Treatment Strategy for Metastatic Spinal Tumors: A Narrative Review
Article information
Abstract
Metastatic spinal tumors are common, and their rising incidence can be attributed to the expanding aging population and increased survival rates among cancer patients. The decision-making process in the treatment of spinal metastasis requires a multidisciplinary approach that includes medical and radiation oncology, surgery, and rehabilitation. Various decision-making systems have been proposed in the literature in order to estimate survival and suggest appropriate treatment options for patients experiencing spinal metastasis. However, recent advances in treatment modalities for spinal metastasis, such as stereotactic radiosurgery and minimally invasive surgical techniques, have reshaped clinical practices concerning patients with spinal metastasis, making a demand for further improvements on current decision-making systems. In this review, recent improvements in treatment modalities and the evolution of decision-making systems for metastatic spinal tumors are discussed.
Introduction
The spine has been identified as the most common site for malignant metastasis in the musculoskeletal system, and, vice versa, spinal metastasis is considered the most common malignant lesion in the spine [1]. The incidence of metastatic spinal tumors has seen an increasing trend due to the growing aging population worldwide and continued improvements in survival rates among cancer patients [2]. Symptomatic spinal metastasis is often the first clinical manifestation for 12%–20% of cancer patients [3], whereas up to 40% of cancer patients may experience spinal metastasis at some point during the course of their disease [4]. The objectives of surgical treatment in patients with metastatic spine tumors are mostly palliative. Spine surgeons make an effort to maintain or improve the patient’s quality of life during the remainder of their survival by reducing pain and preserving ambulatory function via surgical treatment [5].
Because clinical manifestations and treatment responses vary widely among cancer patients, a multidisciplinary decision-making process that integrates medical and radiation oncology together with surgery, along with assistance from pathology and diagnostic radiology, deemed is essential when deciding to conduct surgical treatment for spinal metastasis [6]. In the literature, various decision-making systems have been developed and introduced to date in an effort to aid in this decision-making process [7]. However, recent advancements in the treatment of metastatic spine tumors have injected more complexity into this decision-making process and demanded the evolution of the decision-making system itself; these recent advances include (1) the development of stereotactic spine radiosurgery (SRS), (2) the introduction of minimally invasive surgical techniques, and (3) the evolution of various target therapies for individual primary cancers [8]. In this review article, we discussed the development and evolution of various decision-making systems in spinal metastasis treatment. Current concepts and recent trends in radiotherapy and surgery for spinal metastasis are also included.
Decision-Making Systems for Managing Metastatic Spinal Tumors
Prognostic factors for metastatic spinal tumors
When attempting to choose an appropriate treatment for a patient with spinal metastasis, establishing an accurate estimation of the individual’s life expectancy is the most crucial. To do this, one must first identify prognostic factors associated with the survival of patients with spinal metastasis. As such, many authors have conducted studies to try and identify prognostic factors associated with survival among spinal metastasis patients and have developed various decision-making systems in order to estimate survival based on these prognostic factors [9-14].
In a recently published meta-analysis, Luksanapruksa et al. [15] have identified 17 independent prognostic factors associated with the survival of patients with spinal metastasis. Among these 17 factors, nine factors (52.9%) can be classified as relating to the preoperative performance or neurological status of a patient—for example, the Karnofsky Performance Score or Eastern Cooperative Oncology Group grade [16]. Meanwhile, four factors (23.5%) involve the presence or the number of metastases (spine, bone, or visceral), and two factors (11.8%) were found to be related to primary tumors in the Tomita classification scheme [9]. Finally, male sex and the time interval from cancer diagnosis to the start of radiotherapy are the two remaining prognostic factors independently associated with survival in spinal metastasis patients. Among these, primary tumor histology, the presence and number of metastases, and performance status are proposed as the three most important prognostic factors associated with survival in spinal metastasis patients not only in this study but also in most previous investigations [7].
