segment.
The main disadvantage of plain radiography is the superimposition of the soft tissue and bony structures that interfere with accurate interpretation of the spinal osseous structures, especially at the cervico-thoracic junction and cranio-cervical junction. Moreover, it cannot be detected properly with the visualization of the paravertebral soft tissues, spinal cord, and bone marrow involvement.
2. Computed tomography
CT allows good visualization of spinal pathologies, including compression of neural structures and disease of the laterally situated structures (such as foraminal stenosis) [3-5]. Modern spiral CT with multidetector row allows rapid and continuous data acquisition within few seconds [6]. CT is also useful in the detailed evaluation of bony structures of the spine and is highly sensitive for fracture detection. Thus, CT of the spine is the first choice of imaging for screening trauma patients, especially those at high-risk of spinal injuries (Fig. 1) [7,8].
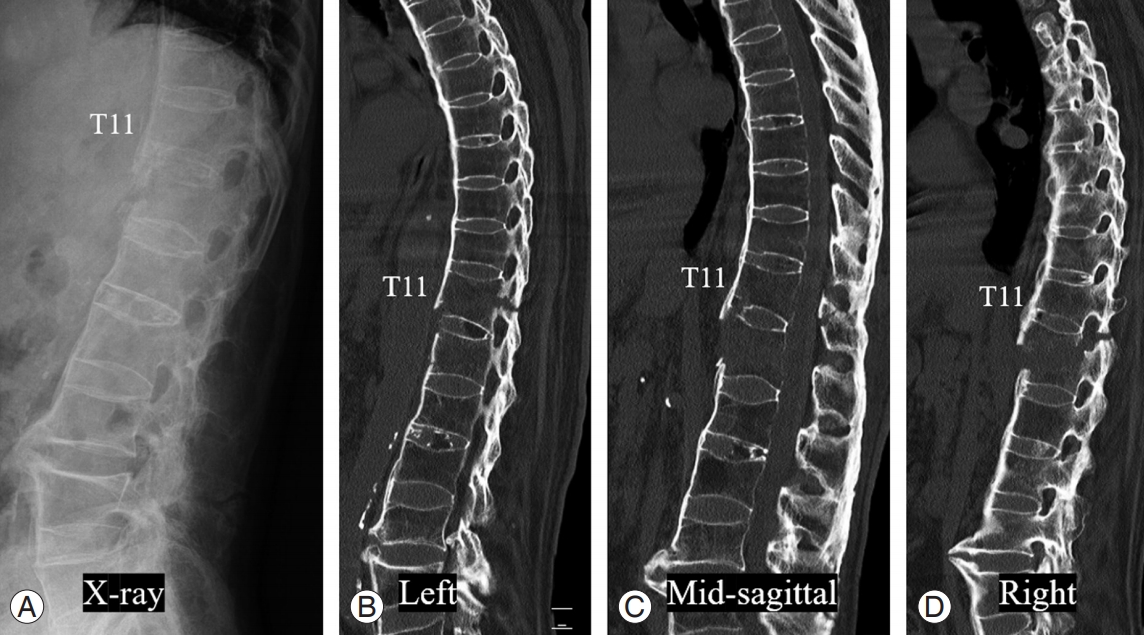
Lateral radiograph (A) and sagittal CT images (B–D) of the thoracolumbar spine showing full ankylosis of the spine, suspiciously ankylosing spondylitis, and multiple fractures at T10–12 levels with traumatic subluxation. The fracture site, pattern, and extent can be detected more clearly at CT images than those at lateral radiograph. CT, computed tomography.
Raw CT acquires image data in the axial plane, generates cross-sectional images, and enables sagittal and coronal reconstruction via post-image acquisition processing. This multiplanar reconstruction allows excellent evaluation of the spine, visualization of the bony anatomy of the lesion, demarcation of the extent of bone destruction, and checking of the alignment of the vertebral column [9]. Tissue density can be accentuated via the manual adjustment of the contrast and window levels, and subtle soft tissue abnormalities such as small disc protrusions can be detected. Three-dimensional (3D) volumetric reconstruction allows intuitive illustrations for clinicians. In particular, 3D reconstructed images of the complex structures involving the bone and soft tissues, such as the occipitocervical junction and C1–2 level, are helpful for establishing an accurate diagnosis and presurgical planning (Fig. 2) [10].

