Neuromonitoring in Cervical Spine Surgery: When Is a Signal Drop Clinically Significant?
Article information
Abstract
Study Design
Retrospective cohort study.
Purpose
To identify the clinical significance of different patterns of intraoperative neuromonitoring (IONM) signal alerts.
Overview of Literature
IONM is a long-established valuable adjunct to complex spine surgeries. IONM for cervical spine surgery is in the form of somatosensory evoked potential (SSEP) and motor evoked potential (MEP). The efficacy of both modalities (individually or in combination) to detect clinically significant neurological compromise is constantly being debated and requires conclusive suggestions.
Methods
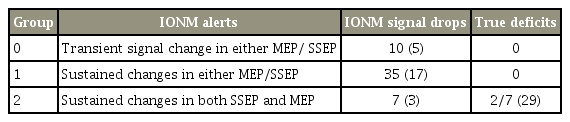
Clinical and neuromonitoring data of 207 consecutive adult patients who underwent cervical spine surgeries at multiple surgical centers using bimodal IONM were analyzed. Signal changes were divided into three groups. Group 0 had transient signal changes in either MEPs or SSEPs, group 1 had sustained unimodal changes, and group 2 had sustained changes in both MEPs and SSEPs. The incidences of true neurological deficits in each group were recorded.
Results
A total of 25% (52/207) had IONM signal alerts. Out of these signal drops, 96% (50/52) were considered to be false positives. Groups 0 and 1 had no incidence of neurological deficits, while group 2 had a 29% (2/7) rate of true neurological deficits. The sensitivities of both MEP and SSEP were 100%. SSEP had a specificity of 96.6%, while MEP had a lower specificity at 76.6%. C5 palsy rate was 6%, and there was no correlation with IONM signal alerts (p=0.73).
Conclusions
This study shows that we can better predict its clinical significance by dividing IONM signal drops into three groups. A sustained, bimodal (MEP and SSEP) signal drop had the highest risk of true neurological deficits and warrants a high level of caution. There were no clear risk factors for false-positive alerts but there was a trend toward patients with cervical myelopathy.
Introduction
The incidence of neurological deficits in cervical spine surgery has been relatively low, ranging between 0.2% and 3.2% [1]. Nevertheless, there has been a continuous effort to improve the safety of cervical spine surgery. Intraoperative neuromonitoring (IONM) is one of the adjuncts hypothesized to improve safety [2–5]. The benefits of IONM in deformity surgery have been long established to reduce incidence of major neurological deficits [2,6,7]. However, current evidence on IONM in cervical spine surgery lacks the strength to give conclusive suggestions, despite showing a reduced rate of new neurological deficits [8,9].
IONM during spinal surgery utilizes three different modalities, namely, somatosensory evoked potential (SSEP), motor evoked potential (MEP), and electromyography (EMG) [10–12]. In view of the devastating consequences of a neurological injury, many surgeons advocate the routine use of IONM to detect possible intraoperative insult; hence immediate remedial measures can be taken [13]. This can be seen in the increasing use of IONM in noncomplex spine surgeries [14,15].
However, there are limitations with using IONM in cervical spine surgery. Fehlings et al. [16] in 2010 showed that multimodal IONM had a wide range of sensitivity between 70% and 100% and specificity ranging from 52.7% to 100%. Previous studies on IONM in cervical spine surgeries showed a higher incidence of false-positive alerts, especially in cases with cervical myelopathy [17]. In addition to a wide specificity range and a low positive predictive value (PPV), the high incidence of false-positive signal alerts makes it challenging for the surgeon to make an intraoperative decision.
This study aimed to determine when an IONM signal alert was clinically significant by comparing the incidence of true neurological deficits in different patterns of signal alerts. In addition, we aimed to identify the risk factors for false-positive signal alerts. The secondary objective of this study was to evaluate the correlation of cervical five (C5) palsies with neuromonitoring signal alerts, as it is generally considered to be a separate entity from a true neurological deficit.
