Invasiveness Reduction of Recent Total En Bloc Spondylectomy: Assessment of the Learning Curve
Article information
Abstract
Study Design
Case-control study.
Purpose
To evaluate the surgical magnitude and learning curve of "second-generation" total en bloc spondylectomy (TES).
Overview of Literature
In June 2010, we developed second-generation TES combined with tumor-induced cryoimmunology, which does not require autograft harvesting.
Methods
TES was performed in 63 patients between June 2010 and September 2013. Three groups of patients were evaluated: 20 undergoing surgery in the first year of development of second-generation TES (group I), 20 in the second year (group II), and 23 in the third year (group III). Patient backgrounds showed no remarkable differences. Operating time, intraoperative blood loss, blood transfusion, and postoperative C-reactive protein and creatine phosphokinase were compared among the groups.
Results
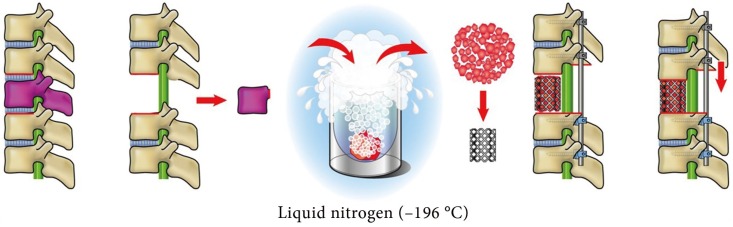
Mean±standard deviation operating time was 486±130 minutes in group I, 441±85 minutes in group II, and 396±75 minutes in group III. The time was significantly shorter in group III than in group I (p<0.05). Intraoperative blood loss was 901±646 mL in group I, 433±177 mL in group II, and 411±167 mL in group III. Blood loss was significantly lower in groups II and III than in group I (p<0.01). Transfusion was not required in 20 of 23 patients in group III, and mean C-reactive protein levels on postoperative day 3 were significantly lower in this group than in group I (6.12 mg/L vs. 10.07 mg/L; p<0.05). Postoperative creatine phosphokinase levels did not differ among the groups.
Conclusions
TES is associated with a significant learning curve. Thus, second-generation TES can no longer be considered highly invasive.
Introduction
Patients with spinal metastasis are generally considered to be in the terminal stage of disease. As a result of local recurrence, poor outcomes are often observed despite surgery like curettage or piecemeal excision. En bloc resection is considered the optimal treatment for malignant spinal tumors, and total en bloc spondylectomy (TES) is a surgical procedure designed to achieve complete en bloc resection while providing an adequate tumor margin [1]. This procedure can decrease the rate of local recurrence and extend survival [23].
As introduced, TES frequently exceeded 10 hours, and was often associated with substantial blood loss exceeding 2,500 mL [2]. In June 2010, we developed the second-generation TES combined with tumor-induced cryoimmunology, which does not require autograft harvesting from the iliac bone or fibula [4]. This greatly reduced the invasiveness of the procedure.
This retrospective study was performed to evaluate the surgical magnitude of second-generation TES, as well as its associated learning curve.
Materials and Methods
1. Ethics statement
This study was approved by our Institutional Ethics Committee. Written informed consent for the surgery and entry into the research study was obtained from each patient.
2. Patient characteristics
TES surgeries were performed by a single surgeon (H.M.) in 82 patients at our university hospital between June 2010 and September 2013. Of these 82 patients, 19 who underwent revision surgery or TES by the anteroposterior double approach were excluded from analysis to assess the surgical magnitude and learning curve more precisely. The remaining 63 consecutive patients who underwent TES by the posterior single approach were enrolled. We evaluated three groups of patients. Group I comprised 20 patients who underwent surgery in the first year of development of second-generation TES. Group II comprised 20 patients in the second year and group III comprised 23 patients in the third year. Patient backgrounds were not notably different among groups. The characteristics of each patient group are presented in Table 1.
Operating time, intraoperative blood loss, volume of concentrated red cell transfusion during surgery, and levels of C-reactive protein (CRP) and creatine phosphokinase (CK) on postoperative days 1, 3, and 7 were compared among the three groups. The level of significance was set at p<0.05, in accordance with the Tukey–Kramer method.