(A, B) Cervical spine magnetic resonance imaging showing posteriorly subluxation of C2 odontoid process and signal change at the spinal cord at C1–2 level. (C, D) Three-dimensional reconstructed computed tomography images at C1–2 level showing bony pathologies as well as surrounding structures (especially, course, proximity, and other abnormalities of vertebral artery).
CT is useful for evaluating posterior elements and bony changes, such as those in Baastrup’s disease [11,12]. Moreover, CT allows easy evaluation of the postoperative status, such as verification of whether spinal fusion has been achieved, locating of the implanted materials, and identification of the hardware-related complications or implant loosening [13]. In particular, CT is superior to MRI in distinguishing the calcified pathology from the surrounding soft tissue, such as OPLL [14,15]. However, its ability to evaluate soft tissue in the spine, especially in neural structures and ligaments, is limited, and it is less sensitive than MRI.
Patients are usually requested to assume a supine position in a CT machine to minimize spine movement. Therefore, limitations to the evaluation of real spinal pathologies that are affected by gravity and standing posture exist, such as spinal stenosis related to spondylolisthesis.
3. Magnetic resonance imaging
MRI is based on the response of the hydrogen nuclei (protons) in intracellular fluids in an artificial magnetic field (rather than on ionizing radiation), and it produces multiplanar images with excellent anatomical and spatial resolution [16]. The proton is temporarily redirected by the magnetic field, and the application of radiofrequency pulses disturbs this alignment. When the pulse is removed, the proton shifts back to its original steady-state position. The MRI machine can distinguish between tissues via the identification of the differences in the shiftback timing of protons. The signal intensity of each tissue depends on the number of protons that is based on the inherent water content of the tissue. Finally, the location and forms of water, fat, bones, and other materials with different resonances can be visualized.
MRI provides high-resolution images of the bone and soft tissues that can be clearly distinguished. Next, MRI can visualize the entire spine and differentiate between individual structures, such as the vertebral body, intervertebral discs, spinal canals, posterior elements, ligaments, paravertebral muscles, nerve roots, and spinal cord. When the contrast in MRI is based on differences in the longitudinal relaxation time, it is called a “T1-weighted” image. Meanwhile, an MRI image is known as a “T2-weighted” image when the image contrast is based on the difference in transverse relaxation time. Further, the release rates of absorbed energy are different for each tissue, and T1 and T2 sequences can be classified accordingly. The routine protocol for MRI of the spine includes axial and sagittal T1- and T2-weighted images. Additional sequences, such as sagittal T2 sequences with fat suppression (e.g., short tau inversion recovery [STIR]) and contrast-enhanced T1 sequences, can be added as required. Knowledge of the signal intensity pattern of each tissue in the T1 and T2 images is vital for reading and interpreting the MRI images [16].
Tissues with high water content, such as the cerebrospinal fluid (CSF), appear as dark structures in T1 images [16]. In contrast, fat-rich tissues have a short T1 relaxation time, and they exhibit a high signal intensity. However, T2 images show high signal intensity in tissues where the extracellular matrix exhibits a higher water content [17]. Thus, bright CSF is the hallmark of T2 images. Next, T1-weighted images provide excellent anatomical details, including bone marrow changes, osseous structures, disks, and soft tissue. The spinal cord and nerves demonstrate an intermediate signal intensity in T2 images, causing maximal contrast between the CSF and neural tissue.
Fat-suppression technology suppresses the high signal of fat (seen in the bone marrow) and is crucial because it allows excellent visualization of the pathological structures [18]. Among the various fat-suppression techniques, the STIR sequence exhibits a high sensitivity in detecting musculoskeletal pathology as it enables the visualization of subtle edematous changes or lesions in the bone marrow or ligamentous structures [19].
The use of intravenous contrast agents, such as gadolinium (Gd), shortens the relaxation time of the adjacent molecules in the magnetic field. Visualization of the contrast enhancement after Gd injection is best visualized on T1 images as an increased signal intensity. Post-contrast imaging can be used to distinguish between postoperative fibrosis of the epidural scar tissues and recurrent herniation of disk fragments [20]. Moreover, post-contrast images are used to evaluate infections, tumors, arteriovenous malformations, and leptomeningeal diseases (Fig. 3) [21-23].