Materials and Methods
This study was approved by the National Healthcare Group-Domain Specific Review Board (NHG-DSRB), Singapore with reference no. 2017/00373. The requirement for informed consent from individual patients was omitted because of the retrospective design of the this study. Clinical and IONM records of a cohort of 257 patients who underwent cervical spine surgeries were retrospectively reviewed. Fellowship-trained spine surgeons performed all procedures. Consecutive adult patients who underwent spinal surgeries involving the cervical spine down to thoracic one (T1) level were selected for analysis. Both MEP and SSEP were monitored intraoperatively. The exclusion criteria were the absence of a recordable baseline MEP or SSEP preoperatively, surgeries involving tumor, and surgeries performed without IONM.
Electronic and paper medical records of each patient were reviewed for demographic data such as gender and age and clinical information such as the presence of myelopathy and American Spinal Injury Association (ASIA) impairment scale preoperatively. Documentation of postoperative neurological assessment done by the surgical team members immediately after the patient recovers from anesthesia and that which was done by the resident physician twice daily throughout inpatient stay were reviewed for deficits. Neurological deficit was defined as any new motor weakness as per Medical Research Council grading or sensory loss which presented immediately after surgery, excluding isolated C5 deficits. The incidence of C5 palsy was recorded and evaluated separately. Surgical data that were collected included the surgical approach and the number of surgical levels. Imaging records of the cervical spine were reviewed for the presence of ossified posterior longitudinal ligament (OPLL). Intraoperative anesthesia charts were also reviewed to identify significant changes in body temperature or intraoperative blood pressure.
A trained neurophysiologist provided by a commercial neurophysiological monitoring service reviewed IONM records of MEPs and SSEPs. Significant signal drops were defined as a 50% loss of amplitude in MEPs or SSEPs after considering all factors stated in the IONM protocol. A sustained alert was defined as a signal change that persisted till the end of surgery, while a nonsustained drop would return to baseline before the end of surgery.
Data was tabulated and stored on a Microsoft Excel spreadsheet without patient identifiers. Two clinicians who were not involved in the patients’ management evaluated all medical records. These two clinicians were medical officers (junior resident equivalent) with at least 3 years’ experience in orthopedic surgery, one of whom was the primary author.
1. Intraoperative neuromonitoring protocol
Multimodal IONM was performed continuously from the time of anesthetic induction to the time the patient emerged from anesthesia, using the NIM-ECLIPSE Spinal System (Medtronic, Minneapolis, MN, USA). A trained neurophysiologist operated the neuromonitoring system.
Efferent MEPs and afferent SSEPs were recorded on a graph. For MEPs, compound muscle action potential (CMAP) responses were recorded from needle electrodes inserted in the hand (abductor pollicis brevis and abductor digiti minimi) and deltoid muscles. CMAPs were stimulated by a brief high-voltage (range, 200 to 1,000 V) anodal electrical stimulus train through C3–4 (10–20 corkscrew electrode system). A train of 3 to 8 pulses ranging between 50 and 75 μS were used at an interval of 1 to 10 milliseconds.
SSEPs were stimulated in the median and ulnar nerve using the same needle electrodes that were placed for MEP monitoring. These were elicited using a 500-μS square-wave electrical pulse at a rate of 3.9 Hz. Stimulation intensity ranged up to 30 mA and the levels were selected as being well within the asymptotic portion of the SSEP intensity versus amplitude plot for each patient. SSEPs were recorded in the international system (C3-FPz, C4-FPz, and Cz-FPz). The same corkscrew electrodes in the scalp were used to stimulate MEPs and record cortical SSEPs.
Total intravenous anesthesia was used as the standard with no further muscle relaxants after intubation. Inhalational anesthesia was not used beyond induction. There was a standard protocol of response to all drops, which first involved an equipment check by the neurophysiologist. The anesthetic team would ensure mean arterial pressure was normotensive (mean arterial pressure >60 mm Hg), avoid hypothermia and hypocapnia, and maintain a balanced depth of anesthesia. Intravenous corticosteroid was not routinely given in response to a signal drop.