3. Surgical procedure
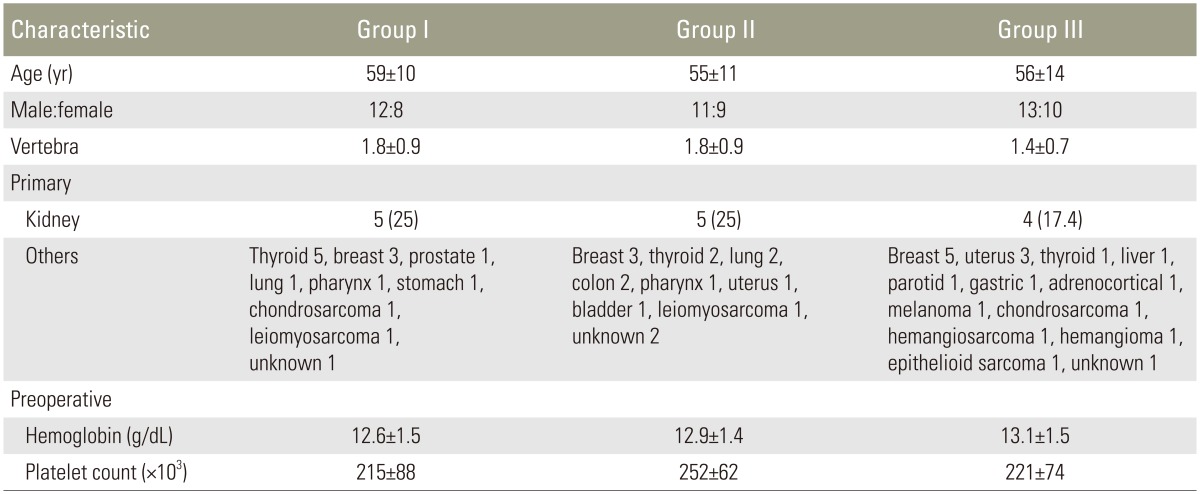
Within 72 hours prior to surgery, preoperative embolization of bilateral segmental arteries was performed for all patients at three levels: in the tumorous vertebra and in two adjacent vertebrae—one cephalad and one caudal. Anesthetic management of intraoperative relative hypotension (systolic blood pressure 80–100 mm Hg) has become common clinical practice. In line with standard practice, the anesthesiologist administered 1 g tranexamic acid (TXA) intravenously at the beginning and closure of TES. In all patients, following en bloc excision of the vertebra or vertebrae, the resected lamina and vertebral body were used as bone graft for anterior spinal reconstruction. After en bloc laminectomy and corpectomy, the tumor and soft tissues including the ligament, disk, and cartilage were curetted away from the resected spine. The spine was then placed in liquid nitrogen (–196℃) for 20 minutes before being crushed and packed into a titanium cage, which was placed between the adjacent healthy vertebral bodies. Finally, spinal shortening was performed to stabilize the cage (Fig. 1).
Results
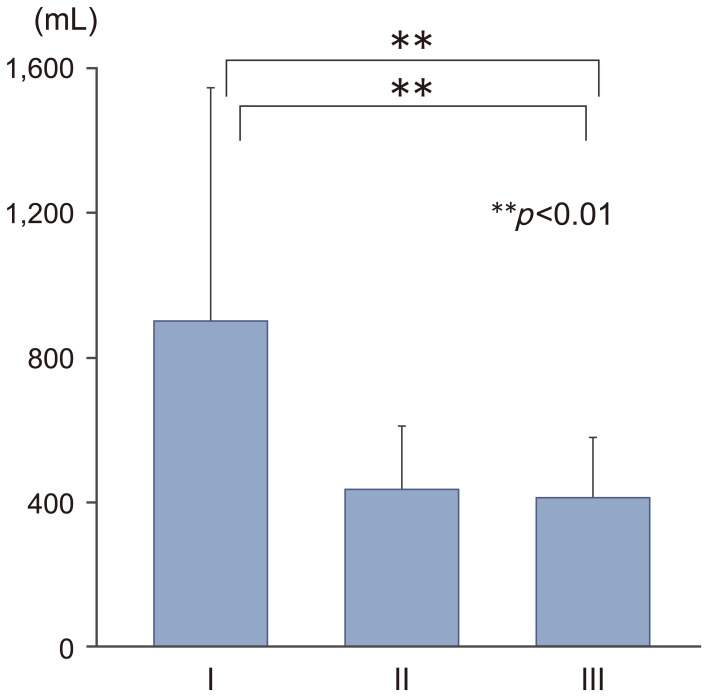
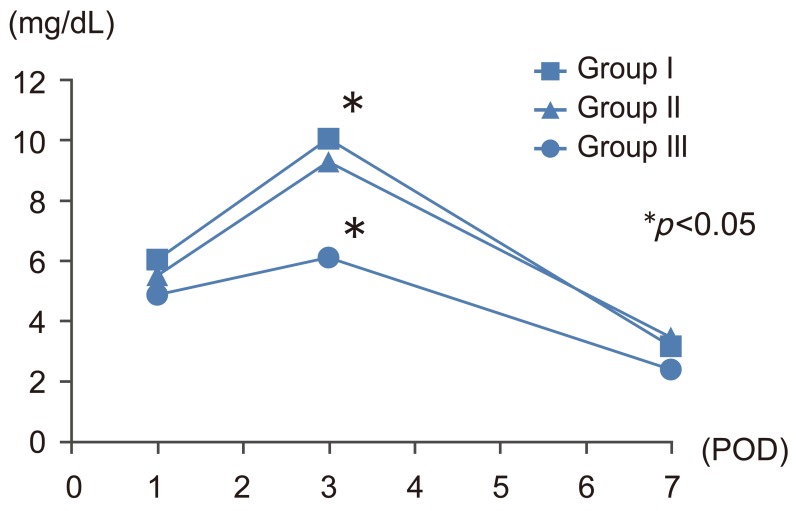
Mean±standard deviation operating time was 486±130 minutes in group I, 441±85 minutes in group II, and 396±75 minutes in group III (Fig. 2). The operating time was significantly shorter in group III than in group I (p<0.05). Intraoperative blood loss was 901±646 mL in group I, 433±177 mL in group II, and 411±167 mL in group III (Fig. 3). Blood loss was significantly lower in groups II and III than in group I (p<0.01). Over the study period, the volume of blood loss diminished in parallel with the surgeon's increasing experience in performing the surgery (y=–310.9ln(x)+1562.9; R2=0.3931; case number, x; intraoperative blood loss, y mL); blood loss exceeding 700 mL was not observed during any operation in group III (Fig. 4). Furthermore, 20 of the 23 patients in group III did not require blood transfusion during surgery, and mean CRP levels on postoperative day 3 were significantly lower in this group than in group I (6.12 mg/L vs. 10.07 mg/L; p<0.05) (Fig. 5). Postoperative CK levels did not differ among the groups (Fig. 6).