In other studies, the patient’s age and comorbidities, assessed using the American Society of Anesthesiologists physical status [17] and Charlson Comorbidity Index [18], have also been identified to be prognostic factors in spinal metastasis [7,19,20]. Other authors have identified laboratory abnormalities such as leukocytosis and low hemoglobulin and albumin levels as prognostic factors and included these into their decision-making systems [7,20,21]. In addition, previous systemic treatment or chemotherapy has also been suggested as an independent prognostic factor by multiple authors [20,22]. Further, not only preoperative chemotherapy but also the presence of available systemic treatments in the postoperative period has been hypothetically regarded as a potential prognostic factor in the literature [8]. In a recently published study by Chang et al. [23], the authors verified the presence of the remaining systemic treatment options to be independently associated with improved postoperative performance status and survival.
Classification-based decision-making systems
Based on these prognostic factors, numerous decision-making systems or “scoring systems” have been developed and introduced to estimate the life expectancy among spinal metastasis patients [9-14]. In these “classification-based” decision-making systems, scores for each prognostic factor identified by multivariate logistic or proportional hazards regression analyses are integrated in order to obtain a total prognostic score that reflects the estimated survival of the patient. Surgeons can adopt these prognostic scores to identify patients with an estimated survival profile that is sufficient to warrant surgical treatment. Although the prognostic factors included in each system vary, primary tumor histology and visceral metastases are included in most systems (Table 1).
In 2015, the New England Spinal Metastasis Score (NESMS) was introduced by Ghori et al. [21]. The NESMS was developed using multicenter data and retrospectively validated in the following investigations [24,25]. The NESMS consists of modified Bauer score components and score, serum albumin level, and ambulatory status of the patient. More recently, the NESMS was validated prospectively in the Prospective Observational Study of Spinal Metastasis Treatment trial, which aimed to verify the NESMS as a reliable predictive tool in spinal metastasis patients [26,27].
There have been efforts to develop novel decision-making systems using evolving methodologies. In 2016, the Skeletal Oncology Research Group (SORG) compared multiple prognostic survival algorithms, including classic, nomogram, and boosting algorithms, using the same retrospective dataset obtained from 649 patients [20]. In their study, the nomogram was found intuitive and demonstrated a comparable level of performance. Then, in 2019, the SORG used machine-learning algorithms to develop a novel prognostic model for metastatic spinal disease [28], which was externally validated in subsequent studies [29,30].
Although these various “classification-based” decision-making systems are helpful and widely used for predicting the survival of spinal metastasis patients, recent studies have reported that the degree of accuracy of these classic systems (e.g., Tomita, Tokuhashi) decreases over time, especially in cancers with poor prognoses, such as lung cancer [19,31-33]. This pitfall of “classification-based” decision-making systems reportedly stems from the inability of these systems to reflect survival improvement due to recent evolutions in systemic treatment for primary cancers [34]. Another existing limitation is that these systems cannot directly guide the selection of specific treatments appropriate for patients with spinal metastasis.
Principle-based decision-making systems
As an alternative to these “classification-based” decision-making systems that are incapable of reflecting recent advances in oncology and guiding specific treatments, several authors have proposed “principle-based” decision-making systems. These “principle-based” systems do not “score the patient” and estimate survival but instead provide advice regarding which treatment is more appropriate in individual cases based on the integration of rapidly evolving treatment modalities, including target therapies, radiosurgery, and minimally invasive surgical techniques.