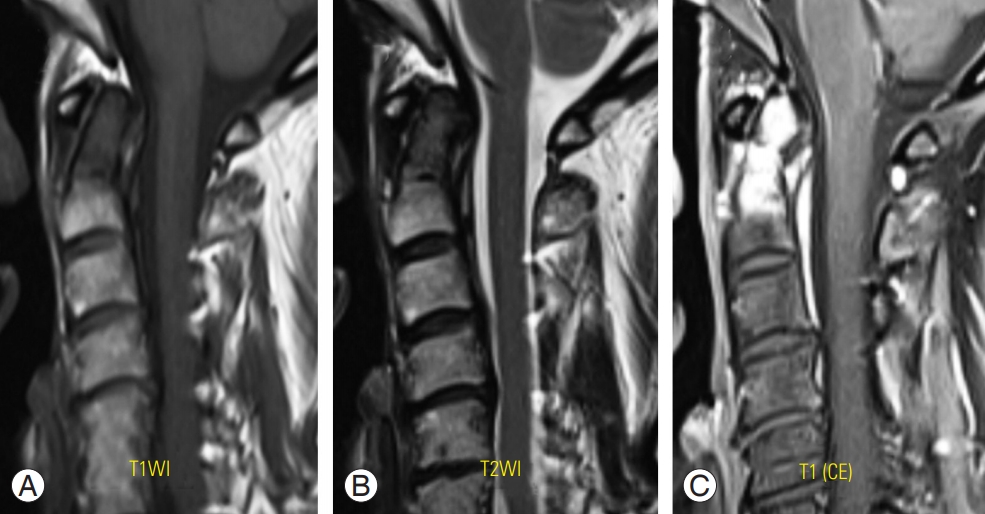
(A–C) Metastatic carcinoma with pathologic fracture on C2 odontoid process. CE T1-weighted MRI (C) showing increased signal intensity of the lesion at C2 odontoid process, that makes detection of the lesion more obviously in comparison with other sequences of images on MRI (A, B). CE, contrast enhanced; MRI, magnetic resonance imaging.
MRI can identify small soft tissue structures in the spine, such as the spinal cord and nerve roots [24-26]. Thus, it is currently the most common method used for diagnosing degenerative spine diseases, including diseases of the intervertebral disc, facet joints, ligamentum flavum, posterior longitudinal ligament, and neural foramen. MRI allows excellent evaluation of the degree of central and foraminal stenosis and the degree of other degenerative changes, such as facet arthropathy and degenerative disk disease [26-28].
For trauma patients, MRI is useful for evaluating traumatic disk rupture, ligamentous or spinal cord injury, and intraspinal hematomas [29-31]. Owing to its high sensitivity in identifying bone marrow edema, it is useful for detecting occult fractures, especially with additional fat suppression, such as STIR sequences (Fig. 4) [29]. A T1-weighted image helps to assess the integrity of the ligamentous structures, especially the anterior and posterior longitudinal ligaments, and the epidural hematoma [32-35].