Learning curve of total en bloc spondylectomy as shown by intraoperative blood loss. POD, postoperative day.

Changes of C-reactive protein at 1, 3, and 7 days after total en bloc spondylectomy. POD, postoperative day.
Discussion
TES is associated with a significant learning curve. The term learning curve was first used in the medical literature in the 1970s, and its use has increased with the development of novel minimally invasive surgical techniques based on laparoscopy, such as laparoscopic cholecystectomy [5]. References to learning curves in the field of spinal surgery became especially prevalent in the mid-1990s, with the development of laparoscopic lumbosacral fusion [6] and arthroscopic discectomy [7]. In recent years, many studies have analyzed the learning curves for surgical techniques, especially since the introduction of thoracoscopic surgery [89]. Operating time is considered the most representative measure of assessing a learning curve because it tends to gradually decrease with the surgeon's experience [5]. In this study, however, regression analysis showed that the coefficient of determination (R2) for operating time (y=–47.92.9ln(x)+591.84; R2=0.1729; case number, x; operating time, y minutes) (Fig. 7) was not as high as that for intraoperative blood loss. This is because we considered reducing intraoperative blood loss to be more important than shortening operating time.
1. Methods of preventing excessive bleeding
1) Preoperative embolization
Intraoperative bleeding during TES can be excessive in patients with hypervascular spinal tumors. Preoperative embolization of the feeding artery at the affected vertebra is undoubtedly critical, but is sometimes insufficient to stop bleeding. In a canine study, ligation of bilateral segmental arteries at three levels reduced blood flow to the middle vertebra by 25% relative to the control group [10]. Total spinal cord blood flow was maintained at 80%, and full spinal cord function was preserved [11].
Based on these data, segmental arteries above and below the tumor were embolized preoperatively at three levels, in addition to the feeding artery [1011]. Our clinical results have shown that this embolization technique considerably reduces intraoperative bleeding from the tumor-involved vertebra, without compromising spinal cord function [12].
2) Perioperative administration of TXA
TXA is a synthetic anti-fibrinolytic drug that is widely-used to reduce perioperative blood loss in clinical surgery [13]. Anti-fibrinolytics reduce the requirement for blood transfusion during cardiac surgery, total knee arthroplasty, and urological surgery [14]. TXA could reasonably be expected to confer similar benefits in spinal surgery. While the use of TXA could theoretically increase the risk of thrombosis, no thrombotic complications have been reported to date [1516]. In line with standard practice, the anesthesiologist administered 1 g TXA intravenously at the beginning and closure of TES.
3) Hypotensive anesthesia
Anesthetic management of intraoperative relative hypotension (systolic blood pressure 80–100 mm Hg) has become common clinical practice. This has not been found to influence spinal cord blood circulation.
4) Careful homeostasis
Controlling bleeding from the epidural venous plexus and paravertebral muscle is essential during TES. To safely and effectively arrest bleeding from the epidural veins, we used curved-tip bipolar forceps (Fig. 8). A correlation between transfusion and postoperative infection in elective surgical patients has previously been reported [1718]. Furthermore, increased recurrence rates in patients with malignant tumors have been related to immunomodulation resulting from transfusion; other consequences like multiple-organ failure and mortality have similarly been attributed to pro-inflammatory immunomodulation following transfusion [1920]. In this investigation, transfusion was not needed in 20 of 23 patients operated in the final and most recent year of the study (group III), demonstrating the increased safety of second-generation TES. There is additional scope to extend surgical indication for compromised cancer patients.
Conclusions
Second-generation TES can no longer be considered highly invasive. In the past, the indication for TES was limited because of its technically demanding procedures and use in patients who often have complicated medical backgrounds, such as cancer. However, we recommend that the indication for TES be extended.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.