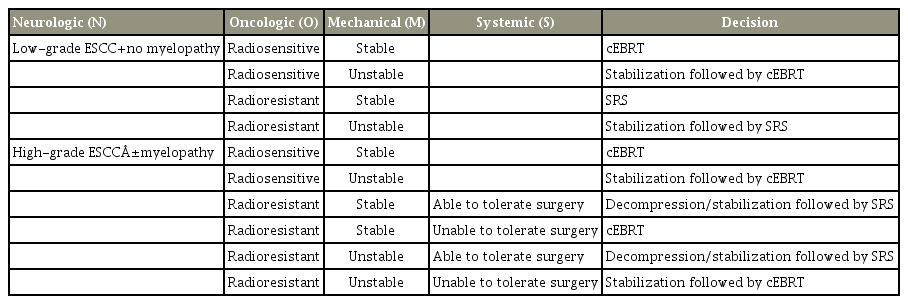
The neurologic, oncologic, mechanical, and systemic (NOMS) decision framework was first introduced in 2006 [35]. The NOMS decision framework consists of neurologic (N), oncologic (O), mechanical (M), and systemic (S) considerations, integrating novel multimodal therapies including SRS and minimally invasive surgical techniques [36]. As a neurological (N) assessment approach, the grading system developed by Bilsky and Smith [35] was used, while surgical decompression is recommended for high-grade spinal cord compressions. During oncological (O) assessment, the responsiveness of spinal metastasis to currently available treatments, especially the level of tumor sensitivity to radiotherapy, is evaluated. Mechanically (M), instability of the spinal column as determined by the Spinal Instability Neoplastic Score (SINS) indicates the need for surgical stabilization, regardless of the neurologic or oncologic status [37]. Finally, systemic (S) assessment focuses on the patient’s ability in tolerating the suggested treatment. Meanwhile, if the general condition, performance status, and medical comorbidities of a patient do not allow surgery to be performed, radiotherapy is instead recommended.
A modification of the NOMS decision framework has also been presented in the literature. Paton et al. introduced the “LMNOP” system as an improvement to the NOMS approach [38]. With this development, these authors added two additional key considerations to the NOMS as follows: (1) the location and levels of metastasis (L) and (2) the patient’s response to previous therapy (P). The P in “LMNOP” stands for not only the response to prior therapy but also includes patient fitness and prognosis, which was considered previously as part of the systemic (S) assessment in the NOMS decision framework. The authors emphasized that the response of primary cancer to previous treatments, including chemotherapy and radiotherapy, is considered a significant factor when determining the appropriate treatment for spinal metastasis patients. For instance, it is anticipated that a patient diagnosed with symptomatic spinal metastasis at the initial presentation of primary cancer (synchronous metastasis), who would have multiple potential treatment options, is more likely to experience a better prognosis than a patient diagnosed with spinal metastasis despite previous treatment (metachronous metastasis). Differences in the survival rates between the patients with synchronous and metachronous spinal metastasis have been confirmed in a previous research [23,39]. Therefore, a more aggressive approach, including surgical treatment, can be considered for patients with a synchronous spinal metastatic lesion. In summary, “principle-based” decision-making systems relative to “classification-based” systems are able to better incorporate evolving treatment modalities and guide the selection of appropriate treatments for patients in a timely fashion (Table 2).
Current trends and future directions in the development of decision-making systems
Advances in cancer biology and treatment modalities are necessitating the evolution of decision-making systems for spinal metastasis. Possible future directions to take to improve decision-making systems include the following: (1) the use of multicenter or multinational databases, (2) the integration of histology-specific data, (3) the application of computational methodologies such as machine-learning algorithms, and (4) the combination of classification-based and principle-based systems. Some recent studies are already covering these trends.
The size of the study sample under assessment determines the performance and accuracy of prognostic models. Although spinal metastasis is found to be relatively common, data from a larger sample population beyond that of just a single institution is usually required to develop a powerful enough prognostic model. For this reason, recently introduced prognostic models or decision-making systems are generated from multicenter databases [20,21]. Future prognostic models should also have the freedom to rely on even larger databases such as multinational tumor registries.
Biologic therapy, including molecular target therapy and immunotherapy, is believed to be an emerging gamechanger in modern cancer treatment. Genetic subtype analysis of the primary cancer histology, which guides selection among t hese therapies, has become more essential [40]. In a revised prognostic system proposed by Katagiri et al. [14], the authors considered the availability of molecular target therapy when classifying the primary tumor. For example, lung cancer treated with targeted drugs was designated as an example of a moderate-growth tumor, while lung cancer without targeted drugs is regarded as an example of a rapid-growth tumor [14]. This application of genetic profiles to decision-making systems is likely to grow more specific and tailored, corresponding to the evolution of molecular genetics in the future.