The surgeon would perform a surgical field and wound exploration and reverse the last surgical step if deemed necessary. A persistent drop would require the surgeon to decide to proceed, perform a wake-up test, or discontinue surgery.
2. Data analysis
Patients were divided into three groups based on IONM interpretation. In group 0, there were unimodal signal drops that were transient and resolved back to baseline by the end of surgery. In group 1, there were sustained signal changes in either MEPs or SSEPs, while in group 2, there were sustained drops in both MEPs and SSEPs (Table 1). The incidence of neurological deficits in these three different groups was evaluated.
Variables within demographic, clinical, and surgical data were evaluated individually as possible risk factors that may affect IONM signals. Sensitivity, specificity, PPV, and negative predictive value (NPV) of MEP and SSEP in cervical spine surgery were calculated. A subanalysis was performed for the incidence C5 palsy and its correlation with neuromonitoring signal alerts.
Graph Pad Prism ver. 5.0 (GraphPad Software Inc., San Diego, CA, USA) was used for all statistical analyses. Continuous variables were reported as means with standard deviations, except “number of surgical levels” which were reported as median with interquartile range. Categorical variables were reported as percentages. Student t-test was used for determining significance between continuous variables. Fisher’s exact test and chi-square test was used for determining significance between categorical variables as appropriate. A p<0.05 was considered statistically significant.
Results
A total of 257 cases were reviewed and 207 patients were analyzed after excluding those without detectable baseline MEP or SSEP signals preoperatively. The mean and median age of patients was 58 years old; 79% (163/207) of patients were males, 50% (104) had cervical myelopathy, and 18% (38/207) had OPLL; 50% (103/207) of surgeries involved three levels or more, 52% (108/207) were done via the anterior approach, 45% (94/207) were done via the posterior approach, and 2% (5/207) were done via both anterior and posterior approach at the same setting. Only 7% (14/207) of patients had severe preoperative neurological deficit with an ASIA impairment scale of either A, B, or C. Emergent surgeries contributed to 12% (25/207) of cases. The incidence of new neurological deficit after surgery was 1% (2/207).
1. Comparison between three groups of intraoperative neuromonitoring signal alerts
A total of 52 (25%) cases had IONM signal drops, 10 (5%) of which were in group 0, 35 (17%) in group 1, and 7 (3%) in group 2. Groups 0 and 1 had no neurological deficits, while 29% (2/7) cases in group 2 had new neurological deficits (Table 2). There was no significant difference between groups in terms of demographics, surgical approach and levels, preoperative ASIA impairment scale, and presence of myelopathy or OPLL.
2. Comparison between false-positive and true-negative intraoperative neuromonitoring
Statistical comparison was made between patients with false-positive IONM signal alerts and those with true-negative IONM. There were no significant differences in age, gender, number of surgical levels and surgical approach, presurgical ASIA impairment scale, presence of myelopathy or OPLL, and rate of emergency surgeries (Table 3). Although there were no significant differences, patients with false-positive signal alerts were older and had a higher incidence of cervical myelopathy at 60% compared with 46% in the true-negative group. There was also a higher incidence of OPLL at 24% compared with 16% in the other group.
3. Efficacy of intraoperative neuromonitoring in cervical spine surgery
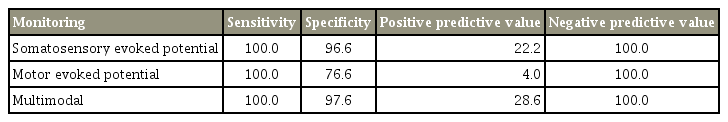
Two by two contingency tables were used to calculate the sensitivity and specificity of IONM in cervical spine surgery. Both MEP and SSEP had a sensitivity of 100%. SSEP had a specificity of 96.6%, while MEP had a lower specificity of 76.6%. The PPV of SSEP was higher at 22.2% compared with 4.0% in MEP. Both tests had NPV of 100%. The specificity of IONM increases to 97.6% and PPV increases to 28.6% when used in conjunction with each other (Table 4).