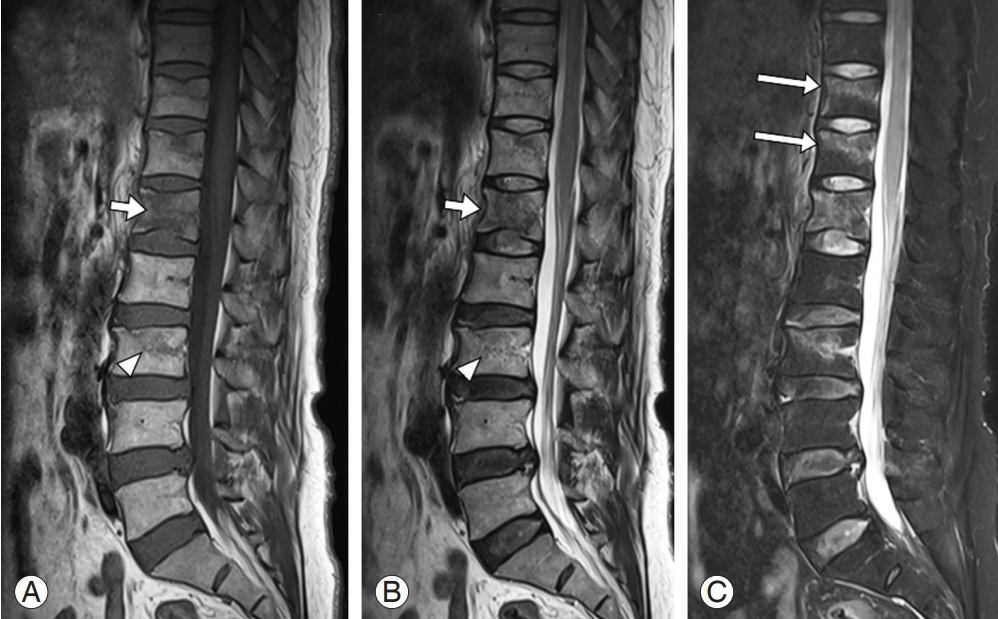
Sagittal T1- (A) and T2- (B) weighted magnetic resonance imaging of the lumbar spine showing definite signal change in L1 vertebral body (short arrows) and subtle marrow edema at the L3 vertebra (arrowheads). Additional T2 sagittal image with fat suppression technique showing the previously identified signal changes of L1 and L3 clearer, (C) while the occult fractures of the T11 and T12 vertebrae are clearly revealed due to the definite contrast of marrow edema (long arrows).
MRI is used to evaluate spine tumors, including not only the spinal cord or the nerve root, but also benign and malignant bone tumors [24]. Whenever a tumor is suspected, post-Gd contrast imaging should be performed. MRI should also be considered in patients suspected to present with spinal column infections. The presence of an abnormally increased T2 signal intensity within the intervertebral disc and the presence of Gd enhancement are diagnostic features that indicate the presence of infectious spondylodiscitis. Epidural extension of infection and abscess formation are also easily visible on MRI. In addition, MRI is used for diagnosing inflammatory diseases, such as multiple sclerosis, sarcoidosis, and transverse myelitis, because it can detect spinal cord edema (acute inflammation) or demyelination (chronic inflammation) [36-38].
In order to perform MRI in a position that can best reveal the pathology, dynamic or axial-loading MRI was introduced. Dynamic (neck extension) MRI is more widely used for diagnosing cervical myelopathy. Similar to the cervical canal, the lumbar canal size decreases in the lumbar extension [39,40].
Despite these advantages, MRI is associated with certain problems and safety concerns. Patients with cardiac pacemakers or other embedded ferromagnetic materials cannot undergo MRI [41,42]. In addition, image quality is inevitably affected in patients with implanted metal artifacts, especially devices containing ferrous metals [42]. Further, performing MRI in patients with claustrophobia is difficult because they are unable to stay in the MRI scanner for the entire duration of the examination. CT is superior to MRI for the assessment of the details of osseous or calcified structures [43]. MRI is relatively contraindicated during pregnancy, especially during the first trimester [44]. Contrast administration with Gd carries a rare but specific risk of nephrogenic systemic fibrosis; thus, it should be administered only in patients with suitable renal function (glomerular filtration rate >30 mL/min) [45].
4. Other imaging modalities (EOS, bone scan, and SPECT/CT)
The EOS imaging system, also called a slot-scanning device or slit-beam DR system, is a biplane radiographic imaging system that uses slot-scanning technology wherein the radiation source and detector move in different planes during image acquisition [46,47]. It uses this ultrasensitive multiwire proportional chamber detector to detect the X-rays, thus limiting the dose of X-rays that is absorbed by the patient. EOS can take posteroanterior and lateral images simultaneously and construct the 3D reconstruction of skeletal structures, using algorithms based on statistical modeling and bone shape recognition [48-50]. The images are taken with the patient in the standing position, allowing the examination of the spine and lower extremities under normal weight-bearing conditions [47]. Therefore, suitable indications for the use of EOS for spine are diseases wherein the shape of the deformity varies as per the weight-bearing and gravity, such as scoliosis or kyphosis. EOS imaging exhibits the following limitations [47]. First, EOS does not provide information on soft tissues. Second, the plain images on radiography films present less contrast, resulting in reduced brightness. Third, 3D reconstructions cannot be obtained for pediatric patients aged <6 years. Fourth, 3D angular measurement of severe deformities or congenital anomalies is not possible because the 3D reconstruction process uses a statistical model based on ‘‘normal’’ bones. Finally, the inner structure or architecture of the bone is not considered in 3D reconstructed image because it only involves the outer bone surface.