As previously described, machine-learning algorithms have been applied in the development of prognostic models. Classically, prognostic models for spinal metastasis have been developed using logistic or proportional hazards regression analyses. As part of its research efforts, the SORG was able to develop prognostic models using machine-learning algorithms such as gradient boosting, decision trees, random forests, and neural networks [20,41], and these algorithms were externally validated elsewhere [29]. Like in other fields of medicine, evolving computational methodologies, including machine-learning algorithms, should be assessed extensively in terms of their potential in the management of spinal metastasis.
Finally, the combination of classification-based and principle-based decision-making systems should be considered. Classification-based systems, or prognostic models, seek to estimate the patient’s remaining survival. Based on this survival estimation, principle-based systems then may suggest the most appropriate treatment option. Until now, surgeons and physicians have been employing these two separate systems in the same decision-making process. A novel decision-making system could integrate these two systems together and provide survival estimations and appropriate treatment options simultaneously.
Radiotherapy for Metastatic Spinal Tumors
Stereotactic spine radiosurgery is triggering a paradigm shift
Evidence in the literature supports that radiosurgery is a safe and effective modality for local tumor control, with low associated complication rates in patients with spinal metastasis [8]. Technical improvements, including intensity-modulated and image-guided radiation delivery and processing software, have allowed SRS to be a gamechanger in the treatment of spinal metastasis [42]. A recent study by Yamada et al. [43] reported that a high-dose single session of SRS achieved durable long-term control of spinal metastasis regardless of histology and tumor size. Of note, the only significant factor associated with tumor control was the radiation dose. These results suggest that SRS can be effective even in cases of metastasis previously considered to be radioresistant.
SRS can also be a definitive treatment for the management of solitary metastasis without spinal cord compression [44]. Excellent local control rates of 84%–88% have allowed SRS to replace curative surgeries with high morbidities, such as total en bloc spondylectomy, for addressing these solitary metastases [45,46]. In patients with high-grade spinal cord compression, SRS can be applied after separation surgery, which will be discussed further in the following section. Overall, the effectiveness of SRS has been changing the role and extent of surgical treatment and, in turn, shifting the focus of the treatment of spinal metastasis.
Vertebral compression fracture following stereotactic spine radiosurgery
A pitfall of SRS is an increase in vertebral compression fracture (VCF) following radiotherapy. Risk estimates for VCF after SRS are reported to be up to 40% as compared with just 5% in relation to conventional radiotherapy [47]. The occurrence of VCF is dose-dependent, and caution is required if the radiation dosage exceeds 20 Gy per fraction in high-risk patients [48]. High-risk patients of older ages, with lytic lesions, and/or with spinal malalignment, can reportedly benefit from undergoing preventive stabilization surgery before SRS. When determining the necessity of stabilization surgery before SRS, SINS can be used to identify potentially unstable lesions [37]. SINS will be further covered in the following section.
Timing of radiotherapy after surgery
Adequate timing of radiotherapy following surgery, whose determination is related to the risk of wound complications, continues to be debated among spine surgeons and radiation oncologists. It is also controversial whether the interval can be shortened in patients receiving SRS. Lee et al. [49] sent questionnaires to 86 radiation oncologists and 27 spine surgeons to gather opinions on the optimal timing of surgery and radiotherapy in spinal metastasis. Based on the procured comments, the authors recommended that the interval be, at minimum, 2 weeks, regardless of radiation modalities. Interestingly, as compared with radiation oncologists, surgeons tended to favor a shorter interval of time between surgery and radiotherapy when SRS is performed, although there was no statistically significant difference in this regard [49].