4. C5 palsy in cervical spine surgery
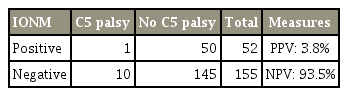
The incidence of C5 palsy in this study was 12 cases (6%), only 2 (17%) of which had any form of IONM signal alert detected (Table 5). A Fisher’s exact test revealed that the occurrence of positive IONM signal alerts in patients who developed C5 palsy and those who did not were similar (p=0.73).
Discussion
The increasing use of IONM in cervical spine surgery in recent years has been contested, mainly because of reports of false negatives and the disadvantages of each of the neuromonitoring modality when used separately [17–19]. It should be noted that some of the studies included isolated C5 palsies as neurological deficits that contributed to false negatives [17]. Despite the controversy, Fehlings et al. [16] revealed a consistently high sensitivity of 70%–100%. In view of a variety of evidence, our study aimed to determine the incidence of positive IONM signal alerts and when it was clinically significant.
In our study, we found that IONM signal alerts were relatively common, occurring in 25% of all cases. However, the overall incidence of postoperative true neurological deficit was only 1% in our study, which fell well within previously reported range of 0.2%–3.2% [17,20]. This meant that most (96%) of the signal alerts were false positives. To help the surgeon interpret these high false-positive rates, we identified that group 2 (sustained, bimodal) signal drops in SSEPs and MEPs had the highest rate of neurological deficits at 29%. There was no occurrence of neurological deficits in unimodal signal alerts, regardless of whether the alert was transient or sustained. Hence, the highest level of caution should be taken if there are concurrent signal drops in MMEP and SSEPs intraoperatively as it is prognostic for a postoperative neurological deficit [12,21].
There were no statistically significant differences in variables that could account for false-positive signal alerts (Table 3). However, a trend was found in patients with cervical myelopathy to have false-positive signal alerts. The incidence of false-positive signal alerts in those with myelopathy was higher, at 29%, compared with those who were not myelopathic, at 19%. This result replicates the higher rate of false-positive IONM alerts in patients with cervical myelopathy seen in previous studies [17,20].
We found an incidence of 6% in C5 palsy, which was also within the previously reported range of 0%–30% [22–25]. No correlation was found between occurrence of C5 palsy and positive IONM signal alerts (p=0.73). The sensitivity of IONM in detecting C5 palsy was 17% with a specificity of 26%. This may be artificially low due to the fact that we chose not to look at EMG records as evidence to show that real-time EMG monitoring was sensitive to C5 palsies, while SSEPs and MEPs were not [16].
There are several limitations in our study. The retrospective nature of this study makes it prone to recall bias. A large proportion of cases were excluded based on not having detectable baseline MEP or SSEP signals, some of which utilized unimodal IONM during surgery. Exclusion of these cases may skewer the efficacy of the individual modalities. Despite having 207 patients, the sample size maybe too small to produce significant results and this may be the reason that there were no statistically significant risk factors for false-positive IONM alerts. In addition, certain pertinent factors such as length of surgery or estimated blood loss could not be obtained from available records.
Conclusions
The incidence of positive signal drops in IONM for cervical spine surgeries was 25%. Bimodal IONM using MEP and SSEP provided sensitivity of 100% and specificity of 97.6% in detecting new neurological deficits, while the sensitivity in detecting C5 palsies was only 17%. Our study showed that by grouping the signal alerts into transient or sustained and unimodal or bimodal, the surgeon will be able to better predict which signal drops are clinically significant, thereby allowing for improved intraoperative decision making for remedial measures.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.