Radionuclide bone scintigraphy, called bone scan, is one of the most widely used molecular imaging modality for specific spine pathologies [51]. This method is noninvasive, less expensive, causes adverse effects rarely, and allows the confirmation of whole-body bone pathology involving the spine. Bone scan uses several types of radioactive materials (radionuclides) that congregate at specific portions of the bone with highly active areas in metabolism [52]. Thus, the positive area in a bone scan is responsible for metabolically active lesions as the pain source. Considering the principle, bone scan is a valuable option for the following spine pathologies: bone tumor and metastasis, infection, subtle or undetectable fracture, unexplained spine pain unexplainable ton radiography, CT, and MRI, and other bone disorders, such as rickets, avascular necrosis, Paget’s disease, and osteoarthritis [53-57].
Bone scintigraphy with single photon emission compute tomography (SPECT/CT) has recently been introduced to define both, the morphology and physiology simultaneously in a single study [58]. As described earlier, CT can provide precise information about bony structures and anatomical changes of the lesion; however, conventional CT scans cannot represent the physiologic status and cannot implicate and localize the source of the spinal pain [59]. In order to overcome the limitation of the conventional imaging modality, SPECT/CT has been developed for diagnosing spine pathologies, with confirming a site of radiotracer uptake. In particular, the uptake finding enables improved accuracy and diagnostic value of the pathology [60]. Based on the basic concept, previous studies demonstrated that SPECT/CT can be a valuable diagnostic modality for spine disease, especially malignancy, active phase of arthritis, subtle trauma, infection, and postoperative pain caused by pseudoarthrosis, minor infection, and minor factors [61,62].
5. Electrodiagnostic study
Electrodiagnostic study facilitates the diagnosis of spinal disorders. The electrodiagnostic test is a useful tool for diagnosing neuropathy because it can reveal the pathophysiological state of the nerves via the measurement of nerve conduction [63]. Further, electrodiagnostic studies include the nerve conduction velocity study/electromyography (NCV/EMG) and central motor conduction time (CMCT) study.
6. Nerve conduction velocity study or electromyography
NCV/EMG is performed to differentiate peripheral neuropathy from muscular disorders [64,65]. The purpose of NCV/EMG is to assess the extent of peripheral nerve damage and identify the lesion site accurately. In the NCV, electrodes are attached to specific sites on the nerves to record their activity, while stimulation is delivered to the nerves with stimulating electrodes [65]. Then, the time taken for the muscles to contract in response to the stimulation of the nerves, conduction velocity, and amplitude are measured. EMG is a test that evaluates the electrophysiological condition of a muscle with the insertion of a thin needle electrode directly into the muscle tissue [66]. Electrophysiological changes occur in a muscle if nerve damage or an abnormality in the muscle itself is present, and needle electrodes are used to examine such changes [66]. NCV/EMG is commonly used for determining the presence or absence of cervical or lumbar radiculopathy; however, no abnormal findings are observed in mild radiculopathy [67]. Furthermore, early stages of neuropathy and chronic neuropathy that persisted for >1 year are not found positive on NCV/EMG [68]. If radiculopathy is detected on NCV/EMG, it suggests that the neuropathy is significant and persisted past the acute stage (Fig. 5). An additional use of NCV/EMG is in cases of radiculopathy at multiple levels (as detected on CT/MRI) to determine the levels to be treated. However, NCV/EMG from the upper limb cannot detect radiculopathy at C4 or a higher level. Further, it cannot differentiate between radiculopathy at C8 and T1; therefore, a diagnosis of C8/T1 radiculopathy was established in both the cases. In a similar manner, in the lower limb, radiculopathies at L2 and L3 cannot be differentiated from each other, and a diagnosis of L2/L3 radiculopathy was established in both the cases. NCV/EMG is useful not only for diagnosing radiculopathy, but also for distinguishing various disorders that can cause pain and motor weakness in the upper or lower limbs [64,69]. Motor neuron disease presents with a clinical pattern similar to that observed in radiculopathy, and NCV/EMG can help differentiate between the two conditions. Moreover, NCV/EMG can help differentiate peripheral nerve disorders, radiculopathy, and myopathy, thus enabling an accurate diagnosis that would allow appropriate treatment.