A recently published systemic review also advocated for 2 weeks (with a minimum of 7 days) between surgery and radiotherapy [50]. When the rates of wound complications were compared between SRS and conventional radiotherapy, many studies reported reduced wound complications in SRS patients [51-53]. However, due to limited high-level evidence, no definite conclusion was made regarding whether the interval could be reduced in patients undergoing SRS.
Surgery for Metastatic Spinal Tumors
Surgical indications
The objective of surgical treatment in spinal metastasis was to provide pain relief; support neurological improvement; and, in turn, enhance the quality of life during the remaining survival period. Clinical benefits of direct surgical decompression in patients with metastatic spinal cord compression (MSCC) have been well described in the literature [54]. The most important prerequisite for surgical treatment in spinal metastasis has been identified as the sufficient enough estimated survival time to make surgery a reasonable approach. Researchers have largely recommended that a minimum of 3 to 6 months of remaining survival should exist when considering whether to perform surgery [55,56]. At this point, a number of prognostic scoring systems previously described are being used to estimate a patient’s survival. The patient should also have a good enough general condition or performance status to in order to endure surgery. If these conditions are satisfied, then surgery can be performed in patients with symptomatic MSCC or mechanical instability.
In spinal metastasis, the instability is assessed by the SINS [37] (Table 3). The SINS is an independent and unique tool that integrates clinical and radiological components to help surgeons decide whether to conduct surgery for stabilization. A score of 7 to 12 points suggests impending instability, while that of 13 points or more indicates existing spinal instability, which requires stabilization. As previously mentioned, the SINS has also been incorporated into principle-based decision-making systems such as the NOMS and LMNOP systems [36,38]. Recent studies have reported the reliability and accuracy of the SINS system in predicting spinal adverse events, including VCF, especially in those patients who received radiotherapy [57-59], although some components may still require revision [60].
Separation surgery and minimally invasive surgery
When considering the surgical techniques used for spinal metastasis, the literature shows a trend toward the adoption of less invasive techniques, which is thought to have primarily resulted from recent advances in radiotherapy, as previously described [8]. With the use of advanced radiation techniques, surgeons can minimize surgical morbidities by avoiding extensive debulking surgery [61] (Fig. 1). During the separation surgery, circumferential spinal cord decompression is performed only to the extent required to facilitate safe radiosurgery. In a study by Laufer et al. [62], separation surgery followed by postoperative SRS resulted in a low local progression rate. Other studies have also reported that this hybrid surgery–radiosurgery approach is a safe and effective treatment option for MSCC [63,64].
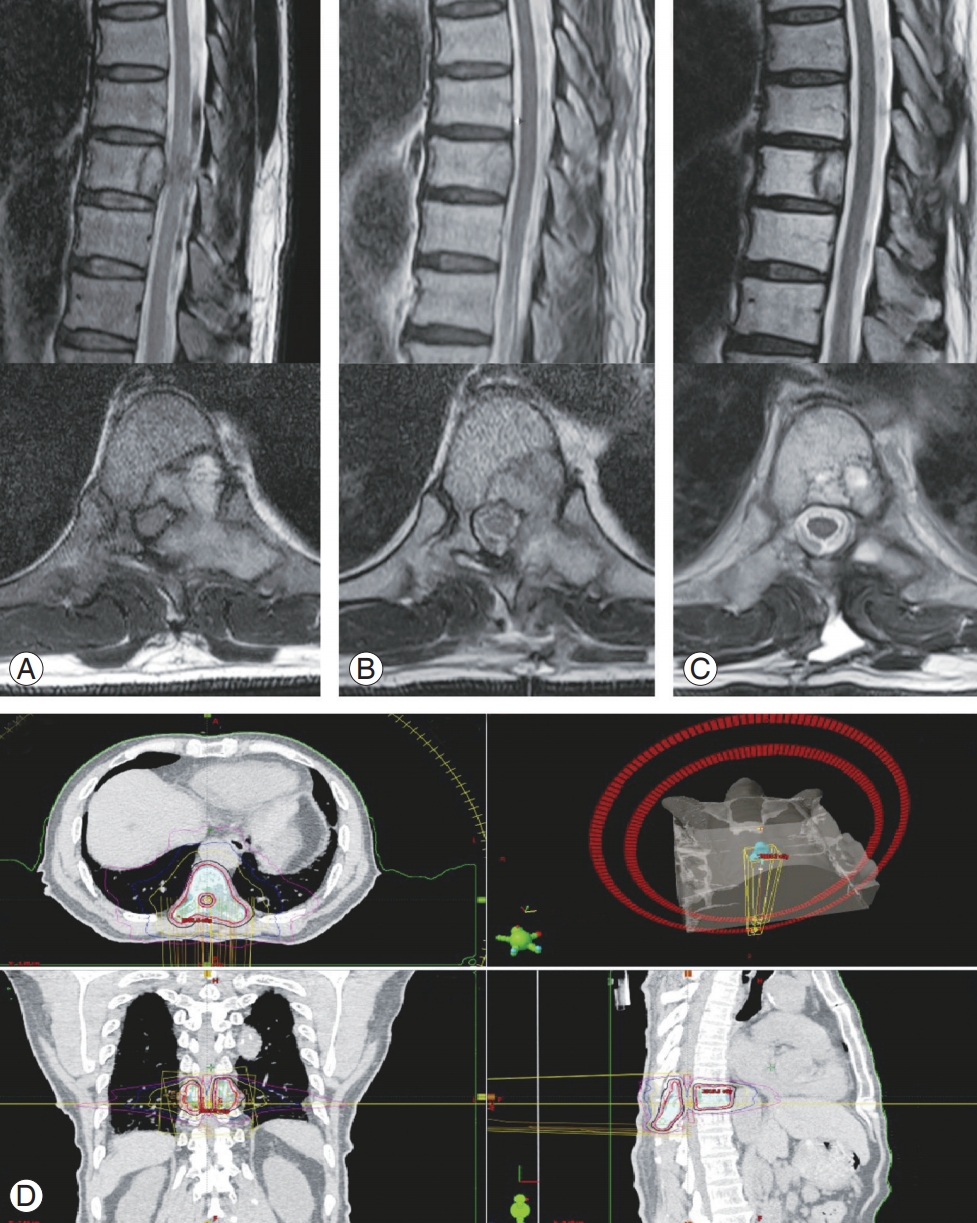
A 68-year-old male with spinal metastasis of renal cell carcinoma at T9. The patient also had lung metastasis. (A) Preoperative MRI shows spinal cord compression and involvement of posterior elements and left rib head at the T9 level. (B) Following a separation surgery without spinal instrumentation, MRI at postoperative 2-weeks shows a decompressed spinal canal but residual metastatic tumor around the left 9th rib head. (C) In the postoperative 1-year MRI, the patient showed a near-complete response following a single session stereotactic radiosurgery (18Gy/1 fraction), which was performed 3 weeks after the separation surgery. MRI, magnetic resonance imaging. (D) Planning images for the postoperative stereotatic radiosurgery following a separation surgery.
“Minimal-access” surgery is also used in treating spinal metastasis while reducing surgical morbidities. In the anterior approach, retractor systems and thoracoscopic assistance have been described [65,66]. Minimally invasive posterior approaches for decompression and corpectomy have also been introduced and reviewed in the literature [67,68]. Other minimally invasive techniques for spine surgery, such as the intraoperative stereotactic navigation system and percutaneous pedicle screw instrumentation, are being incorporated into spinal metastasis surgery as well [64,69].
Studies comparing minimally invasive surgery and open surgery showed that minimally invasive surgery provided equivalent or superior outcomes with reduced surgical morbidity and complications in spinal metastasis patients [70-72]. However, because the quality of evidence is deemed low in the current literature, no definite conclusion regarding the superiority of minimally invasive surgery over open surgery can be derived, and no strong recommendations have been made at this point [73].