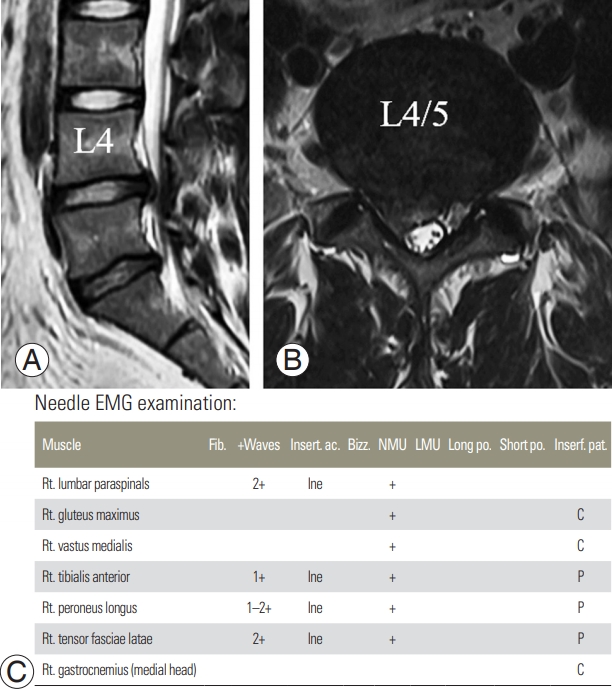
MRI and EMG of a 46-male patients having right lumbar radicular pain. Both examinations were conducted 1 month after the symptom onset. (A, B) T2-weighted MRIs at L4–5 disc level showed right central protrusion type disc herniation. (C) Positive sharp waves are manifested on right lumbar paraspinals and the muscles (right tensor fascia latae, tibialis anterior, and peroneus longus) innervated by right L5 nerve root, which is indicative finding of right L5 radiculopathy. MRI, magnetic resonance imaging; EMG, electromyography; Fib., fibrillation; Insert. ac., insertional activity; Bizz, bizzar potential; NMU, normal motor unit; LMU, large motor unit; Long po., long duration polyphasic potential; Short po., short duration polyphasic potential; Interf. pat., interference pattern.
7. Central motor conduction time study
CMCT is a test based on motor evoked potentials (MEPs), and it is used to determine the presence or absence of pathology in the brain and the spine [70]. In order to induce an MEP, electrodes are attached to the muscles in the upper or lower limbs, and magnetic stimulation is delivered to the scalp [70]. Electric stimulation is initiated in the corticospinal tract of the brain cortex and is delivered to the muscles in the limb where the electrodes are attached, inducing muscle contraction. Muscle contractions are recorded in the form of MEPs on the monitor; then, the MEP amplitude and latency are calculated. CMCT is estimated based on the MEP latency. The conduction velocity from the cerebral cortex to the spinal nerve root (that is, between the brain and spine) can be estimated by subtracting the latency of nerve conduction between the spinal nerve root around the intervertebral foramen and the muscle where an electrode is attached from the latency of nerve conduction from the cerebral cortex to the muscle via the corticospinal tract with magnetic stimulation. Slowed conduction velocity indicates the presence of a central nervous system disorder. Thus, a CMCT study is useful for determining whether a central nervous system disorder is present; however, it includes a limitation in that minor lesions can be missed because of false negative results.
Conclusions
Accurate diagnosis and proper management of spinal disease is necessary; however, it is occasionally challenging. Next, spine physicians can use several diagnostic imaging options, such as radiography, CT, MRI, and electrophysiological test. Patient (or case)-specific selection of the diagnostic modality is vital because it can help in proper patient management and accurate determination of the prognosis. Therefore, being aware of the various diagnostic modalities of the spine is important in order to be able to determine the best approach for each patient.
Notes
No potential conflict of interest relevant to this article was reported.