Role of curative surgery (en bloc resection)
Most surgeries for spinal metastasis are found palliative, and the role of en bloc resection in spinal metastasis is decreasing even further due to improvements in radiotherapy. Generally, curative surgical resection of spinal metastasis has been considered in the context of a single metastasis of a slow-growing tumor, such as in renal cell, thyroid, and breast cancers (Fig. 2). Favorable outcomes in this regard have been reported in the literature [74-76]. However, some authors recently reported that curative surgical resection (en bloc spondylectomy) did not impact the oncologic outcomes of spinal metastasis patients [77]. Therefore, a more careful and thorough decision-making process is required before performing curative resection surgery for spinal metastasis, especially when the extended role of radiosurgery is considered.

A 63-year-old male with spinal metastasis of thyroid carcinoma at T8. (A) Preoperative MRI shows pathologic fracture and spinal cord compression at the T8 level. (B) Postoperative X-ray at 1 month shows removal T8 vertebra and reconstruction with an expandable cage following total en bloc spondylectomy. (C) MRI at postoperative 3-years shows a widely decompressed spinal canal with no tumor recurrence. (D) Postoperative X-ray at postoperative 5-years shows well-maintained instrumentation. (E) Bone scan at postoperative 5-years shows no evidence of bone metastasis. MRI, magnetic resonance imaging.
Postoperative complications and preventive measures
The overall complication rate following surgery for spinal metastasis ranges from 10% to 66.7% in the literature [78]. Because surgery for spinal metastasis is performed to improve the quality of life of a patient, surgeons should try to minimize all possible surgical and medical complications by implementing multidisciplinary interventions. Among diverse complications, those that require additional attention when found in spinal metastasis patients (e.g., wound infection, instrumentation failure, and intraoperative bleeding) are briefly discussed here.
The incidence of surgical site infection (SSI) is determined to be higher in spinal metastasis surgery, reaching up to 30%, as compared with during other spine surgeries [78]. SSI has also been identified as the most common cause for reoperation (in 42% of reoperations) following surgery for spinal metastasis [79]. Poor nutrition and exposure to adjuvant therapies (chemotherapy and radiotherapy) put spinal metastasis patients at risk for SSI [80]. Surgeons should provide adequate nutritional support perioperatively and secure a sufficient time interval between radiation and surgery, as previously discussed, to minimize SSIs. Additional risk factors, such as smoking, obesity, and medical comorbidities, should also be considered [81].
The second cause for reoperation in spinal metastasis surgery is the failure of instrumentation [79]. Risk factors associated with instrumentation failure include the number of operated levels, prior chest wall resection, and history of radiotherapy [82,83]. The necessity of additional fusion procedures while performing surgery for spinal metastasis is debated, but high-level evidence remains to be lacking at this time [80]. In future studies, we suggest that instances of early and late failure be distinguished when assessing instrumentation failure because the mechanisms of failure between the two types seem to differ, i.e., insufficient stability of the construct in the context of early failure versus the progression of deformity or lack of fusion in the context of late failure.
Intraoperative bleeding during spinal metastasis surgeries can be massive and can further lead to serious complications such as cardiovascular or cerebral events [84]. For the spinal metastasis of hyper-vascular tumors, such as renal cell, hepatocellular, and thyroid cancers, preoperative embolization is recommended. Previous studies have verified the effectiveness of preoperative embolization in reducing intraoperative bleeding in spinal metastasis surgeries [85-87]. Surgery should be performed within 24 hours following embolization to avoid the diminished effect of preoperative embolization [88].
Conclusions
The determination of appropriate treatment for a patient with spinal metastasis is a challenging task that requires multidisciplinary considerations. Recent advances in radiotherapy and surgery for spinal metastasis have brought about improvements in the management of these patients. Evolving decision-making systems are also crucial contributors to state-of-the-art care of patients with metastatic spinal tumors.
Notes
No potential conflict of interest relevant to this article was reported.